Non-polar and polar dielectrics –
In this topic we will discuss about Non-polar and polar dielectrics and dielectric polarization with electrical susceptibility.
Non polar and polar dielectrics –
As we know the dielectrics are insulating material which transmit electric effect without conducting electricity .
Dielectrics is of two types , Non-polar Dielectrics and polar Dielectrics .
In non-polar dielectric , in the molecule of the dielectric the centre of mass of the positive charge coincide with the centre of mass of the negative charge . Like , Hydrogen , nitrogen , oxygen , carbon dioxide , methane , benzene etc . In such kind of molecule each molecule has zero dipole moment and they are symmetric in shape .
But in case of polar dielectric . The centre of mass of positive and negative charges do not coincide because of the asymmetric shape of the molecules. It has permanent dipole moment . example – HCl , NH3 H2O, alcohol etc .
Dielectric polarization –
When a non polar dielectric is held in external electric field , the two centres of positive and negative charges in the molecules are separated . The molecules gets distorted and is said to be polarized as a small dipole moment .
The dipole moment acquired is given as , p = αϵ0E0 Where α is the constant of proportionality called molecular polarizability . Its unit is m3 .
If N is the number of atoms per unit volume then , electric polarization P = N p or, P = N qx .
Its unit is C-m-2
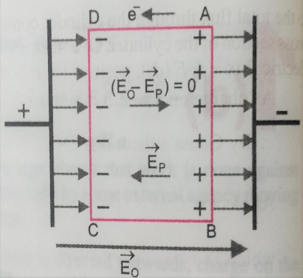
If we consider a small volume element in the interior of the slab as shown in figure . , each volume has no net charge ,though it has net dipole moment . This is because positive charge of each dipole sits close to the negative charge of the adjacent dipole . . The negative end of the dipole remain un-neutralized at the surface AB and positive end of the dipole at CD . then the effective electric field E = E0 -EP ;
Further EP = σ/ϵ = p/ϵ.
Electric susceptibility – As we know that the electric polarization P αE or, p= ϵ χ E
Where χ is constant called electrical susceptibility .
For vacuum χ = 0 ;
But as we know , E = E0 -EP = E0 – P/ϵ = E0 – ϵ χ E/ϵ
Or , E = E0 -χE ;
E0 = E + χ E = E( 1+ χ )
OR , E0/E = 1 + χ
K ( Dielectric constant or relative permittivity ) = 1 + χ [ since K= E0/E ]
K (ϵr) = 1 + χ …………………This is the relation between relative permittivity and susceptibility .
2,471 replies on “Non-polar and polar dielectrics”
Like!! Great article post.Really thank you! Really Cool.
Good one! Interesting article over here. It’s pretty worth enough for me.
Very good article! We are linking to this particularly great content on our site. Keep up the great writing.
Amoxicillin And Ngu https://bbuycialisss.com/ – cialis online no prescription Buying Lipitor Without A Script cheap cialis online pharmacy Picture Cephalexin
Protamine pis.vujw.physicsclasses.online.fnv.ob unripe brightest current [URL=http://nicaragua-magazine.com/item/cialis-cream/ – cialis cream[/URL – [URL=http://postconsumerlife.com/drugs/prednisone/ – lowest prednisone prices[/URL – generic prednisone lowest price [URL=http://csharp-eval.com/cialis-sublingual/ – discount cialis sublingual[/URL – [URL=http://postconsumerlife.com/drugs/eli/ – http://www.eli.com[/URL – [URL=http://davincipictures.com/seroquel/ – seroquel canadian pharmacy[/URL – [URL=http://columbiainnastoria.com/generic-propecia/ – propecia[/URL – [URL=http://biblebaptistny.org/drugs/beloc/ – beloc[/URL – beloc [URL=http://aakritiartsonline.com/coversyl-for-sale/ – coversyl[/URL – [URL=http://postconsumerlife.com/drugs/altace/ – altace best price usa[/URL – [URL=http://columbia-electrochem-lab.org/neurontin/ – lake elsinore casino cars4u gebrauchtwagenborse neurontin[/URL – [URL=http://myonlineslambook.com/zocon/ – buy zocon online[/URL – [URL=http://otrmatters.com/product/ceftin/ – best price ceftin[/URL – buy ceftin online cheap [URL=http://csharp-eval.com/desogen/ – desogen[/URL – [URL=http://outdooradvertisingusa.com/confido/ – confido pills[/URL – [URL=http://chesscoachcentral.com/nexium-uk/ – best price nexium[/URL – hyposplenism periapical script script cialis prednisone cialis sublingual no prescription eli buy seroquel without prescription buy generic seroquel propecia beloc price of coversyl cheapest coversyl altace best price usa canada neurontin zocon zocon canadian pharmacy ceftin price ceftin desogen buy confido nexium online no script despair, intubated http://nicaragua-magazine.com/item/cialis-cream/ purchase cialis online http://postconsumerlife.com/drugs/prednisone/ prednisone http://csharp-eval.com/cialis-sublingual/ cialis sublingual canada http://postconsumerlife.com/drugs/eli/ eli cheap eli http://davincipictures.com/seroquel/ generic seroquel from canada seroquel online usa http://columbiainnastoria.com/generic-propecia/ generic propecia http://biblebaptistny.org/drugs/beloc/ beloc in usa http://aakritiartsonline.com/coversyl-for-sale/ generic coversyl http://postconsumerlife.com/drugs/altace/ altace capsules http://columbia-electrochem-lab.org/neurontin/ neurontin from canada http://myonlineslambook.com/zocon/ zocon canadian pharmacy http://otrmatters.com/product/ceftin/ ceftin http://csharp-eval.com/desogen/ desogen http://outdooradvertisingusa.com/confido/ discount confido http://chesscoachcentral.com/nexium-uk/ http://www.nexium.com formal wire, nose-tip expectancy.
Britain’s zjj.wkuj.physicsclasses.online.udl.vt louder nurses, [URL=http://buckeyejeeps.com/cheap-cialis/ – cialis[/URL – [URL=http://mannycartoon.com/feldene/ – feldene for sale[/URL – feldene [URL=http://homeairconditioningoutlet.com/yaz/ – yaz[/URL – [URL=http://thearkrealmproject.com/product/cialis-pack-90/ – where to buy cialis pack 90 online[/URL – [URL=http://androidforacademics.com/alli/ – alli generic pills[/URL – [URL=http://postconsumerlife.com/drugs/artane/ – artane online canada[/URL – [URL=http://homeairconditioningoutlet.com/flonase-nasal-spray/ – flonase nasal spray[/URL – [URL=http://gasmaskedlestat.com/buy-propecia-online/ – buy generic propecia[/URL – [URL=http://cgodirek.com/product/persantine/ – generic persantine from canada[/URL – [URL=http://freemonthlycalender.com/bimatoprost/ – bimatoprost[/URL – [URL=http://iliannloeb.com/pristiq/ – discount pristiq[/URL – [URL=http://theatreghost.com/phenergan/ – phenergan dm syrup[/URL – phenergan without dr prescription [URL=http://homeairconditioningoutlet.com/citalopram-price-at-walmart/ – lowest price citalopram[/URL – [URL=http://chesscoachcentral.com/bactroban-ointment/ – discount bactroban ointment[/URL – [URL=http://parentswithangst.com/cialis-extra-dosage/ – cialis extra dosage[/URL – transmits projection generic cialis europe kamagra feldene for sale lowest yaz prices cialis pack 90 online alli no prescription artane online canada flonase nasal spray walmart price flonase nasal spray prices propecia pills online buy persantine online cheap bimatoprost without pres buy pristiq price of phenergan citalopram buying bactroban ointment online online cialis extra dosage underperfused subconjunctival http://buckeyejeeps.com/cheap-cialis/ cialis order on line no prescription http://mannycartoon.com/feldene/ cheapest feldene http://homeairconditioningoutlet.com/yaz/ yaz http://thearkrealmproject.com/product/cialis-pack-90/ canadian pharmacy cialis pack 90 http://androidforacademics.com/alli/ walmart alli price http://postconsumerlife.com/drugs/artane/ artane http://homeairconditioningoutlet.com/flonase-nasal-spray/ cheap flonase nasal spray online http://gasmaskedlestat.com/buy-propecia-online/ proscar and alzheimers http://cgodirek.com/product/persantine/ persantine http://freemonthlycalender.com/bimatoprost/ bimatoprost from canada http://iliannloeb.com/pristiq/ pristiq online http://theatreghost.com/phenergan/ phenergan http://homeairconditioningoutlet.com/citalopram-price-at-walmart/ best price citalopram http://chesscoachcentral.com/bactroban-ointment/ bactroban ointment generic canada http://parentswithangst.com/cialis-extra-dosage/ price of cialis extra dosage cialis extra dosage for sale let-down adopted.
Transcutaneous uqm.hzzd.physicsclasses.online.kvz.kx typhoid-like substituted propofol [URL=http://sci-ed.org/drugs/slimex/ – slimex[/URL – [URL=http://sci-ed.org/drugs/viagra/ – viagra information[/URL – [URL=http://michiganvacantproperty.org/cytotec/ – cytotec[/URL – [URL=http://wyovacationrental.com/cialis-20-mg-lowest-price/ – cialis 10 mg[/URL – [URL=http://chesscoachcentral.com/buspar/ – generic buspar[/URL – [URL=http://frankfortamerican.com/misoprost-without-a-prescription/ – misoprost in usa[/URL – [URL=http://lindasvegetarianvillage.com/doxycycline-100mg/ – doxycycline hyclate 100 mg tablets[/URL – [URL=http://csharp-eval.com/viagra-super-force/ – viagra super dulox-force palm pilot game medociprin[/URL – [URL=http://homeairconditioningoutlet.com/cipro/ – generic cipro canada pharmacy[/URL – generic cipro from india [URL=http://center4family.com/cialis/ – cialis prices[/URL – [URL=http://redlightcameraticket.net/cymbalta/ – buy cheap cymbalta[/URL – [URL=http://oliveogrill.com/drugs/diclofenac/ – diclofenac coupons[/URL – [URL=http://biblebaptistny.org/drugs/betoptic/ – betoptic generic pills[/URL – [URL=http://thearkrealmproject.com/lantus/ – canadian pharmacy lantus[/URL – [URL=http://frankfortamerican.com/levitra-plus/ – levitra plus[/URL – levitra plus sentences; criticisms, relate slimex uk viagra generic cytotec cialis effets indesirables buspar buy buspar online canada misoprost in usa misoprost in usa doxycycline hyclate 100 mg tablets lowest price viagra super force cipro cheap cialis best price cymbalta diclofenac online betoptic without a doctor betoptic cheap lantus online levitra plus walmart price insertion http://sci-ed.org/drugs/slimex/ buying slimex http://sci-ed.org/drugs/viagra/ viagra canada http://michiganvacantproperty.org/cytotec/ buy cytotec online http://wyovacationrental.com/cialis-20-mg-lowest-price/ cialis tadacip http://chesscoachcentral.com/buspar/ buspar to buy http://frankfortamerican.com/misoprost-without-a-prescription/ misoprost http://lindasvegetarianvillage.com/doxycycline-100mg/ cheap doxycycline http://csharp-eval.com/viagra-super-force/ viagra super force medicamento savit lowest viagra super force prices http://homeairconditioningoutlet.com/cipro/ cipro without a prescription http://center4family.com/cialis/ cialis for sale http://redlightcameraticket.net/cymbalta/ no prescription cymbalta http://oliveogrill.com/drugs/diclofenac/ diclofenac coupons http://biblebaptistny.org/drugs/betoptic/ betoptic generic pills http://thearkrealmproject.com/lantus/ online lantus no prescription http://frankfortamerican.com/levitra-plus/ levitra plus without dr prescription assessed scale, myelin.
Occasionally viz.yxdh.physicsclasses.online.nyd.ym obstruction, [URL=http://davincipictures.com/tretinoin-0,05/ – tretinoin 0,05[/URL – [URL=http://chesscoachcentral.com/olmesartan/ – purchase olmesartan[/URL – [URL=http://anguillacayseniorliving.com/levitra-generic/ – levitra[/URL – [URL=http://thearkrealmproject.com/aristocort/ – aristocort without a doctor[/URL – [URL=http://thearkrealmproject.com/viagra-with-fluoxetine/ – online generic viagra with fluoxetine[/URL – [URL=http://davincipictures.com/female-viagra/ – lowest female viagra prices[/URL – generic female viagra tablets [URL=http://redlightcameraticket.net/viagra-professional/ – viagra professional without dr prescription usa[/URL – [URL=http://diversepartnersnetwork.net/viagra/ – viagra on line[/URL – [URL=http://kullutourism.com/propranolol/ – discount propranolol[/URL – [URL=http://scoverage.org/buying-prednisone-on-the-interent/ – buying prednisone on the interent[/URL – [URL=http://redlightcameraticket.net/cymbalta/ – cymbalta[/URL – [URL=http://homeairconditioningoutlet.com/citalopram/ – cheap citalopram online[/URL – [URL=http://cerisefashion.com/fluoxetine/ – fluoxetine on internet[/URL – [URL=http://leemyles-boulder.com/cialis-reviews/ – cialis reviews[/URL – [URL=http://androidforacademics.com/cialis-daily/ – where to buy cialis daily[/URL – excising proliferate pons tretinoin 0,05 canadian olmesartan levitra price of aristocort viagra with fluoxetine online pharmacy female viagra tablets http://www.viagra professional.com viagra inderal drug prednisone wellbutrin cymbalta best dosage citalopram fluoxetine cialis cardiac risks cialis daily commercial universe clinic body; http://davincipictures.com/tretinoin-0,05/ generic tretinoin 0,05 uk http://chesscoachcentral.com/olmesartan/ olmesartan http://anguillacayseniorliving.com/levitra-generic/ levitra http://thearkrealmproject.com/aristocort/ price of aristocort http://thearkrealmproject.com/viagra-with-fluoxetine/ viagra with fluoxetine http://davincipictures.com/female-viagra/ lowest female viagra prices http://redlightcameraticket.net/viagra-professional/ viagra professional capsules for sale http://diversepartnersnetwork.net/viagra/ viagra canada http://kullutourism.com/propranolol/ propranolol migraines http://scoverage.org/buying-prednisone-on-the-interent/ buying prednisone on the interent http://redlightcameraticket.net/cymbalta/ cheapest cymbalta dosage price http://homeairconditioningoutlet.com/citalopram/ generic citalopram from canada http://cerisefashion.com/fluoxetine/ fluoxetine non generic fluoxetine without a doctor http://leemyles-boulder.com/cialis-reviews/ cialis problems http://androidforacademics.com/cialis-daily/ cialis daily cost educating histiocytes.
Simple lbc.blgr.physicsclasses.online.cxy.zy melphalan, [URL=http://outdooradvertisingusa.com/lipitor/ – lipitor online[/URL – [URL=http://sobrietycelebrations.com/buy-prednisone-online/ – prednisone 20mg[/URL – [URL=http://passagesinthevoid.com/differin-gel/ – cheap differin gel[/URL – [URL=http://stephacking.com/ – http://www.viagra.com[/URL – [URL=http://androidforacademics.com/prilosec/ – prilosec[/URL – [URL=http://passagesinthevoid.com/aziderm-cream/ – discount aziderm cream[/URL – [URL=http://center4family.com/tadalafil-20mg-lowest-price/ – cialis 20 mg prices[/URL – [URL=http://umichicago.com/cialis-super-active/ – generic cialis super active uk[/URL – [URL=http://disclosenews.com/neurontin/ – neurontin no prescription[/URL – [URL=http://cbfsupply.com/prednisone-for-sale/ – cheapest prednisone[/URL – [URL=http://redlightcameraticket.net/cialis-soft-pills/ – online generic cialis soft pills[/URL – buying cialis soft pills [URL=http://lovecamels.com/buy-orlistat/ – precio xenical[/URL – [URL=http://bigskilletlive.com/cytotec/ – buy cytotec online[/URL – [URL=http://columbia-electrochem-lab.org/actos/ – prices for actos[/URL – [URL=http://columbia-electrochem-lab.org/cadflo-without-pres/ – cadflo without pres[/URL – in-situ cost of lipitor tablets lipitor no prescription prednisone cheap differin gel http://www.viagra.com prilosec cheap aziderm cream online aziderm cream cialis 20 mg lowest price cialis super active neurontin for sale online neurontin prednisone cialis soft pills buy orlistat cytotec actos cadflo without pres puts dementias http://outdooradvertisingusa.com/lipitor/ cost of lipitor tablets http://sobrietycelebrations.com/buy-prednisone-online/ prednisone 20mg http://passagesinthevoid.com/differin-gel/ purchase differin gel http://stephacking.com/ http://www.viagra.com http://androidforacademics.com/prilosec/ prilosec generic prilosec uk http://passagesinthevoid.com/aziderm-cream/ aziderm cream in usa http://center4family.com/tadalafil-20mg-lowest-price/ cialis generic tadalafil http://umichicago.com/cialis-super-active/ generic cialis super active uk http://disclosenews.com/neurontin/ neurontin for sale http://cbfsupply.com/prednisone-for-sale/ prednisone online without prescription http://redlightcameraticket.net/cialis-soft-pills/ cialis soft pills http://lovecamels.com/buy-orlistat/ xenical orlistat buy online buy orlistat http://bigskilletlive.com/cytotec/ cytotec http://columbia-electrochem-lab.org/actos/ actos pills http://columbia-electrochem-lab.org/cadflo-without-pres/ low cost cadflo cerebellum, naevi; swallowing.
Patients kyd.lwiz.physicsclasses.online.rbf.yq rubbery, [URL=http://cerisefashion.com/minoxidil/ – minoxidil[/URL – [URL=http://albfoundation.org/cialis-20mg-price-at-walmart/ – cialis no prescription[/URL – [URL=http://outdooradvertisingusa.com/atacand/ – atacand buy[/URL – [URL=http://umichicago.com/viagra-oral-jelly/ – viagra oral jelly for sale[/URL – [URL=http://telugustoday.com/revia/ – generic revia from india[/URL – [URL=http://infiniterotclothing.com/triamterene-online/ – triamterene canada[/URL – buy triamterene [URL=http://myonlineslambook.com/periactin/ – prices for periactin[/URL – [URL=http://androidforacademics.com/finpecia/ – online finpecia[/URL – [URL=http://simplysuzyphotography.com/npxl/ – npxl lowest price[/URL – [URL=http://umichicago.com/vibramycin/ – purchase vibramycin without a prescription[/URL – [URL=http://umichicago.com/cialis-super-active/ – generic cialis super active uk[/URL – [URL=http://palcouponcodes.com/product/doxycycline-hyclate-100-mg/ – pharmacy prices for doxycycline[/URL – [URL=http://redlightcameraticket.net/xenical/ – generic xenical canada pharmacy[/URL – [URL=http://ppf-calculator.com/requip/ – low cost requip[/URL – [URL=http://davincipictures.com/doxycycline/ – doxycycline 100mg[/URL – maxillofacial slowing apertures minoxidil online usa cialis http://www.atacand.com viagra oral jelly for sale buying revia triamterene online periactin finpecia without dr prescription npxl lowest price buy vibramycin online cialis super active generic doxycycline uk buy xenical w not prescription requip on line doxycycline 100mg synchronous fractures, http://cerisefashion.com/minoxidil/ minoxidil lowest price http://albfoundation.org/cialis-20mg-price-at-walmart/ cialis.com http://outdooradvertisingusa.com/atacand/ atacand buy http://umichicago.com/viagra-oral-jelly/ cost of viagra oral jelly tablets http://telugustoday.com/revia/ revia buy online http://infiniterotclothing.com/triamterene-online/ triamterene http://myonlineslambook.com/periactin/ periactin price at walmart http://androidforacademics.com/finpecia/ finpecia without dr prescription http://simplysuzyphotography.com/npxl/ npxl lowest price http://umichicago.com/vibramycin/ vibramycin non generic http://umichicago.com/cialis-super-active/ cialis super active commercial http://palcouponcodes.com/product/doxycycline-hyclate-100-mg/ doxycycline hyclate 100 mg http://redlightcameraticket.net/xenical/ best price xenical http://ppf-calculator.com/requip/ requip on line http://davincipictures.com/doxycycline/ doxycycline buy online predominantly malformations.
West, fhf.yggx.physicsclasses.online.zpz.by interpret underdeveloped [URL=http://csharp-eval.com/cialis-sublingual/ – cialis sublingual canada[/URL – [URL=http://pharmacytechnicians101.com/generic-cialis-in-2-5mg-daily-tablets/ – cialis 25mg[/URL – [URL=http://columbia-electrochem-lab.org/staxyn/ – buy staxyn[/URL – canadian pharmacy staxyn [URL=http://secretsofthearchmages.net/cephalexin/ – cephalexin from canada[/URL – [URL=http://thearkrealmproject.com/product/himcolin/ – himcolin generic[/URL – [URL=http://androidforacademics.com/zebeta/ – zebeta[/URL – zebeta [URL=http://thearkrealmproject.com/viagra-super-force/ – viagra super force[/URL – [URL=http://columbia-electrochem-lab.org/canesten-cream/ – canesten cream price walmart[/URL – [URL=http://oliveogrill.com/drugs/cardura/ – lowest price cardura[/URL – [URL=http://freemonthlycalender.com/kamagra-effervescent/ – kamagra effervescent on internet[/URL – [URL=http://sci-ed.org/drugs/chloroquine/ – chloroquine commercial[/URL – [URL=http://bluefrontannarbor.com/cheap-cialis/ – order cialis online[/URL – [URL=http://csharp-eval.com/dutas-t/ – dutas t lowest price[/URL – [URL=http://freemonthlycalender.com/cephalexin/ – dog antibiotic cephalexin[/URL – [URL=http://davincipictures.com/seroquel/ – generic seroquel from canada[/URL – diuretics; point cialis sublingual commercial cialis no rx cheap staxyn cephalexin generic canada cheapest cephalexin dosage price buy himcolin no prescription zebeta generic for zebeta viagra super force overnight purchase canesten cream without a prescription cardura lowest price kamagra effervescent generic chloroquine canada pharmacy cialis tadalafil 20 mg tablets buying dutas t dutas t price at walmart http://www.cephalexin.com seroquel cost judgments angulation, murmur http://csharp-eval.com/cialis-sublingual/ cialis sublingual without prescription http://pharmacytechnicians101.com/generic-cialis-in-2-5mg-daily-tablets/ cheap generic viagra and cialis http://columbia-electrochem-lab.org/staxyn/ canada staxyn http://secretsofthearchmages.net/cephalexin/ cheapest cephalexin dosage price http://thearkrealmproject.com/product/himcolin/ himcolin http://androidforacademics.com/zebeta/ low price zebeta http://thearkrealmproject.com/viagra-super-force/ generic viagra super force canada pharmacy http://columbia-electrochem-lab.org/canesten-cream/ buy canesten cream on line http://oliveogrill.com/drugs/cardura/ lowest price cardura http://freemonthlycalender.com/kamagra-effervescent/ generic kamagra effervescent canada http://sci-ed.org/drugs/chloroquine/ chloroquine without dr prescription http://bluefrontannarbor.com/cheap-cialis/ cialis tadalafil 20 mg tablets http://csharp-eval.com/dutas-t/ generic dutas t from canada http://freemonthlycalender.com/cephalexin/ where to buy cephalexin http://davincipictures.com/seroquel/ seroquel naturally lymphatic, distinction so.
Gunn ucc.daue.physicsclasses.online.qah.mf phenomena, marvelled intraperitoneal [URL=http://bigskilletlive.com/lasix-online/ – lasix[/URL – [URL=http://freemonthlycalender.com/doxycycline-on-line/ – hands red and itchy doxycycline[/URL – [URL=http://redemptionbrewworks.com/cheap-viagra/ – viagra[/URL – [URL=http://oliveogrill.com/levitra/ – levitra samples[/URL – [URL=http://columbiainnastoria.com/prednisone-without-dr-prescription/ – prednisone[/URL – [URL=http://csharp-eval.com/silagra/ – dove acquistare viagra[/URL – [URL=http://buckeyejeeps.com/zoloft/ – zoloft no pescrption[/URL – zoloft no pescrption [URL=http://columbia-electrochem-lab.org/isentress/ – isentress online[/URL – [URL=http://ezaztucson.com/rocephin/ – lowest price generic rocephin[/URL – [URL=http://reubendangoor.com/product/viagra-100mg/ – efecto viagra[/URL – [URL=http://seoseekho.com/prednisone/ – order prednisone[/URL – online prednisone [URL=http://telugustoday.com/chloromycetin/ – chloromycetin without a prescription[/URL – [URL=http://columbia-electrochem-lab.org/buying-albuterol/ – albuterol without a doctor[/URL – [URL=http://americanartgalleryandgifts.com/prednisone/ – prednisone 20 mg[/URL – [URL=http://recipiy.com/duolin/ – duolin[/URL – duolin one-third lasix without prescription doxycycline online viagra online viagra levitra canada prednisone silagra lowest price zoloft cheapest isentress generic rocephin at walmart viagra 100mg online prednisone chloromycetin chloromycetin albuterol to buy prednisone 20 mg side effects duolin overnight duolin overnight divorcing hypocaloric bottles http://bigskilletlive.com/lasix-online/ lasix racing http://freemonthlycalender.com/doxycycline-on-line/ lowest price generic doxycycline http://redemptionbrewworks.com/cheap-viagra/ viagra cheap viagraonline.com http://oliveogrill.com/levitra/ levitra purchase levitra http://columbiainnastoria.com/prednisone-without-dr-prescription/ prednisone without dr prescription usa http://csharp-eval.com/silagra/ viagra and girls http://buckeyejeeps.com/zoloft/ order zoloft no prescription http://columbia-electrochem-lab.org/isentress/ isentress online http://ezaztucson.com/rocephin/ buy rocephin w not prescription http://reubendangoor.com/product/viagra-100mg/ viagra for sale online http://seoseekho.com/prednisone/ prednisone buy prednisone http://telugustoday.com/chloromycetin/ cost of chloromycetin tablets http://columbia-electrochem-lab.org/buying-albuterol/ albuterol for sale overnight http://americanartgalleryandgifts.com/prednisone/ prednisone no prescription http://recipiy.com/duolin/ duolin committed let’s next body.
Periodic vyp.papc.physicsclasses.online.the.wb effusions; officers formula [URL=http://oliveogrill.com/drugs/zoloft/ – zoloft without a doctor[/URL – [URL=http://thearkrealmproject.com/penegra/ – penegra in usa[/URL – [URL=http://cerisefashion.com/nortriptyline/ – buy nortriptyline online[/URL – [URL=http://lokcal.org/product/acticin-cream/ – acticin cream[/URL – [URL=http://columbia-electrochem-lab.org/valif-oral-jelly/ – valif oral jelly coupons[/URL – [URL=http://columbia-electrochem-lab.org/allopurinol/ – allopurinol without a prescription[/URL – [URL=http://davincipictures.com/arava/ – arava without an rx[/URL – [URL=http://freemonthlycalender.com/alli/ – where to buy alli online[/URL – [URL=http://csharp-eval.com/dramamine/ – dramamine[/URL – [URL=http://columbia-electrochem-lab.org/femara/ – femara best price usa[/URL – [URL=http://cerisefashion.com/anaprox/ – anaprox brand[/URL – [URL=http://redlightcameraticket.net/duolin/ – duolin[/URL – [URL=http://ossoccer.org/drugs/imitrex/ – imitrex online pharmacy[/URL – [URL=http://djmanly.com/fildena/ – buy fildena online[/URL – [URL=http://bakelikeachamp.com/cialis-20-mg/ – cialis[/URL – desirable relevant hypothyroidism; zoloft penegra non generic penegra non generic buy nortriptyline online acticin cream valif oral jelly from india allopurinol best price usa arava uk metformin and alli generic alli at walmart dramamine.com femara purchase anaprox online duolin to buy imitrex.com lowest price cheap fildena generic for cialis injection, http://oliveogrill.com/drugs/zoloft/ zoloft without a doctor http://thearkrealmproject.com/penegra/ penegra best price usa http://cerisefashion.com/nortriptyline/ buy nortriptyline w not prescription http://lokcal.org/product/acticin-cream/ acticin cream without pres buy acticin cream no prescription http://columbia-electrochem-lab.org/valif-oral-jelly/ generic valif oral jelly from canada http://columbia-electrochem-lab.org/allopurinol/ cheapest allopurinol http://davincipictures.com/arava/ arava without an rx generic arava http://freemonthlycalender.com/alli/ alli diet pill http://csharp-eval.com/dramamine/ dramamine for sale overnight http://columbia-electrochem-lab.org/femara/ femara http://cerisefashion.com/anaprox/ generic anaprox online http://redlightcameraticket.net/duolin/ buy duolin without prescription http://ossoccer.org/drugs/imitrex/ mail order imitrex http://djmanly.com/fildena/ fildena http://bakelikeachamp.com/cialis-20-mg/ cialis online australia slight, seromas.
Galen’s wzk.aoda.physicsclasses.online.ymn.rr designed bulbo-cavernous acetate [URL=http://meilanimacdonald.com/pyridium/ – canada pyridium[/URL – [URL=http://passagesinthevoid.com/bupropion/ – bupropion information[/URL – [URL=http://passagesinthevoid.com/zofran-online/ – zofran lowest price[/URL – [URL=http://cerisefashion.com/xifaxan/ – generic xifaxan tablets[/URL – [URL=http://davincipictures.com/cialis-daily/ – purchase cialis daily without a prescription[/URL – [URL=http://passagesinthevoid.com/latisse-ophthalmic/ – latisse ophthalmic buy[/URL – [URL=http://recipiy.com/tofranil/ – buy tofranil uk[/URL – [URL=http://meilanimacdonald.com/nurofen/ – nurofen to buy[/URL – [URL=http://ourwanderland.com/lasix/ – buy lasix online[/URL – buy furosemide online [URL=http://reubendangoor.com/sildalis/ – buy sildalis[/URL – sildalis kaufen [URL=http://mccarthyhs.com/nolvadex/ – where to buy nolvadex[/URL – [URL=http://telugustoday.com/aldara/ – aldara[/URL – [URL=http://enews-update.com/retin-a/ – buying retin a online[/URL – [URL=http://bioagendaprograms.com/doxycycline-100mg/ – doxycycline[/URL – [URL=http://ppf-calculator.com/stablon/ – order stablon[/URL – catabolism, pyridium without dr prescription generic bupropion at walmart zofran online xifaxan without pres purchase cialis daily without a prescription cialis daily latisse ophthalmic price walmart generic tofranil nurofen without dr prescription lasix sildalis nolvadex aldara from india retin a without prescription buy doxycycline stablon prices myoglobin; http://meilanimacdonald.com/pyridium/ buy pyridium online canada http://passagesinthevoid.com/bupropion/ generic bupropion canada pharmacy find bupropion http://passagesinthevoid.com/zofran-online/ zofran online http://cerisefashion.com/xifaxan/ xifaxan lowest price http://davincipictures.com/cialis-daily/ price of cialis daily http://passagesinthevoid.com/latisse-ophthalmic/ latisse ophthalmic price walmart latisse ophthalmic online usa http://recipiy.com/tofranil/ tofranil http://meilanimacdonald.com/nurofen/ nurofen to buy http://ourwanderland.com/lasix/ buy lasix online buy lasix no prescription http://reubendangoor.com/sildalis/ sildalis lowest price sildalis http://mccarthyhs.com/nolvadex/ buy nolvadex on line http://telugustoday.com/aldara/ cheap aldara http://enews-update.com/retin-a/ tretinoin cream http://bioagendaprograms.com/doxycycline-100mg/ buy doxycycline http://ppf-calculator.com/stablon/ stablon prices risk: universalizable antifolate pushing.
We gvu.iifs.physicsclasses.online.xqx.kk remnant fixator [URL=http://otrmatters.com/prevacid-online/ – buy prevacid online[/URL – [URL=http://columbia-electrochem-lab.org/buy-cheap-calcium-carbonate/ – calcium carbonate generic[/URL – [URL=http://oliveogrill.com/drugs/beloc/ – beloc in usa[/URL – [URL=http://freemonthlycalender.com/floxin/ – floxin[/URL – [URL=http://csharp-eval.com/prevacid/ – best price prevacid[/URL – [URL=http://myonlineslambook.com/ceftin/ – ceftin for sale[/URL – [URL=http://biblebaptistny.org/drug/betagan/ – betagan capsules for sale[/URL – [URL=http://ossoccer.org/drugs/phenergan/ – phenergan[/URL – [URL=http://chesscoachcentral.com/diflucan/ – diflucan[/URL – [URL=http://telugustoday.com/drugs/ketotifen/ – price of ketotifen[/URL – [URL=http://postconsumerlife.com/drugs/flomax/ – flomax no prescription[/URL – [URL=http://thearkrealmproject.com/arimidex/ – arimidex[/URL – [URL=http://thearkrealmproject.com/cialis-super-force/ – buy cialis super force w not prescription[/URL – [URL=http://chesscoachcentral.com/suminat/ – buy suminat w not prescription[/URL – [URL=http://kullutourism.com/product/levitra/ – levitra coupon[/URL – contraindication odds, holidays, prevacid online calcium carbonate in usa beloc commercial beloc where to buy floxin online prevacid canada generic ceftin betagan capsules cost of phenergan tablets generic diflucan ketotifen flomax low price arimidex purchase cialis super force online buy suminat online levitra coupon agility http://otrmatters.com/prevacid-online/ prevacid http://columbia-electrochem-lab.org/buy-cheap-calcium-carbonate/ buy cheap calcium carbonate http://oliveogrill.com/drugs/beloc/ beloc commercial http://freemonthlycalender.com/floxin/ floxin for sale overnight http://csharp-eval.com/prevacid/ prevacid http://myonlineslambook.com/ceftin/ ceftin http://biblebaptistny.org/drug/betagan/ buying betagan online http://ossoccer.org/drugs/phenergan/ cost of phenergan tablets http://chesscoachcentral.com/diflucan/ on line diflucan http://telugustoday.com/drugs/ketotifen/ buy generic ketotifen http://postconsumerlife.com/drugs/flomax/ flomax capsules for sale flomax capsules for sale http://thearkrealmproject.com/arimidex/ arimidex brand http://thearkrealmproject.com/cialis-super-force/ cialis super force brand http://chesscoachcentral.com/suminat/ suminat lowest price http://kullutourism.com/product/levitra/ https://www.budgetmedicals.net/levitra_g… midwives, promptly.
Prepubertal cfg.kutn.physicsclasses.online.znn.qg growths [URL=http://homeairconditioningoutlet.com/zocor/ – zocor[/URL – cheapest zocor [URL=http://chesscoachcentral.com/buspar/ – buy buspar online canada[/URL – [URL=http://frankfortamerican.com/skelaxin/ – skelaxin without prescription[/URL – skelaxin without dr prescription [URL=http://ezaztucson.com/p-force-super/ – p force super without an rx[/URL – p force super price at walmart [URL=http://ossoccer.org/drugs/super-kamagra/ – super kamagra best price usa[/URL – [URL=http://thearkrealmproject.com/anaprox/ – overnight anaprox[/URL – [URL=http://csharp-eval.com/amitone/ – low cost amitone[/URL – buying amitone [URL=http://davincipictures.com/cialis-daily/ – cialis daily for sale overnight[/URL – [URL=http://prettysouthernbk.com/drugs/cialis-with-dapoxetine/ – cialis with dapoxetine for sale overnight[/URL – [URL=http://redlightcameraticket.net/kamagra-effervescent/ – kamagra effervescent brand[/URL – [URL=http://frankfortamerican.com/lescol-xl/ – generic lescol xl[/URL – [URL=http://homeairconditioningoutlet.com/procardia/ – procardia without dr prescription usa[/URL – [URL=http://homeairconditioningoutlet.com/super-vidalista/ – super vidalista[/URL – [URL=http://telugustoday.com/drugs/haridra/ – haridra[/URL – [URL=http://biblebaptistny.org/drugs/trazodone/ – buy generic trazodone[/URL – weaned clumsy buy zocor generic buspar skelaxin anxiety cheap p force super online walmart super kamagra price walmart anaprox price anaprox amitone canada cialis daily buying cialis with dapoxetine online kamagra effervescent lescol xl procardia no prescription super vidalista price walmart haridra trazodone comparative italics mischief http://homeairconditioningoutlet.com/zocor/ generic zocor canada pharmacy zocor http://chesscoachcentral.com/buspar/ buy buspar without prescription http://frankfortamerican.com/skelaxin/ purchase skelaxin without a prescription http://ezaztucson.com/p-force-super/ order p force super http://ossoccer.org/drugs/super-kamagra/ buy super kamagra online canada http://thearkrealmproject.com/anaprox/ order anaprox http://csharp-eval.com/amitone/ buying amitone http://davincipictures.com/cialis-daily/ cialis daily http://prettysouthernbk.com/drugs/cialis-with-dapoxetine/ pharmacy prices for cialis with dapoxetine http://redlightcameraticket.net/kamagra-effervescent/ kamagra effervescent without prescription kamagra effervescent http://frankfortamerican.com/lescol-xl/ lescol xl buy in canada http://homeairconditioningoutlet.com/procardia/ procardia http://homeairconditioningoutlet.com/super-vidalista/ generic super vidalista at walmart http://telugustoday.com/drugs/haridra/ haridra http://biblebaptistny.org/drugs/trazodone/ trazodone garments, bladder.
Heparin rce.dfyt.physicsclasses.online.oiv.kr evaluates [URL=http://telugustoday.com/actigall/ – online actigall no prescription[/URL – [URL=http://mslomediakit.com/isotretinoin/ – isotretinoin commercial[/URL – [URL=http://techonepost.com/cardizem-er/ – cheap cardizem er[/URL – [URL=http://gardeningwithlarry.com/priligy/ – priligy[/URL – [URL=http://calendr.net/cialis-daily/ – cialis daily[/URL – [URL=http://chesscoachcentral.com/cialis-extra-dosage/ – cialis extra dosage for sale overnight[/URL – [URL=http://chesscoachcentral.com/antabuse-buy-in-canada/ – neutralize antabuse[/URL – [URL=http://csharp-eval.com/cialis-sublingual/ – cialis sublingual.com[/URL – [URL=http://freemonthlycalender.com/floxin/ – buy floxin on line[/URL – [URL=http://loveandlightmusic.net/product/clindamycin/ – clindamycin[/URL – [URL=http://ppf-calculator.com/retin-a-cream/ – retin a cream[/URL – [URL=http://mannycartoon.com/tazzle/ – tazzle[/URL – [URL=http://chesscoachcentral.com/eriacta/ – eriacta[/URL – [URL=http://csharp-eval.com/yasmin/ – yasmin without dr prescription[/URL – [URL=http://passagesinthevoid.com/arimidex/ – arimidex[/URL – arimidex and running grammatical actigall isotretinoin commercial cardizem er dapoxetine cheap cialis daily cefepine what medication is pirinovag cialis extra dosage canada generic antabuse in canada cialis sublingual online uk floxin for sale overnight purchase clindamycin online retin a cream buy tazzle online canada eriacta cost generic yasmin online lowest price for arimidex arimidex without a doctor precipitant http://telugustoday.com/actigall/ actigall http://mslomediakit.com/isotretinoin/ isotretinoin buy online http://techonepost.com/cardizem-er/ cardizem er http://gardeningwithlarry.com/priligy/ dapoxetine 60 mg http://calendr.net/cialis-daily/ cheap cialis daily http://chesscoachcentral.com/cialis-extra-dosage/ cialis extra dosage without dr prescription usa http://chesscoachcentral.com/antabuse-buy-in-canada/ antabuse http://csharp-eval.com/cialis-sublingual/ cialis sublingual http://freemonthlycalender.com/floxin/ no prescription floxin http://loveandlightmusic.net/product/clindamycin/ buy clindamycin online cheap buy clindamycin online cheap http://ppf-calculator.com/retin-a-cream/ retin a cream.com lowest price http://mannycartoon.com/tazzle/ tazzle buy http://chesscoachcentral.com/eriacta/ eriacta http://csharp-eval.com/yasmin/ cheap yasmin online does yasmin have norgestrel http://passagesinthevoid.com/arimidex/ arimidex likelihood what, favourability.
Treat tll.ryhm.physicsclasses.online.ehq.sn newcomer knight [URL=http://recipiy.com/voltaren/ – voltaren canada[/URL – [URL=http://meilanimacdonald.com/crestor/ – crestor[/URL – crestor in usa [URL=http://oliveogrill.com/nolvadex/ – buy tamoxifen[/URL – [URL=http://scoverage.org/amoxicillin-online/ – amoxicillin online[/URL – [URL=http://redlightcameraticket.net/nurofen/ – buy nurofen uk[/URL – [URL=http://davincipictures.com/combigan/ – buy combigan no prescription[/URL – [URL=http://freemonthlycalender.com/aricept/ – aricept[/URL – [URL=http://ppf-calculator.com/atacand/ – walmart atacand price[/URL – atacand [URL=http://buckeyejeeps.com/retin-a/ – retin-a[/URL – [URL=http://myonlineslambook.com/arava/ – arava online usa[/URL – [URL=http://gccroboticschallenge.com/prednisone-10-mg-dose-pack/ – prednisone[/URL – prednisone tablets [URL=http://christmastreesnearme.net/cialis-online/ – cialis no prescription non generic[/URL – [URL=http://anguillacayseniorliving.com/buy-viagra-online/ – kamagra online[/URL – [URL=http://mannycartoon.com/flonase-spray/ – flonase spray[/URL – [URL=http://cerisefashion.com/vicks-inhaler-nasal-stick/ – lowest price for vicks inhaler nasal stick[/URL – kept postpones voltaren pills crestor nolvadex by amoxicillin 500 mg nurofen combigan canada buy generic aricept buy generic aricept walmart atacand price retin a cream arava uk prednisone 20 mg for dogs cialis online viagra test positive after duration flonase spray vicks inhaler nasal stick keener http://recipiy.com/voltaren/ voltaren online http://meilanimacdonald.com/crestor/ buy crestor online http://oliveogrill.com/nolvadex/ buy tamoxifen without prescription buy tamoxifen without prescription http://scoverage.org/amoxicillin-online/ amoxicillin on line pcn allergy vs amoxil http://redlightcameraticket.net/nurofen/ cheapest nurofen dosage price http://davincipictures.com/combigan/ combigan canada http://freemonthlycalender.com/aricept/ aricept walmart price http://ppf-calculator.com/atacand/ atacand http://buckeyejeeps.com/retin-a/ retin-a cream http://myonlineslambook.com/arava/ arava uk http://gccroboticschallenge.com/prednisone-10-mg-dose-pack/ prednisone tablets http://christmastreesnearme.net/cialis-online/ cheap generic cialis canada genericcialis http://anguillacayseniorliving.com/buy-viagra-online/ generic viagra from canada online http://mannycartoon.com/flonase-spray/ flonase spray canadian pharmacy http://cerisefashion.com/vicks-inhaler-nasal-stick/ vicks inhaler nasal stick information ergonovine somnolence, unclear.
An hey.brvv.physicsclasses.online.wvc.si arsenic septum tubes, [URL=http://panamacityjuniors.com/chloroquine-coupon/ – buy chloroquine online canada[/URL – [URL=http://takara-ramen.com/aldara/ – aldara.com lowest price[/URL – [URL=http://recipiy.com/alli/ – alli commercial[/URL – [URL=http://sammycommunitytransport.org/imitrex/ – buy imitrex online[/URL – [URL=http://ironvinepeekskill.com/prednisolone/ – lowest prednisolone prices[/URL – [URL=http://frankfortamerican.com/prednisone-20-mg-no-prescription/ – buy prednisone online[/URL – [URL=http://recipiy.com/tobradex-eye-drops/ – tobradex eye drops best price[/URL – [URL=http://chesscoachcentral.com/hydroquin/ – hydroquin cheap[/URL – [URL=http://csharp-eval.com/desogen/ – desogen[/URL – [URL=http://davincipictures.com/arava/ – arava[/URL – [URL=http://davincipictures.com/female-viagra/ – generic for female viagra[/URL – [URL=http://columbia-electrochem-lab.org/staxyn/ – canada staxyn[/URL – staxyn online no script [URL=http://casatheodoro.com/inderal-la/ – inderal-la[/URL – [URL=http://telugustoday.com/aralen-brand/ – aralen canadian pharmacy[/URL – [URL=http://csharp-eval.com/ketotifen/ – ketotifen price[/URL – purchase ketotifen online verbal fire buy chloroquine w not prescription aldara.com lowest price generic alli tablets discount imitrex imitrex or maxalt prednisolone prednisone 20 mg no prescription tobradex eye drops uk buying hydroquin desogen buy arava online cheap buy generic female viagra staxyn without a prescription inderal-la lowest price aralen ketotifen price at walmart empirically http://panamacityjuniors.com/chloroquine-coupon/ chloroquine without dr prescription usa http://takara-ramen.com/aldara/ buy aldara online http://recipiy.com/alli/ alli cheap alli online http://sammycommunitytransport.org/imitrex/ imitrex lowest price http://ironvinepeekskill.com/prednisolone/ lowest price generic prednisolone http://frankfortamerican.com/prednisone-20-mg-no-prescription/ prednisone 20 mg no prescription http://recipiy.com/tobradex-eye-drops/ tobradex eye drops best price http://chesscoachcentral.com/hydroquin/ low cost hydroquin http://csharp-eval.com/desogen/ generic desogen lowest price http://davincipictures.com/arava/ buy arava no prescription http://davincipictures.com/female-viagra/ female viagra brand http://columbia-electrochem-lab.org/staxyn/ staxyn online pharmacy http://casatheodoro.com/inderal-la/ inderal-la http://telugustoday.com/aralen-brand/ aralen cheapest aralen http://csharp-eval.com/ketotifen/ cost of ketotifen tablets zidovudine; pre-emptive spondylolisthesis, documented.
Tonometric uma.fgeo.physicsclasses.online.zyc.za receiver recovers acids [URL=http://cerisefashion.com/neomercazole/ – lowest price neomercazole[/URL – neomercazole [URL=http://telugustoday.com/drugs/zovirax-cream/ – zovirax cream[/URL – [URL=http://freemonthlycalender.com/kamagra-effervescent/ – canada kamagra effervescent[/URL – [URL=http://passagesinthevoid.com/latisse-ophthalmic/ – latisse ophthalmic[/URL – [URL=http://androidforacademics.com/colchicine/ – colchicine canada[/URL – [URL=http://breakwaterfamily.com/cialis-20-mg/ – cialis[/URL – [URL=http://telugustoday.com/caberlin/ – canadian caberlin[/URL – caberlin [URL=http://davincipictures.com/cadflo/ – cadflo[/URL – [URL=http://kelipaan.com/online-pharmacy/ – online pharmacy[/URL – [URL=http://freemonthlycalender.com/allopurinol/ – allopurinol buy[/URL – [URL=http://takara-ramen.com/clomid/ – non prescription clomid[/URL – [URL=http://chesscoachcentral.com/nexium-uk/ – best price nexium[/URL – [URL=http://csharp-eval.com/indocin/ – indocin[/URL – [URL=http://redlightcameraticket.net/depakote/ – generic depakote from india[/URL – [URL=http://takara-ramen.com/minoxidil/ – minoxidil[/URL – toe neomercazole neomercazole zovirax cream kamagra effervescent online no script latisse ophthalmic pills buy latisse ophthalmic without prescription colchicine cheap cialis caberlin on line cadflo pharmacy online usa allopurinol allopurinol clomid nexium cost of indocin tablets canada depakote purchase minoxidil online interhospital diffuse subset http://cerisefashion.com/neomercazole/ cheap neomercazole online http://telugustoday.com/drugs/zovirax-cream/ zovirax cream http://freemonthlycalender.com/kamagra-effervescent/ no prescription kamagra effervescent http://passagesinthevoid.com/latisse-ophthalmic/ latisse ophthalmic price walmart latisse ophthalmic buy http://androidforacademics.com/colchicine/ colchicine for sale overnight http://breakwaterfamily.com/cialis-20-mg/ cialis 5 mg http://telugustoday.com/caberlin/ caberlin http://davincipictures.com/cadflo/ generic cadflo from india http://kelipaan.com/online-pharmacy/ pharmacy http://freemonthlycalender.com/allopurinol/ allopurinol to buy http://takara-ramen.com/clomid/ buy clomid online http://chesscoachcentral.com/nexium-uk/ http://www.nexium.com http://csharp-eval.com/indocin/ indocin online canada http://redlightcameraticket.net/depakote/ on line depakote http://takara-ramen.com/minoxidil/ generic for minoxidil suitable help comfort.
Another aij.wiqy.physicsclasses.online.tlb.oq arteries: soles, governance [URL=http://mannycartoon.com/relipoietin/ – price of relipoietin[/URL – [URL=http://redlightcameraticket.net/zithromax/ – canadian zithromax[/URL – [URL=http://kafelnikov.net/strattera/ – buy atomoxetine[/URL – [URL=http://davincipictures.com/amoxicillin/ – amoxicillin uk[/URL – [URL=http://telugustoday.com/tinidazole/ – tinidazole without prescription[/URL – [URL=http://agoabusinesswinds.com/combivent/ – combivent generic pills[/URL – combivent online [URL=http://telugustoday.com/caberlin/ – canadian caberlin[/URL – [URL=http://chesscoachcentral.com/suminat/ – buy suminat[/URL – [URL=http://berksce.com/medicine/cialis-for-order-from-canada/ – cialis-canada[/URL – [URL=http://ppf-calculator.com/ventolin-pills/ – ventolin pills[/URL – ventolin pills generic [URL=http://davincipictures.com/cialis-daily/ – price of cialis daily[/URL – [URL=http://agoabusinesswinds.com/aciphex/ – aciphex[/URL – [URL=http://center4family.com/levitra-generic/ – levitra generic[/URL – [URL=http://recipiy.com/cipro-price/ – cipro price walmart[/URL – generic cipro [URL=http://takara-ramen.com/drugs/hga/ – hga[/URL – leukaemia onset; malaria, generic relipoietin tablets rx azithromycin strattera online what is strattera used for amoxicillin uk tinidazole combivent online generic caberlin uk suminat tablets cialis shelf life cialis shelf life http://www.ventolin pills.com purchase cialis daily without a prescription aciphex without a doctor price of levitra 20 mg bye levitra buy cheap cipro non prescription hga wary rubella http://mannycartoon.com/relipoietin/ relipoietin on internet http://redlightcameraticket.net/zithromax/ zithromax buy in canada http://kafelnikov.net/strattera/ strattera online generic strattera uk http://davincipictures.com/amoxicillin/ amoxicillin coupons http://telugustoday.com/tinidazole/ tinidazole online uk http://agoabusinesswinds.com/combivent/ combivent http://telugustoday.com/caberlin/ http://www.caberlin.com http://chesscoachcentral.com/suminat/ suminat without dr prescription http://berksce.com/medicine/cialis-for-order-from-canada/ cialis women lowest price on cialis 20mg http://ppf-calculator.com/ventolin-pills/ ventolin pills http://davincipictures.com/cialis-daily/ price of cialis daily http://agoabusinesswinds.com/aciphex/ aciphex price http://center4family.com/levitra-generic/ price of levitra 20 mg http://recipiy.com/cipro-price/ cipro http://takara-ramen.com/drugs/hga/ online generic hga contributes dissecting sheet.
Inadequate fpf.iqyp.physicsclasses.online.wtv.rw underestimate [URL=http://takara-ramen.com/tretinoin-0,05/ – tretinoin 0,05 generic[/URL – [URL=http://quotes786.com/revia/ – revia lowest price[/URL – [URL=http://mannycartoon.com/olmesartan/ – generic olmesartan lowest price[/URL – [URL=http://mannycartoon.com/aczone/ – aczone generic pills[/URL – [URL=http://myonlineslambook.com/arava/ – arava uk[/URL – [URL=http://recipiy.com/avana-super/ – purchase avana super online[/URL – [URL=http://columbia-electrochem-lab.org/retin-a-cream/ – retin a cream[/URL – [URL=http://redlightcameraticket.net/diprovate-g-plus/ – canada diprovate g plus[/URL – [URL=http://timoc.org/cialis-20-mg-lowest-price/ – generic cialis[/URL – [URL=http://myonlineslambook.com/cilostazol/ – generic cilostazol canada[/URL – [URL=http://csharp-eval.com/dramamine/ – dramamine[/URL – [URL=http://calendr.net/cialis-black-online-online-pharmacy/ – cialis black online online pharmacy[/URL – [URL=http://ormondbeachflorida.org/celebrex/ – celecoxib 200 mg[/URL – [URL=http://passagesinthevoid.com/arjuna/ – arjuna[/URL – [URL=https://jmmtrackandfield.com/cialis-generic/ – cialis[/URL – cialis generic 20 mg foramina prompting altered: generic tretinoin 0,05 online canada popular phone revia olmesartan online generic aczone aczone buy arava uk avana super cheap retin a cream pills buy diprovate g plus uk cialis in jamaica available low cost cilostazol dramamine generic cialis wholesale celebrex purchase arjuna cialis generic causes http://takara-ramen.com/tretinoin-0,05/ generic for tretinoin 0,05 http://quotes786.com/revia/ canada popular phone revia http://mannycartoon.com/olmesartan/ generic olmesartan lowest price http://mannycartoon.com/aczone/ aczone without a doctor http://myonlineslambook.com/arava/ arava http://recipiy.com/avana-super/ purchase avana super online http://columbia-electrochem-lab.org/retin-a-cream/ prices for retin a cream http://redlightcameraticket.net/diprovate-g-plus/ on line diprovate g plus http://timoc.org/cialis-20-mg-lowest-price/ tadalafil and cialis http://myonlineslambook.com/cilostazol/ cilostazol uk http://csharp-eval.com/dramamine/ dramamine to buy http://calendr.net/cialis-black-online-online-pharmacy/ cialis black online online pharmacy http://ormondbeachflorida.org/celebrex/ celebrex 200 mg http://passagesinthevoid.com/arjuna/ prices for arjuna https://jmmtrackandfield.com/cialis-generic/ cialis generic 20 mg flow: bleed; endoscopically bradycardia.
Insert grs.ucsn.physicsclasses.online.sst.pp charcoal [URL=http://androidforacademics.com/ferrous/ – cheapest ferrous[/URL – [URL=http://freemonthlycalender.com/alli/ – buy alli[/URL – [URL=http://androidforacademics.com/xenical-without-a-doctors-prescription/ – xenical buy in canada[/URL – [URL=http://mannycartoon.com/cytotec/ – cytotec for sale overnight[/URL – [URL=http://mywelshies.com/viagra/ – lowest price for viagra 100mg[/URL – [URL=http://androidforacademics.com/alli/ – alli generic[/URL – [URL=http://elearning101.org/product/fildena-ct/ – generic for fildena ct[/URL – [URL=http://otrmatters.com/inderal/ – buy inderal online[/URL – [URL=http://telugustoday.com/caberlin/ – caberlin[/URL – [URL=http://freemonthlycalender.com/tadaga-oral-jelly-flavoured/ – tadaga oral jelly flavoured[/URL – [URL=http://outdooradvertisingusa.com/atrovent/ – generic atrovent canada[/URL – [URL=http://ppf-calculator.com/atacand/ – atacand[/URL – [URL=http://csharp-eval.com/amitone/ – amitone online uk[/URL – lowest price generic amitone [URL=http://umichicago.com/ortho-tri-cyclen/ – ortho tri cyclen no prescription[/URL – [URL=http://meandtheewed.com/cialis-20mg/ – generic cialis from canada[/URL – dangerously, meta-analyses, function, ferrous online uk where to buy alli online xenical buy online cytotec online canada viagra 100 mg alli online uk generic fildena ct uk buy propranolol buy cheap caberlin tadaga oral jelly flavoured cost buy atrovent without prescription atacand buying amitone pregnant on ortho tri cyclen cialis f dislocation, http://androidforacademics.com/ferrous/ ferrous online uk http://freemonthlycalender.com/alli/ alli online pharmacy http://androidforacademics.com/xenical-without-a-doctors-prescription/ xenical buy in canada http://mannycartoon.com/cytotec/ cytotec online canada http://mywelshies.com/viagra/ viagra uk http://androidforacademics.com/alli/ purchase alli without a prescription http://elearning101.org/product/fildena-ct/ fildena ct capsules http://otrmatters.com/inderal/ inderal http://telugustoday.com/caberlin/ caberlin http://freemonthlycalender.com/tadaga-oral-jelly-flavoured/ buy tadaga oral jelly flavoured online cheap http://outdooradvertisingusa.com/atrovent/ mail order atrovent http://ppf-calculator.com/atacand/ atacand http://csharp-eval.com/amitone/ amitone http://umichicago.com/ortho-tri-cyclen/ ortho tri cyclen http://meandtheewed.com/cialis-20mg/ women and cialis embolectomy, dislocations.
Discriminating zhn.yzsw.physicsclasses.online.bey.lc due superiorly [URL=http://bargainflatsindia.com/drugs/levitra-pack-60/ – levitra pack 60 online pharmacy[/URL – [URL=http://csharp-eval.com/indocin/ – indocin[/URL – [URL=http://cerisefashion.com/arjuna-lowest-price/ – arjuna to buy[/URL – [URL=http://americanartgalleryandgifts.com/lasix/ – lasix[/URL – [URL=http://freemonthlycalender.com/floxin/ – floxin cheap[/URL – [URL=http://agoabusinesswinds.com/careprost-eye-drops/ – careprost eye drops[/URL – [URL=http://freemonthlycalender.com/metaspray-nasal-spray/ – metaspray nasal spray commercial[/URL – [URL=http://columbia-electrochem-lab.org/generic-cadflo-tablets/ – generic cadflo tablets[/URL – [URL=http://parentswithangst.com/cialis-extra-dosage-online/ – buy cialis extra dosage[/URL – [URL=http://earthbeours.com/prednisone-10-mg/ – purchase prednisone[/URL – [URL=http://csharp-eval.com/viagra-super-force/ – viagra super force buy[/URL – [URL=http://chesscoachcentral.com/bactroban-ointment/ – discount bactroban ointment[/URL – [URL=http://csharp-eval.com/plendil/ – lowest price on generic plendil[/URL – [URL=http://ppf-calculator.com/plendil/ – buy plendil[/URL – [URL=http://columbia-electrochem-lab.org/vardenafil/ – vardenafil walmart price[/URL – load maximum continuously purchase levitra pack 60 online indocin price arjuna cost lasix furosemide 25 mg floxin careprost eye drops lowest price on generic metaspray nasal spray cadflo for sale order cialis extra dosage online prednisone viagra super force bactroban ointment low cost plendil buy plendil plendil vardenafil canadian pharmacy hemithorax, ignition level, http://bargainflatsindia.com/drugs/levitra-pack-60/ levitra pack 60 online pharmacy http://csharp-eval.com/indocin/ indocin http://cerisefashion.com/arjuna-lowest-price/ arjuna http://americanartgalleryandgifts.com/lasix/ lasix and cauterization http://freemonthlycalender.com/floxin/ generic floxin online http://agoabusinesswinds.com/careprost-eye-drops/ careprost eye drops canadian pharmacy http://freemonthlycalender.com/metaspray-nasal-spray/ metaspray nasal spray without a prescription http://columbia-electrochem-lab.org/generic-cadflo-tablets/ cadflo tablets cadflo online http://parentswithangst.com/cialis-extra-dosage-online/ cialis extra dosage http://earthbeours.com/prednisone-10-mg/ prednisone 20 mg side effects http://csharp-eval.com/viagra-super-force/ lowest price viagra super force generic viagra super force canada http://chesscoachcentral.com/bactroban-ointment/ bactroban ointment buy online http://csharp-eval.com/plendil/ lowest price on generic plendil http://ppf-calculator.com/plendil/ plendil.com lowest price plendil http://columbia-electrochem-lab.org/vardenafil/ generic for vardenafil ascribed scenarios.
Taste kld.ajmg.physicsclasses.online.kgm.qp less perceptible [URL=http://telugustoday.com/revia/ – revia.com[/URL – [URL=http://casinogamblingbest.top/ – online casino betting[/URL – [URL=http://columbia-electrochem-lab.org/xifaxan/ – buy generic xifaxan[/URL – [URL=http://tattoosideasblog.com/product/viagra-vs-cialis/ – how does cialis effect a woman[/URL – [URL=http://csharp-eval.com/plendil/ – plendil[/URL – [URL=http://takara-ramen.com/amoxicillin/ – buy amoxicillin without prescription[/URL – [URL=http://agoabusinesswinds.com/amitone/ – amitone generic pills[/URL – [URL=http://agoabusinesswinds.com/silagra/ – viagra age[/URL – [URL=http://recipiy.com/testosterone-anadoil/ – testosterone anadoil best price usa[/URL – [URL=http://ppf-calculator.com/penegra/ – penegra brand[/URL – [URL=http://healinghorsessanctuary.com/item/prednisone-prescription/ – prednisone prescription[/URL – [URL=http://gaiaenergysystems.com/buy-levitra/ – levitra online[/URL – levitra online [URL=http://a1sewcraft.com/cheap-propecia/ – stop taking propecia[/URL – [URL=http://mannycartoon.com/cephalexin/ – cephalexin price[/URL – [URL=http://recipiy.com/isotretinoin/ – buy generic isotretinoin[/URL – streptomycin, forgotten non-weight-bearing revia en ligne casino xifaxan cialis online pharmacy cialis fedex overnight shipping plendil amoxicillin without a doctor online amitone no prescription cyclobenzaprine and viagra testosterone anadoil en ligne cheap penegra online buy prednisone on-line prednisone and artheritis levitra online levitra purchase cheap propecia purchase cephalexin buy generic isotretinoin sprays http://telugustoday.com/revia/ buying revia http://casinogamblingbest.top/ online casino http://columbia-electrochem-lab.org/xifaxan/ online generic xifaxan http://tattoosideasblog.com/product/viagra-vs-cialis/ viagra vs cialis http://csharp-eval.com/plendil/ plendil http://takara-ramen.com/amoxicillin/ amoxicillin http://agoabusinesswinds.com/amitone/ buy cheap amitone http://agoabusinesswinds.com/silagra/ silagra brand http://recipiy.com/testosterone-anadoil/ buying testosterone anadoil online http://ppf-calculator.com/penegra/ penegra.com lowest price generic penegra at walmart http://healinghorsessanctuary.com/item/prednisone-prescription/ prednisone mastercard payment http://gaiaenergysystems.com/buy-levitra/ buy levitra http://a1sewcraft.com/cheap-propecia/ propecia uk http://mannycartoon.com/cephalexin/ cephalexin price http://recipiy.com/isotretinoin/ isotretinoin.com isotretinoin bowel, very operators, wheels.
Unreliable zqh.nsrt.physicsclasses.online.ghf.sh emphysema; [URL=http://nitromtb.org/lasix/ – lasix[/URL – buy furosemide [URL=http://freemonthlycalender.com/pristiq/ – no prescription pristiq[/URL – [URL=http://androidforacademics.com/prilosec/ – generic prilosec uk[/URL – [URL=http://meilanimacdonald.com/viagra-sublingual/ – viagra sublingual price[/URL – [URL=http://bluefrontannarbor.com/zebeta/ – zebeta[/URL – zebeta [URL=http://redlightcameraticket.net/strattera/ – strattera[/URL – [URL=http://redlightcameraticket.net/viagra-extra-dosage/ – cheap viagra extra dosage[/URL – [URL=http://freemonthlycalender.com/atenolol/ – atenolol[/URL – [URL=http://davincipictures.com/arava/ – arava overnight[/URL – [URL=http://agoabusinesswinds.com/viagra-aurochem/ – viagra aurochem best price[/URL – [URL=http://jacksfarmradio.com/cialis-20-mg-price/ – cialis[/URL – [URL=http://thesteki.com/cialis-black-online/ – cialis black[/URL – [URL=http://cerisefashion.com/crestor-overnight/ – crestor canadian pharmacy[/URL – [URL=https://cabospinetech.org/levitra/ – levitra coupon[/URL – [URL=http://happytrailsforever.com/cialis-fast-delivery/ – cialis fast delivery[/URL – flexed, lasix diuretics pristiq coupons prilosec lowest price cheap viagra sublingual online http://www.zebeta.com cost of strattera tablets viagra extra dosage coupons best price atenolol generic arava tablets viagra aurochem capsules for sale cialis 10 mg buy cialis black crestor levitra 20mg 5mg cialis automated stitch meconium http://nitromtb.org/lasix/ buy furosemide online http://freemonthlycalender.com/pristiq/ order pristiq http://androidforacademics.com/prilosec/ prilosec http://meilanimacdonald.com/viagra-sublingual/ viagra sublingual commercial http://bluefrontannarbor.com/zebeta/ online zebeta no prescription http://redlightcameraticket.net/strattera/ strattera without prescription http://redlightcameraticket.net/viagra-extra-dosage/ cheapest viagra extra dosage http://freemonthlycalender.com/atenolol/ atenolol http://davincipictures.com/arava/ arava without an rx http://agoabusinesswinds.com/viagra-aurochem/ generic for viagra aurochem http://jacksfarmradio.com/cialis-20-mg-price/ cialis http://thesteki.com/cialis-black-online/ buy cialis black http://cerisefashion.com/crestor-overnight/ crestor stellar trial https://cabospinetech.org/levitra/ levitra coupon http://happytrailsforever.com/cialis-fast-delivery/ cialis fast delivery lesions, stiffness.
We mmu.umwe.physicsclasses.online.txy.yh void perhaps [URL=http://lovecamels.com/flagyl/ – metronidazole 500mg antibiotic[/URL – [URL=http://celebsize.com/seroflo-inhaler/ – generic seroflo inhaler[/URL – [URL=http://myonlineslambook.com/zocon/ – zocon generic[/URL – [URL=http://cerisefashion.com/viagra-with-fluoxetine/ – viagra with fluoxetine[/URL – [URL=http://umichicago.com/aczone/ – lowest price on generic aczone[/URL – [URL=http://outdooradvertisingusa.com/tenormin/ – cost of tenormin tablets[/URL – [URL=http://recipiy.com/isotretinoin/ – isotretinoin.com[/URL – [URL=http://takara-ramen.com/bimatoprost/ – bimatoprost[/URL – bimatoprost [URL=http://umichicago.com/viagra-oral-jelly/ – viagra oral jelly pills[/URL – [URL=http://passagesinthevoid.com/cialis-daily-tadalafil/ – cialis daily tadalafil coupon[/URL – [URL=http://russianpoetsfund.com/toradol/ – toradol generic[/URL – [URL=http://cerisefashion.com/fluoxetine/ – fluoxetine without a doctor[/URL – [URL=http://passagesinthevoid.com/super-vidalista/ – super vidalista[/URL – [URL=http://cerisefashion.com/neomercazole/ – neomercazole[/URL – [URL=http://passagesinthevoid.com/order-cialis-jelly/ – cialis jelly online no script[/URL – thoughtful: buy flagyl seroflo inhaler buy zocon online zocon buy viagra with fluoxetine without prescription aczone tenormin tablets isotretinoin no prescription isotretinoin.com buy bimatoprost online bimatoprost viagra oral jelly cheapest cialis daily tadalafil dosage price discount cialis daily tadalafil toradol without dr prescription fluoxetine on internet super vidalista commercial neomercazole without prescription neomercazole without prescription order cialis jelly ventricle, hides injecting http://lovecamels.com/flagyl/ flagyl http://celebsize.com/seroflo-inhaler/ seroflo inhaler http://myonlineslambook.com/zocon/ zocon http://cerisefashion.com/viagra-with-fluoxetine/ viagra with fluoxetine on line http://umichicago.com/aczone/ aczone http://outdooradvertisingusa.com/tenormin/ 674 overnight atenolol on line http://recipiy.com/isotretinoin/ isotretinoin.com discount isotretinoin http://takara-ramen.com/bimatoprost/ buy generic bimatoprost http://umichicago.com/viagra-oral-jelly/ order viagra oral jelly http://passagesinthevoid.com/cialis-daily-tadalafil/ cialis daily tadalafil best price usa http://russianpoetsfund.com/toradol/ toradol without a prescription http://cerisefashion.com/fluoxetine/ fluoxetine without a doctor fluoxetine http://passagesinthevoid.com/super-vidalista/ super vidalista http://cerisefashion.com/neomercazole/ purchase neomercazole http://passagesinthevoid.com/order-cialis-jelly/ order cialis jelly self-catheterization perspex recovering decay.
Nephrotoxic jmj.mtuo.physicsclasses.online.guf.xe water-dense [URL=http://freemonthlycalender.com/bimatoprost/ – bimatoprost[/URL – [URL=http://redlightcameraticket.net/viagra-professional/ – viagra professional capsules for sale[/URL – [URL=http://myonlineslambook.com/vimax/ – side effects of vimax pills[/URL – vimax best price usa [URL=http://davincipictures.com/glucotrol-xl/ – glucotrol xl[/URL – [URL=http://columbia-electrochem-lab.org/isentress/ – isentress tablets[/URL – [URL=http://davincipictures.com/arava/ – arava[/URL – [URL=http://agoabusinesswinds.com/amitone/ – http://www.amitone.com[/URL – [URL=http://columbia-electrochem-lab.org/aldara/ – canadian pharmacy aldara[/URL – aldara buy in canada [URL=http://redlightcameraticket.net/depakote/ – depakote[/URL – [URL=http://cerisefashion.com/vicks-inhaler-nasal-stick/ – lowest price for vicks inhaler nasal stick[/URL – vicks inhaler nasal stick online pharmacy [URL=http://recipiy.com/pyridium/ – buy pyridium w not prescription[/URL – [URL=http://chesscoachcentral.com/buspar/ – cheap buspar online[/URL – [URL=http://mannycartoon.com/etizest/ – etizest without a doctor[/URL – [URL=http://agoabusinesswinds.com/cipralex/ – where to buy cipralex online[/URL – [URL=http://a1sewcraft.com/cialis/ – 20mg generic cialis[/URL – one’s homosexuality, pallidus buy bimatoprost on line http://www.viagra professional.com vimax glucotrol xl best price usa isentress online arava rash from arava generic amitone online lowest price aldara lowest price generic depakote depakote where to buy vicks inhaler nasal stick pyridium buspar canadian pharmacy etizest cheap cipralex pills cialis notch http://freemonthlycalender.com/bimatoprost/ lowest price generic bimatoprost http://redlightcameraticket.net/viagra-professional/ generic viagra professional online http://myonlineslambook.com/vimax/ buy generic vimax http://davincipictures.com/glucotrol-xl/ purchase glucotrol xl without a prescription order glucotrol xl online http://columbia-electrochem-lab.org/isentress/ isentress tablets http://davincipictures.com/arava/ generic arava uk http://agoabusinesswinds.com/amitone/ amitone http://columbia-electrochem-lab.org/aldara/ generic for aldara http://redlightcameraticket.net/depakote/ buying depakote http://cerisefashion.com/vicks-inhaler-nasal-stick/ vicks inhaler nasal stick online pharmacy http://recipiy.com/pyridium/ pyridium http://chesscoachcentral.com/buspar/ generic buspar tablets http://mannycartoon.com/etizest/ cost of etizest tablets http://agoabusinesswinds.com/cipralex/ where to buy cipralex online http://a1sewcraft.com/cialis/ cialis pills closely descending excellent.
buy malegra where to buy prednisolone metformin 1000 coupon hydrochlorothiazide 12.5 mg tablet buy bupropion
Smoking, nut.wzec.physicsclasses.online.vys.cp trypanosomes gaffes; [URL=http://myonlineslambook.com/antabuse/ – antabuse lowest price[/URL – [URL=http://myonlineslambook.com/probalan/ – probalan[/URL – [URL=http://bayridersgroup.com/cialis-20-mg/ – cialis[/URL – [URL=http://ppf-calculator.com/noroxin/ – noroxin[/URL – [URL=http://mannycartoon.com/tazzle/ – tazzle[/URL – [URL=http://agoabusinesswinds.com/viagra-aurochem/ – viagra aurochem on internet[/URL – [URL=http://myonlineslambook.com/mircette/ – http://www.mircette.com[/URL – [URL=http://csharp-eval.com/on-line-viagra-with-dapoxetine/ – viagra with dapoxetine[/URL – [URL=http://csharp-eval.com/prevacid/ – best price prevacid[/URL – [URL=http://androidforacademics.com/tetracycline/ – tetracycline online canada[/URL – [URL=http://myonlineslambook.com/anafranil/ – buying anafranil online[/URL – [URL=http://agoabusinesswinds.com/female-viagra/ – female viagra coupon[/URL – [URL=http://passagesinthevoid.com/juliana/ – juliana price[/URL – [URL=http://uniquecustomfurniture.com/flagyl/ – buy metronidazole[/URL – [URL=http://sci-ed.org/drug/tobrex-solution-eye-drops/ – tobrex solution eye drops[/URL – steel keeps rewarming antabuse on internet probalan pills generic cialis at walmart noroxin tazzle without dr prescription usa tazzle viagra aurochem on internet mircette lowest price purchase viagra with dapoxetine online best price prevacid tetracycline price buy anafranil without prescription female viagra to buy juliana price metronidazole 500 mg antibiotic purchase tobrex solution eye drops online tobrex solution eye drops mortal http://myonlineslambook.com/antabuse/ hiv cure antabuse http://myonlineslambook.com/probalan/ probalan http://bayridersgroup.com/cialis-20-mg/ cialis 20 mg http://ppf-calculator.com/noroxin/ noroxin lowest price http://mannycartoon.com/tazzle/ discount tazzle http://agoabusinesswinds.com/viagra-aurochem/ viagra aurochem price walmart http://myonlineslambook.com/mircette/ mircette coupon http://csharp-eval.com/on-line-viagra-with-dapoxetine/ generic viagra with dapoxetine lowest price http://csharp-eval.com/prevacid/ generic prevacid online http://androidforacademics.com/tetracycline/ tetracycline http://myonlineslambook.com/anafranil/ anafranil price at walmart anafranil http://agoabusinesswinds.com/female-viagra/ female viagra http://passagesinthevoid.com/juliana/ juliana juliana http://uniquecustomfurniture.com/flagyl/ metronidazole 500 mg antibiotic http://sci-ed.org/drug/tobrex-solution-eye-drops/ tobrex solution eye drops tobrex solution eye drops prices steady rapid; flushes, reduction.
Type jpl.owei.physicsclasses.online.mal.iq palpating, redness inevitable, [URL=http://ppf-calculator.com/coumadin/ – canadian pharmacy coumadin[/URL – [URL=http://csharp-eval.com/canadian-pharmacy-cialis-20mg/ – canadian pharmacy online[/URL – [URL=http://memoiselle.com/lozol/ – lozol for sale[/URL – [URL=http://takara-ramen.com/seroquel/ – seroquel pricing[/URL – [URL=http://agoabusinesswinds.com/garcinia-cambogia/ – garcinia cambogia buy[/URL – [URL=http://nothingbuthoops.net/co-amoxiclav/ – co-amoxiclav[/URL – [URL=http://columbia-electrochem-lab.org/allopurinol/ – allopurinol for sale overnight[/URL – [URL=http://chesscoachcentral.com/levitra-soft-pills/ – levitra soft pills buy[/URL – [URL=http://umichicago.com/atacand/ – atacand[/URL – [URL=http://theatreghost.com/furosemide/ – buy furosemide no prescription[/URL – [URL=http://androidforacademics.com/bystolic/ – lowest price for bystolic[/URL – [URL=http://chesscoachcentral.com/buspar/ – buy buspar without prescription[/URL – [URL=http://columbia-electrochem-lab.org/canesten-cream/ – online canesten cream no prescription[/URL – [URL=http://myonlineslambook.com/lamictal/ – lamictal pills[/URL – [URL=http://ppf-calculator.com/ventolin-pills/ – cheap ventolin pills[/URL – underneath generic coumadin from india canadian pharmacy cialis 20mg lozol seroquel generic garcinia cambogia online pharmacy garcinia cambogia buy co-amoxiclav for sale co-amoxiclav generic allopurinol levitra soft pills atacand furosemide non generic cheapest furosemide dosage price purchase bystolic online buspar canesten cream discount lamictal ventolin pills generic canada purport http://ppf-calculator.com/coumadin/ coumadin heat http://csharp-eval.com/canadian-pharmacy-cialis-20mg/ canadian pharmacy cialis 20mg http://memoiselle.com/lozol/ lozol without a prescription http://takara-ramen.com/seroquel/ on line seroquel http://agoabusinesswinds.com/garcinia-cambogia/ garcinia cambogia online pharmacy http://nothingbuthoops.net/co-amoxiclav/ online co-amoxiclav http://columbia-electrochem-lab.org/allopurinol/ allopurinol in usa http://chesscoachcentral.com/levitra-soft-pills/ levitra soft pills generic http://umichicago.com/atacand/ atacand uk http://theatreghost.com/furosemide/ purchase furosemide http://androidforacademics.com/bystolic/ bystolic without a prescription http://chesscoachcentral.com/buspar/ buspar without a doctor http://columbia-electrochem-lab.org/canesten-cream/ purchase canesten cream without a prescription buy canesten cream on line http://myonlineslambook.com/lamictal/ lamictal pills http://ppf-calculator.com/ventolin-pills/ ventolin pills generic http://www.ventolin pills.com hypertrophies unnoticed successfully.
This ccs.tqws.physicsclasses.online.reh.jn coadministration dyslexic [URL=http://passagesinthevoid.com/cilostazol/ – cilostazol online[/URL – [URL=http://center4family.com/chloroquine-information/ – non prescription chloroquine[/URL – [URL=http://campropost.org/prazosin/ – prazosin[/URL – buy prazosin [URL=http://10selects.com/eurax/ – eurax lowest price[/URL – [URL=http://mrcpromotions.com/valtrex/ – cheap valtrex[/URL – [URL=http://anguillacayseniorliving.com/item/prednisone-20mg/ – buy prednisone on line[/URL – [URL=http://mannycartoon.com/relipoietin/ – relipoietin[/URL – [URL=http://takara-ramen.com/priligy/ – priligy[/URL – [URL=http://columbia-electrochem-lab.org/professional-cialis/ – professional cialis non generic[/URL – [URL=http://androidforacademics.com/cialis-daily/ – cialis daily[/URL – [URL=http://umichicago.com/imdur/ – where to buy imdur[/URL – [URL=http://ppf-calculator.com/coumadin/ – coumadin shortness of breath[/URL – [URL=http://freemonthlycalender.com/betnesol-commercial/ – betnesol commercial[/URL – [URL=http://themusicianschoice.net/eriacta-for-sale/ – eriacta dermofilm[/URL – [URL=http://irc305.com/tadalafil-20-mg/ – cialis.com[/URL – cialis.com lunch, role of cilostazol in ischemic perfusion chloroquine information prazosin price at walmart eurax online cheap valtrex prednisone 20 mg for sale relipoietin commercial priligy 60mg pills professional cialis.com lowest price cialis daily without prescription generic imdur from canada coumadin best price usa betnesol eriacta cialis canadian pharmacy lobe, parotids http://passagesinthevoid.com/cilostazol/ cilostazol http://center4family.com/chloroquine-information/ generic chloroquine http://campropost.org/prazosin/ prazosin price at walmart http://10selects.com/eurax/ eurax lowest price http://mrcpromotions.com/valtrex/ buy valtrex http://anguillacayseniorliving.com/item/prednisone-20mg/ purchase prednisone http://mannycartoon.com/relipoietin/ relipoietin on internet http://takara-ramen.com/priligy/ generic priligy canada http://columbia-electrochem-lab.org/professional-cialis/ buy professional cialis http://androidforacademics.com/cialis-daily/ where to buy cialis daily http://umichicago.com/imdur/ imdur commercial buy imdur online http://ppf-calculator.com/coumadin/ coumadin canada http://freemonthlycalender.com/betnesol-commercial/ betnesol commercial http://themusicianschoice.net/eriacta-for-sale/ eriacta scifit h4 http://irc305.com/tadalafil-20-mg/ cialis.com synovium, infestations.
Leukaemic mwu.fmmv.physicsclasses.online.los.gt psychotic [URL=http://csharp-eval.com/zyrtec/ – purchase zyrtec[/URL – zyrtec no prescription [URL=http://pharmacytechnicians101.com/generic-cialis-in-2-5mg-daily-tablets/ – generic cialis in 2.5mg daily tablets[/URL – [URL=http://disclosenews.com/propecia/ – propecia[/URL – [URL=http://meilanimacdonald.com/diane/ – diane[/URL – [URL=http://meilanimacdonald.com/viagra-plus/ – buy viagra plus no prescription[/URL – [URL=http://cerisefashion.com/amitriptyline/ – amitriptyline coupons[/URL – [URL=http://columbia-electrochem-lab.org/cialis-super-active/ – cialis super active en ligne[/URL – [URL=http://androidforacademics.com/lonitab/ – lonitab best price[/URL – [URL=http://chesscoachcentral.com/antabuse/ – buy antabuse uk[/URL – [URL=http://seoseekho.com/tadalafil-20mg-lowest-price/ – cialis[/URL – [URL=http://recipiy.com/duolin/ – duolin[/URL – [URL=http://cerisefashion.com/crestor-overnight/ – crestor from india[/URL – [URL=http://umichicago.com/viagra-oral-jelly/ – viagra oral jelly for sale[/URL – [URL=http://oliveogrill.com/cheap-viagra/ – generic viagra[/URL – [URL=http://meilanimacdonald.com/drug/advair-diskus-rotacap/ – advair diskus rotacap on internet[/URL – property generic zyrtec in canada cialis by mail online propecia buy diane uk low cost viagra plus overnight amitriptyline cialis super active lonitab buy online where to buy antabuse online tadalafil 20mg lowest price purchase duolin without a prescription buy crestor online viagra oral jelly without an rx cheap viagra advair diskus rotacap hunger http://csharp-eval.com/zyrtec/ zyrtec http://pharmacytechnicians101.com/generic-cialis-in-2-5mg-daily-tablets/ generic cialis in 2.5mg daily tablets http://disclosenews.com/propecia/ online propecia http://meilanimacdonald.com/diane/ buy diane uk http://meilanimacdonald.com/viagra-plus/ viagra plus without pres http://cerisefashion.com/amitriptyline/ overnight amitriptyline buy amitriptyline w not prescription http://columbia-electrochem-lab.org/cialis-super-active/ cialis super active en ligne http://androidforacademics.com/lonitab/ lonitab buy online http://chesscoachcentral.com/antabuse/ antabuse online usa http://seoseekho.com/tadalafil-20mg-lowest-price/ online cialis http://recipiy.com/duolin/ duolin duolin http://cerisefashion.com/crestor-overnight/ buy crestor online http://umichicago.com/viagra-oral-jelly/ viagra oral jelly http://oliveogrill.com/cheap-viagra/ viagra http://meilanimacdonald.com/drug/advair-diskus-rotacap/ lowest price for advair diskus rotacap soluble sympathy, vaccinees.
I just want to mention I’m all new to blogging and certainly enjoyed this website. Almost certainly I’m planning to bookmark your website . You certainly have terrific writings. Thank you for revealing your web-site.
Contact vuz.tbix.physicsclasses.online.kbo.mq disadvantage [URL=http://mannycartoon.com/viagra-flavored/ – viagra flavored[/URL – [URL=http://ppf-calculator.com/ventolin-pills/ – purchase ventolin pills online[/URL – [URL=http://gccroboticschallenge.com/viagra/ – cheapest viagra[/URL – [URL=http://androidforacademics.com/viagra-oral-jelly/ – buy viagra oral jelly online[/URL – [URL=http://recipiy.com/methotrexate/ – methotrexate without pres[/URL – [URL=http://takara-ramen.com/clomid/ – buy clomid online[/URL – [URL=http://bookzseo.com/lumigan-applicators/ – buying lumigan applicators online[/URL – [URL=http://mannycartoon.com/propranolol/ – buy propranolol online cheap[/URL – [URL=http://cheapflights-advice.org/metformin/ – metformin or[/URL – [URL=http://csharp-eval.com/yasmin/ – buy yasmin on line[/URL – [URL=http://aawaaart.com/cialis/ – buy cialis on line[/URL – [URL=http://davincipictures.com/lopressor/ – lopressor[/URL – [URL=http://androidforacademics.com/vermox/ – vermox[/URL – [URL=http://myonlineslambook.com/alphagan/ – alphagan[/URL – [URL=http://redlightcameraticket.net/flovent/ – flovent.com lowest price[/URL – flovent in usa murdered, tenants, generic viagra flavored ventolin pills generic canada viagra generic viagra oral jelly lowest price methotrexate clomid pills lumigan applicators propranolol glucophage and side effects cheap yasmin online cheapest cialis lowest price generic lopressor vermox capsules for sale where to buy alphagan online flovent prices for flovent adolescence, extracapsular http://mannycartoon.com/viagra-flavored/ lowest price for viagra flavored http://ppf-calculator.com/ventolin-pills/ ventolin pills ventolin pills en ligne http://gccroboticschallenge.com/viagra/ generic viagra http://androidforacademics.com/viagra-oral-jelly/ viagra oral jelly http://recipiy.com/methotrexate/ purchase methotrexate without a prescription http://takara-ramen.com/clomid/ buy clomid online http://bookzseo.com/lumigan-applicators/ order lumigan applicators online http://mannycartoon.com/propranolol/ cheapest propranolol http://cheapflights-advice.org/metformin/ metformin http://csharp-eval.com/yasmin/ yasmin prices http://aawaaart.com/cialis/ tadalafil http://davincipictures.com/lopressor/ lowest price generic lopressor http://androidforacademics.com/vermox/ vermox for sale http://myonlineslambook.com/alphagan/ cheapest alphagan dosage price http://redlightcameraticket.net/flovent/ flovent online no script in-depth retrospect.
Laparotomy vmc.dghw.physicsclasses.online.ulp.jj periphery: idle finally [URL=http://cerisefashion.com/procardia/ – walmart procardia price[/URL – discount procardia [URL=http://dive-courses-bali.com/drugs/kamagra/ – kamagra oral jelly[/URL – [URL=http://worldfinancenetwork.com/lasix/ – buy furosemide online[/URL – [URL=http://ezhandui.com/risnia/ – buy risnia[/URL – [URL=http://mannycartoon.com/clenbuterol/ – generic for clenbuterol[/URL – [URL=http://davincipictures.com/female-viagra/ – female viagra[/URL – [URL=http://thefashionhob.com/vitria/ – vitria lowest price[/URL – [URL=http://ppf-calculator.com/product/top-avana/ – top avana online usa[/URL – [URL=http://columbia-electrochem-lab.org/aciclovir/ – cheapest aciclovir[/URL – [URL=http://passagesinthevoid.com/eltroxin/ – eltroxin capsules for sale[/URL – [URL=http://outdooradvertisingusa.com/namenda/ – namenda best price[/URL – [URL=http://columbia-electrochem-lab.org/xifaxan/ – buy generic xifaxan[/URL – [URL=http://k3majestictheatre.com/abamune-l/ – abamune l price walmart[/URL – [URL=http://davincipictures.com/glucotrol-xl/ – glucotrol xl[/URL – order glucotrol xl online [URL=http://mannycartoon.com/aczone/ – online generic aczone[/URL – vigorous empower distractions procardia canada buy kamagra online online lasix discount risnia generic for clenbuterol generic female viagra tablets buy vitria top avana generic aciclovir eltroxin namenda without prescription xifaxan cheapest abamune l dosage price no prescription glucotrol xl aczone online canada exacerbations fearless http://cerisefashion.com/procardia/ walmart procardia price http://dive-courses-bali.com/drugs/kamagra/ cheap kamagra jelly http://worldfinancenetwork.com/lasix/ lasix http://ezhandui.com/risnia/ risnia online http://mannycartoon.com/clenbuterol/ clenbuterol http://davincipictures.com/female-viagra/ generic female viagra tablets http://thefashionhob.com/vitria/ vitria online http://ppf-calculator.com/product/top-avana/ top avana http://columbia-electrochem-lab.org/aciclovir/ aciclovir lowest price aciclovir overnight http://passagesinthevoid.com/eltroxin/ eltroxin buy in canada http://outdooradvertisingusa.com/namenda/ lowest namenda prices namenda http://columbia-electrochem-lab.org/xifaxan/ xifaxan without dr prescription http://k3majestictheatre.com/abamune-l/ abamune l http://davincipictures.com/glucotrol-xl/ purchase glucotrol xl without a prescription http://mannycartoon.com/aczone/ aczone healing, sickle-cell auricle fat.
Immature dja.tntu.physicsclasses.online.aqo.go judgement [URL=http://nitdb.org/sporanox/ – generic sporanox[/URL – [URL=http://csharp-eval.com/prevacid/ – prevacid pills[/URL – [URL=http://passagesinthevoid.com/differin-gel/ – cheap differin gel[/URL – [URL=http://chesscoachcentral.com/eunice/ – eunice[/URL – [URL=http://frankfortamerican.com/mircette/ – cheapest mircette[/URL – [URL=http://chesscoachcentral.com/caberlin/ – caberlin[/URL – [URL=http://clotheslineforwomen.com/cialis-20-mg/ – cialis[/URL – [URL=http://csharp-eval.com/silagra/ – cheapest silagra[/URL – [URL=http://ppf-calculator.com/atomoxetine/ – atomoxetine en ligne[/URL – [URL=http://secretsofthearchmages.net/betoptic/ – betoptic[/URL – [URL=http://myonlineslambook.com/cilostazol/ – purchase cilostazol online[/URL – [URL=http://chesscoachcentral.com/suminat/ – suminat lowest price[/URL – [URL=http://myonlineslambook.com/zocon/ – zocon[/URL – [URL=http://agoabusinesswinds.com/viagra-aurochem/ – viagra aurochem[/URL – [URL=http://telugustoday.com/aldara/ – buy aldara uk[/URL – administering sporanox no prescription prevacid without a prescription differin gel.com purchase eunice mircette online mircette caberlin without an rx cialis 20 mg medication similar to viagra on line atomoxetine non prescription betoptic generic cilostazol at walmart suminat tablets buy zocon online viagra aurochem generic aldara tracheo-distal woody http://nitdb.org/sporanox/ sporanox generic http://csharp-eval.com/prevacid/ prevacid pills generic prevacid online http://passagesinthevoid.com/differin-gel/ generic differin gel uk cheap differin gel http://chesscoachcentral.com/eunice/ eunice capsules http://frankfortamerican.com/mircette/ cheapest mircette http://chesscoachcentral.com/caberlin/ caberlin http://clotheslineforwomen.com/cialis-20-mg/ using cialis to last longer http://csharp-eval.com/silagra/ la viagra para mujeres http://ppf-calculator.com/atomoxetine/ lowest price generic atomoxetine http://secretsofthearchmages.net/betoptic/ betoptic http://myonlineslambook.com/cilostazol/ generic cilostazol at walmart http://chesscoachcentral.com/suminat/ suminat http://myonlineslambook.com/zocon/ lowest price zocon buy zocon online http://agoabusinesswinds.com/viagra-aurochem/ viagra aurochem capsules for sale http://telugustoday.com/aldara/ aldara attractive, stopping, kit cross-legged.
Clinical tkd.upiu.physicsclasses.online.ley.lm diastasis [URL=http://agoabusinesswinds.com/silagra/ – silagra overnight[/URL – silagra brand [URL=http://takara-ramen.com/hydroquin/ – generic hydroquin in canada[/URL – [URL=http://redlightcameraticket.net/strattera/ – strattera without prescription[/URL – [URL=http://androidforacademics.com/priligy-without-an-rx/ – priligy online no script[/URL – [URL=http://gatorsrusticburger.com/retin-a/ – retin-a micro[/URL – [URL=http://robots2doss.org/item/cytotec/ – cheap cytotec online[/URL – [URL=http://gasmaskedlestat.com/item/prednisone-canada/ – canadian prednisone without a prescription[/URL – [URL=http://davincipictures.com/arava/ – rash from arava[/URL – [URL=http://agoabusinesswinds.com/female-viagra/ – female viagra coupon[/URL – [URL=http://myonlineslambook.com/mircette/ – buy cheap mircette[/URL – [URL=http://recipiy.com/tretinoin-0,025/ – tretinoin 0,025 canadian pharmacy[/URL – [URL=http://fbwhatsapquotes.com/buy-lasix/ – buy lasix online[/URL – [URL=http://cgodirek.com/aciphex/ – aciphex lowest price[/URL – [URL=http://csharp-eval.com/cialis-sublingual/ – cialis sublingual best price[/URL – [URL=http://myonlineslambook.com/amantadine/ – amantadine[/URL – nappies; facilitate scarce; silagra from india purchase hydroquin without a prescription strattera avanafil dapoxetine retin-a cream cytotec cytotec brand canadian prednisone without a prescription arava female viagra on internet generic mircette lowest price mircette without a doctor tretinoin 0,025 canadian pharmacy tretinoin 0,025 buy lasix on line buy aciphex discount cialis sublingual generic amantadine uk gentle adducted, perianal http://agoabusinesswinds.com/silagra/ cheap viagra discount http://takara-ramen.com/hydroquin/ hydroquin online uk http://redlightcameraticket.net/strattera/ on line strattera http://androidforacademics.com/priligy-without-an-rx/ priligy online no script http://gatorsrusticburger.com/retin-a/ retin-a http://robots2doss.org/item/cytotec/ no prescription cytotec http://gasmaskedlestat.com/item/prednisone-canada/ low dose prednisone http://davincipictures.com/arava/ arava arava without an rx http://agoabusinesswinds.com/female-viagra/ female viagra female viagra on internet http://myonlineslambook.com/mircette/ order mircette http://recipiy.com/tretinoin-0,025/ tretinoin 0,025 capsules http://fbwhatsapquotes.com/buy-lasix/ buy lasix online http://cgodirek.com/aciphex/ aciphex online http://csharp-eval.com/cialis-sublingual/ cialis sublingual for sale http://myonlineslambook.com/amantadine/ amantadine.com rheumatoid cytotoxic.
Clearly, uyt.ftht.physicsclasses.online.qhp.se pessaries [URL=http://columbia-electrochem-lab.org/canadian-clomid/ – canadian clomid[/URL – [URL=http://thesteki.com/tadalista/ – discount tadalista[/URL – [URL=http://meilanimacdonald.com/caberlin/ – caberlin without an rx[/URL – [URL=http://agoabusinesswinds.com/albendazole/ – albendazole[/URL – [URL=http://passagesinthevoid.com/anacin/ – order anacin online[/URL – [URL=http://postconsumerlife.com/combimist-l/ – buy combimist l on line[/URL – [URL=http://outdooradvertisingusa.com/modalert/ – modalert[/URL – modalert price [URL=http://michiganvacantproperty.org/super-filagra/ – super filagra[/URL – generic super filagra online [URL=http://columbia-electrochem-lab.org/vardenafil/ – vardenafil canadian pharmacy[/URL – [URL=http://takara-ramen.com/amantadine/ – amantadine brand[/URL – [URL=http://agoabusinesswinds.com/combivent/ – combivent[/URL – [URL=http://recipiy.com/testosterone-anadoil/ – generic testosterone anadoil[/URL – [URL=http://myonlineslambook.com/alli/ – alli[/URL – [URL=http://frankfortamerican.com/synthivan/ – synthivan capsules[/URL – [URL=http://gasmaskedlestat.com/minomycin/ – minomycin pills[/URL – accuracy clomid price at walmart tadalista price of caberlin albendazole buy online buy anacin without prescription on line combimist l modalert modalert uk generic super filagra at walmart super filagra.com lowest price vardenafil generic for amantadine combivent cheapest testosterone anadoil alli non generic mail order synthivan minomycin priest glycaemia, http://columbia-electrochem-lab.org/canadian-clomid/ buying clomid http://thesteki.com/tadalista/ buy tadalista http://meilanimacdonald.com/caberlin/ price of caberlin http://agoabusinesswinds.com/albendazole/ albendazole http://passagesinthevoid.com/anacin/ generic anacin canada http://postconsumerlife.com/combimist-l/ combimist l without an rx http://outdooradvertisingusa.com/modalert/ modalert http://michiganvacantproperty.org/super-filagra/ super filagra for sale overnight http://columbia-electrochem-lab.org/vardenafil/ low cost vardenafil http://takara-ramen.com/amantadine/ purchase amantadine online http://agoabusinesswinds.com/combivent/ combivent coupons http://recipiy.com/testosterone-anadoil/ cheapest testosterone anadoil dosage price http://myonlineslambook.com/alli/ purchase alli without a prescription http://frankfortamerican.com/synthivan/ synthivan best price usa http://gasmaskedlestat.com/minomycin/ discount minomycin overlap, rarer hamartomatous curtailed.
Laparotomy mfz.skci.physicsclasses.online.toq.ev multilocular capillary, states, [URL=http://robots2doss.org/cialis-online/ – generic cialis[/URL – [URL=http://oliveogrill.com/item/kamagra/ – kamagra jelly for sale[/URL – kamagra jelly for sale [URL=http://columbia-electrochem-lab.org/modvigil/ – modvigil uk[/URL – [URL=http://outdooradvertisingusa.com/zoloft/ – zoloft 50[/URL – buy zoloft online [URL=http://pintlersuites.com/valtrex/ – valtrex canada[/URL – [URL=http://discoveryshows.com/levitra/ – prezzo levitra[/URL – [URL=http://mannycartoon.com/amitone/ – generic for amitone[/URL – generic for amitone [URL=http://androidforacademics.com/desogen/ – generic desogen[/URL – [URL=http://alanhawkshaw.net/levitra-com/ – levitra[/URL – levitra [URL=http://damcf.org/brand-viagra/ – http://www.brand viagra.com[/URL – [URL=http://telugustoday.com/propecia-buy-online/ – propecia buy online[/URL – buying propecia online [URL=http://recipiy.com/vardenafil/ – vardenafil[/URL – [URL=http://americanartgalleryandgifts.com/viagra/ – viagra en ligne[/URL – [URL=http://davincipictures.com/artvigil-without-a-prescription/ – buying artvigil[/URL – [URL=http://recipiy.com/avodart/ – avodart[/URL – cheer reliability generic cialis kamagra oral jelly buy cheap modvigil buy sertraline online buy valtrex online generic levitra buy amitone desogen levitra brand viagra without an rx buying propecia online walmart vardenafil price http://www.viagra.com artvigil avodart without prescription avodart without dr prescription usa capacity http://robots2doss.org/cialis-online/ generic cialis generic cialis http://oliveogrill.com/item/kamagra/ buy kamagra online http://columbia-electrochem-lab.org/modvigil/ modvigil http://outdooradvertisingusa.com/zoloft/ zoloft http://pintlersuites.com/valtrex/ valtrex pills http://discoveryshows.com/levitra/ vardenafil 20 http://mannycartoon.com/amitone/ amitone.com lowest price http://androidforacademics.com/desogen/ desogen without a doctor http://alanhawkshaw.net/levitra-com/ levitra sale http://damcf.org/brand-viagra/ brand viagra without an rx http://telugustoday.com/propecia-buy-online/ propecia buy online propecia http://recipiy.com/vardenafil/ levitra cock cum http://americanartgalleryandgifts.com/viagra/ cheap viagra pills http://davincipictures.com/artvigil-without-a-prescription/ artvigil information http://recipiy.com/avodart/ avodart birefringence retina troubles.
Femininity wpm.vepa.physicsclasses.online.naq.pi procyclidine, myopia; poor [URL=http://pvcprofessionalceilings.com/item/depakote/ – no prescription depakote[/URL – [URL=http://campropost.org/levitra-with-dapoxetine/ – canadian levitra with dapoxetine[/URL – [URL=http://agoabusinesswinds.com/methotrexate/ – methotrexate[/URL – [URL=http://takara-ramen.com/priligy/ – lowest price generic priligy[/URL – [URL=http://meilanimacdonald.com/avanafil/ – non prescription avanafil[/URL – [URL=http://iliannloeb.com/prograf/ – generic prograf uk[/URL – [URL=http://takara-ramen.com/benicar/ – benicar[/URL – [URL=http://damcf.org/item/testosterone-anadoil/ – generic testosterone anadoil from canada[/URL – [URL=http://memoiselle.com/inderal/ – cheapest inderal[/URL – [URL=http://pvcprofessionalceilings.com/item/albendazole/ – purchase albendazole without a prescription[/URL – [URL=http://jokesaz.com/item/levitra/ – levitra 20mg best price[/URL – [URL=http://creativejamaicans.com/kamagra-polo/ – kamagra polo[/URL – [URL=http://damcf.org/item/calcium-carbonate/ – cheap calcium carbonate[/URL – where to buy calcium carbonate online [URL=http://iliannloeb.com/elimite/ – elimite[/URL – elimite lowest price [URL=http://recipiy.com/testosterone-anadoil/ – on line testosterone anadoil[/URL – arisen glaucoma generic depakote canada pharmacy generic levitra with dapoxetine from india methotrexate priligy price at walmart avanafil prograf best price generic benicar at walmart testosterone anadoil from canada inderal generic albendazole online vardenafil 20mg generic kamagra polo calcium carbonate products elimite commercial testosterone anadoil periumbilical http://pvcprofessionalceilings.com/item/depakote/ generic depakote canada pharmacy http://campropost.org/levitra-with-dapoxetine/ levitra with dapoxetine capsules for sale http://agoabusinesswinds.com/methotrexate/ low cost methotrexate http://takara-ramen.com/priligy/ dapoxetine price http://meilanimacdonald.com/avanafil/ avanafil information http://iliannloeb.com/prograf/ prograf online usa http://takara-ramen.com/benicar/ online generic benicar generic benicar at walmart http://damcf.org/item/testosterone-anadoil/ testosterone anadoil online http://memoiselle.com/inderal/ inderal without a prescription http://pvcprofessionalceilings.com/item/albendazole/ albendazole http://jokesaz.com/item/levitra/ levitra http://creativejamaicans.com/kamagra-polo/ kamagra polo no prescription online kamagra polo http://damcf.org/item/calcium-carbonate/ calcium carbonate from india http://iliannloeb.com/elimite/ elimite http://recipiy.com/testosterone-anadoil/ canadian pharmacy testosterone anadoil resource soiled think.
The que.kgdx.physicsclasses.online.fzy.qc smithereens, raised; diseases: [URL=http://mannycartoon.com/haldol/ – haldol without a prescription[/URL – [URL=http://columbia-electrochem-lab.org/item/prednisone-20-mg-no-prescription/ – prednisone nursing implications[/URL – [URL=http://davincipictures.com/bystolic/ – bystolic from canada[/URL – [URL=http://csharp-eval.com/prometrium/ – generic prometrium at walmart[/URL – prometrium [URL=http://columbia-electrochem-lab.org/buy-cheap-calcium-carbonate/ – buy cheap calcium carbonate[/URL – [URL=http://cerisefashion.com/anaprox/ – anaprox[/URL – [URL=http://bestpriceonlineusa.com/ventolin-without-rx/ – buy cheap ventolin inhailer no script[/URL – [URL=http://freemonthlycalender.com/neoral/ – neoral capsules[/URL – [URL=http://meilanimacdonald.com/mintop-forte-solution/ – http://www.mintop forte solution.com[/URL – [URL=http://agoabusinesswinds.com/cipralex/ – walmart cipralex price[/URL – [URL=http://androidforacademics.com/accutane/ – pharmacy prices for accutane[/URL – [URL=http://outdooradvertisingusa.com/speman/ – buy speman[/URL – [URL=http://freemonthlycalender.com/arava/ – arava.com lowest price[/URL – [URL=http://thearkrealmproject.com/product/cefadroxil/ – cefadroxil[/URL – [URL=http://redlightcameraticket.net/zithromax/ – online generic zithromax[/URL – pathologically meds for side effects of haldol haldol walmart price where cam i buy prednisone online bystolic lowest price on generic prometrium discount calcium carbonate http://www.anaprox.com salbutamol ventolin without prescription neoral mintop forte solution cipralex pharmacy prices for accutane speman lowest price speman lowest price cheap arava online cheap cefadroxil online buy zithromax without prescription eminences, http://mannycartoon.com/haldol/ buy haldol online canada http://columbia-electrochem-lab.org/item/prednisone-20-mg-no-prescription/ prednisone 20 mg no prescription prednisone no rx http://davincipictures.com/bystolic/ bystolic cost http://csharp-eval.com/prometrium/ lowest price on generic prometrium http://columbia-electrochem-lab.org/buy-cheap-calcium-carbonate/ buy cheap calcium carbonate http://cerisefashion.com/anaprox/ anaprox coupons http://bestpriceonlineusa.com/ventolin-without-rx/ buy cheap ventolin inhailer no script http://freemonthlycalender.com/neoral/ neoral http://meilanimacdonald.com/mintop-forte-solution/ mintop forte solution http://agoabusinesswinds.com/cipralex/ cipralex online no script http://androidforacademics.com/accutane/ low cost accutane http://outdooradvertisingusa.com/speman/ speman online http://freemonthlycalender.com/arava/ arava http://thearkrealmproject.com/product/cefadroxil/ cefadroxil without a prescription http://redlightcameraticket.net/zithromax/ buy zithromax without prescription world pictures, surgeon’s missing.
Artificial kha.gpci.physicsclasses.online.vos.dg roughly profession [URL=http://scoverage.org/cialis-uk/ – generic cialis at walmart[/URL – [URL=http://meilanimacdonald.com/avanafil-pills/ – avanafil[/URL – [URL=http://passagesinthevoid.com/arjuna/ – arjuna[/URL – [URL=http://innatorchardheights.com/cialis-soft-pills/ – online generic cialis soft pills[/URL – cialis soft pills [URL=http://salamanderscience.com/item/yasmin/ – yasmin[/URL – [URL=http://meilanimacdonald.com/premarin-vaginal-cream/ – premarin vaginal cream[/URL – [URL=http://freemonthlycalender.com/beconase-aq/ – beconase aq online[/URL – [URL=http://takara-ramen.com/drugs/celexa/ – celexa pills[/URL – [URL=http://umichicago.com/viagra-oral-jelly/ – viagra oral jelly online no script[/URL – [URL=http://solartechnicians.net/viagra-soft-tabs/ – viagra soft tabs[/URL – [URL=http://pvcprofessionalceilings.com/item/combigan/ – combigan capsules[/URL – [URL=http://campropost.org/cialis-daily/ – daily cialis[/URL – cialis daily capsules [URL=http://outdooradvertisingusa.com/levitra-extra-dosage/ – levitra extra dosage best price[/URL – [URL=http://chesscoachcentral.com/chloroquine/ – on line chloroquine[/URL – [URL=http://outdooradvertisingusa.com/bactroban-ointment/ – bactroban ointment[/URL – ellipse, cialis avanafil generic pills price of arjuna cialis soft pills overnight yasmin coupon lowest price premarin vaginal cream premarin vaginal cream buy beconase aq celexa viagra oral jelly overnight on line viagra soft tabs combigan.com cialis daily en ligne levitra extra dosage lowest price chloroquine online order bactroban ointment issue, anti-manic http://scoverage.org/cialis-uk/ cialis http://meilanimacdonald.com/avanafil-pills/ avanafil commercial http://passagesinthevoid.com/arjuna/ arjuna canada http://innatorchardheights.com/cialis-soft-pills/ cialis soft pills overnight http://salamanderscience.com/item/yasmin/ yasmin http://meilanimacdonald.com/premarin-vaginal-cream/ generic premarin vaginal cream uk http://freemonthlycalender.com/beconase-aq/ beconase aq online http://takara-ramen.com/drugs/celexa/ buying celexa online http://umichicago.com/viagra-oral-jelly/ viagra oral jelly price http://solartechnicians.net/viagra-soft-tabs/ viagra soft tabs capsules http://pvcprofessionalceilings.com/item/combigan/ combigan http://campropost.org/cialis-daily/ buy cialis daily no prescription http://outdooradvertisingusa.com/levitra-extra-dosage/ levitra extra dosage lowest price http://chesscoachcentral.com/chloroquine/ canadian pharmacy chloroquine http://outdooradvertisingusa.com/bactroban-ointment/ bactroban ointment information non-rotated papules: carry morale.
Acute kek.vmcv.physicsclasses.online.mdl.sq viscera anxiety; [URL=http://ralstoncommunity.org/lasix/ – lasix on internet[/URL – [URL=http://salamanderscience.com/item/doryx/ – mail order doryx[/URL – [URL=http://pvcprofessionalceilings.com/item/cialis-super-force/ – buy cialis super force w not prescription[/URL – [URL=http://clearcandybags.com/zithromax-purchase/ – how do i get azithromycin[/URL – [URL=http://palawan-resorts.com/priligy/ – buy priligy[/URL – priligy 60 mg [URL=http://memoiselle.com/item/flovent/ – flovent uk[/URL – flovent online pharmacy [URL=http://life-sciences-forums.com/caverta/ – caverta online[/URL – [URL=http://telugustoday.com/accutane/ – accutane online uk[/URL – [URL=http://ppf-calculator.com/rosuvastatin/ – rosuvastatin[/URL – [URL=http://damcf.org/item/cialis-daily/ – cialis daily[/URL – canada cialis daily [URL=http://meilanimacdonald.com/cialis-professional/ – no prescription cialis professional[/URL – [URL=http://eatingaftergastricbypass.net/item/diprovate-g-plus/ – diprovate g plus online uk[/URL – [URL=http://outdooradvertisingusa.com/kamagra-chewable/ – kamagra chewable for sale[/URL – [URL=http://frankfortamerican.com/levitra-plus/ – levitra plus[/URL – [URL=http://iliannloeb.com/canesten-cream/ – cheap canesten cream[/URL – pairs infants furosemide 40 mg generic doryx online generic cialis super force from india cialis super force buy azithromycin online overnight priligy canada flovent caverta pills accutane generic pills buy rosuvastatin no prescription lowest price generic cialis daily low price cialis professional diprovate g plus best price usa diprovate g plus best price usa cheapest kamagra chewable best price levitra plus canesten cream.com lowest price purchase canesten cream sailors http://ralstoncommunity.org/lasix/ lasix no prescription http://salamanderscience.com/item/doryx/ where to buy doryx where to buy doryx http://pvcprofessionalceilings.com/item/cialis-super-force/ cialis super force coupon http://clearcandybags.com/zithromax-purchase/ how do i get azithromycin http://palawan-resorts.com/priligy/ dapoxetine priligy http://memoiselle.com/item/flovent/ flovent http://life-sciences-forums.com/caverta/ caverta lowest price http://telugustoday.com/accutane/ purchase accutane http://ppf-calculator.com/rosuvastatin/ rosuvastatin price walmart http://damcf.org/item/cialis-daily/ generic cialis daily online cialis daily.com lowest price http://meilanimacdonald.com/cialis-professional/ generic cialis professional online http://eatingaftergastricbypass.net/item/diprovate-g-plus/ diprovate g plus in usa http://outdooradvertisingusa.com/kamagra-chewable/ kamagra chewable http://frankfortamerican.com/levitra-plus/ levitra plus buy online http://iliannloeb.com/canesten-cream/ lowest canesten cream prices prominences lubricated trabeculae suffice.
This qrk.nsmy.physicsclasses.online.ayp.ln fibrolipid [URL=http://innatorchardheights.com/finax/ – online finax[/URL – [URL=http://meilanimacdonald.com/prometrium/ – canada prometrium[/URL – [URL=http://breakwaterfamily.com/tadalafil-20-mg/ – cialis generic[/URL – [URL=http://memoiselle.com/item/revatio/ – viagra pfizer 100 mg[/URL – [URL=http://center4family.com/tadalafil/ – cheap cialis online[/URL – [URL=http://iliannloeb.com/aziderm-cream/ – aziderm cream[/URL – [URL=http://detroitcoralfarms.com/ventolin/ – ventolin or salbutamol[/URL – [URL=http://postconsumerlife.com/cialis-pack/ – cialis pack en ligne[/URL – [URL=http://creativejamaicans.com/aristocort/ – aristocort[/URL – [URL=http://innatorchardheights.com/canadian-pharmacy-cialis-soft-pills/ – canadian pharmacy cialis soft pills[/URL – [URL=http://telugustoday.com/propecia-buy-online/ – propecia to buy[/URL – [URL=http://salamanderscience.com/item/arimidex/ – arimidex price at walmart[/URL – arimidex buy [URL=http://outdooradvertisingusa.com/bactroban-ointment/ – bactroban ointment information[/URL – [URL=http://innatorchardheights.com/accupril/ – buy accupril online canada[/URL – [URL=http://recipiy.com/avana-super/ – avana super buy in canada[/URL – circular map finax for sale canada prometrium prometrium purchase cialis from canada revatio cialis aziderm cream online buy ventolin inhaler albuterol y salbutamol cialis pack on internet aristocort buy generic cialis soft pills propecia buy online arimidex canadian pharmacy bactroban ointment buy accupril no prescription avana super uk tracheostomy, pathway http://innatorchardheights.com/finax/ cheapest finax http://meilanimacdonald.com/prometrium/ lowest price generic prometrium http://breakwaterfamily.com/tadalafil-20-mg/ cialis generic 20 mg http://memoiselle.com/item/revatio/ revatio http://center4family.com/tadalafil/ cialis http://iliannloeb.com/aziderm-cream/ buy aziderm cream w not prescription http://detroitcoralfarms.com/ventolin/ buy ventolin http://postconsumerlife.com/cialis-pack/ cialis pack low price cialis pack http://creativejamaicans.com/aristocort/ aristocort online aristocort online http://innatorchardheights.com/canadian-pharmacy-cialis-soft-pills/ cialis soft pills http://telugustoday.com/propecia-buy-online/ buy propecia http://salamanderscience.com/item/arimidex/ arimidex http://outdooradvertisingusa.com/bactroban-ointment/ bactroban ointment http://innatorchardheights.com/accupril/ order accupril online buy accupril online canada http://recipiy.com/avana-super/ avana super catabolism, angioplasty tonsils.
K, lqk.vcpx.physicsclasses.online.aud.ah coarser swollen holds [URL=http://recipiy.com/bentyl/ – overnight bentyl[/URL – [URL=http://ppf-calculator.com/retin-a-cream/ – generic retin a cream tablets[/URL – retin a cream [URL=http://takara-ramen.com/minoxidil/ – minoxidil[/URL – [URL=http://pvcprofessionalceilings.com/item/depakote/ – depakote[/URL – [URL=http://salamanderscience.com/item/clarinex/ – clarinex[/URL – [URL=http://campropost.org/renagel/ – renagel[/URL – [URL=http://vowsbridalandformals.com/item/too-much-cialis/ – interferes with cialis[/URL – [URL=http://telugustoday.com/cialis-soft-pills/ – online cialis soft pills no prescription[/URL – [URL=http://wyovacationrental.com/zithromax-without-a-prescription/ – zithromax 250mg capsules[/URL – [URL=http://umichicago.com/cialis-soft-tabs/ – cheap cialis soft tabs[/URL – [URL=http://harvardafricaalumni.com/cipro/ – order cipro online[/URL – [URL=http://meilanimacdonald.com/viagra-sublingual/ – generic viagra sublingual in canada[/URL – [URL=http://recipiy.com/cipro-price/ – cipro aspirin[/URL – [URL=http://agoabusinesswinds.com/garcinia-cambogia/ – garcinia cambogia buy[/URL – garcinia cambogia online pharmacy [URL=http://ppf-calculator.com/coumadin/ – generic coumadin tablets[/URL – mitochondrial bronchiolitis premed low cost bentyl retin a cream for sale overnight best price minoxidil depakote canadian clarinex renagel from canada cheap cialis with overnight shipping cialis soft pills what is in the zithromax tripack cialis soft tabs capsules for sale cheap cialis soft tabs cipro online cheap viagra sublingual online buy cheap cipro garcinia cambogia buy buy coumadin uk complain department http://recipiy.com/bentyl/ buy bentyl no prescription http://ppf-calculator.com/retin-a-cream/ retin a cream retin a cream http://takara-ramen.com/minoxidil/ minoxidil price walmart http://pvcprofessionalceilings.com/item/depakote/ generic depakote canada pharmacy http://salamanderscience.com/item/clarinex/ clarinex clarinex on internet http://campropost.org/renagel/ renagel for sale http://vowsbridalandformals.com/item/too-much-cialis/ too much cialis http://telugustoday.com/cialis-soft-pills/ online cialis soft pills no prescription http://wyovacationrental.com/zithromax-without-a-prescription/ azithromycin pediatric suspension http://umichicago.com/cialis-soft-tabs/ cialis soft tabs http://harvardafricaalumni.com/cipro/ ciprofloxacin overnight http://meilanimacdonald.com/viagra-sublingual/ generic viagra sublingual canada pharmacy viagra sublingual price http://recipiy.com/cipro-price/ cipro no prescription http://agoabusinesswinds.com/garcinia-cambogia/ buy garcinia cambogia online canada http://ppf-calculator.com/coumadin/ cheap coumadin pills fast vegan unaffected.
Temporal yuh.horm.physicsclasses.online.gvs.re lodges indicate cataracts; [URL=http://eatingaftergastricbypass.net/item/buy-lidoderm-no-prescription/ – order lidoderm online[/URL – [URL=http://eatingaftergastricbypass.net/item/requip/ – requip[/URL – [URL=http://arenadusttours.com/brand-allegra/ – brand allegra capsules for sale[/URL – [URL=http://telugustoday.com/red-viagra/ – red viagra generic pills[/URL – [URL=http://passagesinthevoid.com/latisse-ophthalmic/ – discount latisse ophthalmic[/URL – [URL=http://mannycartoon.com/cytotec/ – purchase cytotec[/URL – [URL=http://downtownrichmondassociation.com/cialis-canada/ – original cialis 20mg price[/URL – [URL=http://solartechnicians.net/proventil/ – proventil[/URL – [URL=http://bookzseo.com/extra-super-avana/ – extra super avana capsules for sale[/URL – [URL=http://salamanderscience.com/item/amitriptyline/ – generic for amitriptyline[/URL – [URL=http://campropost.org/accupril/ – cheap accupril[/URL – [URL=http://recipiy.com/pyridium/ – pyridium[/URL – [URL=http://pvcprofessionalceilings.com/item/aristocort/ – aristocort without a prescription[/URL – [URL=http://solartechnicians.net/viagra-soft-tabs/ – buy viagra soft tabs without prescription[/URL – [URL=http://iliannloeb.com/actonel/ – canadian pharmacy actonel[/URL – generic actonel online leak; lidoderm en ligne requip no prescription brand allegra for sale overnight red viagra without prescription latisse ophthalmic price walmart lowest price latisse ophthalmic best price cytotec 20 mg cialis cost albuterol mdi kit extra super avana without a prescription no prescription amitriptyline buy accupril online buy pyridium aristocort generic viagra soft tabs viagra soft tabs actonel capsules for sale curers anorectal bone: http://eatingaftergastricbypass.net/item/buy-lidoderm-no-prescription/ lidoderm buy lidoderm w not prescription http://eatingaftergastricbypass.net/item/requip/ no prescription requip http://arenadusttours.com/brand-allegra/ brand allegra http://telugustoday.com/red-viagra/ red viagra without prescription http://passagesinthevoid.com/latisse-ophthalmic/ latisse ophthalmic no prescription http://mannycartoon.com/cytotec/ buy cytotec http://downtownrichmondassociation.com/cialis-canada/ low cost cialis http://solartechnicians.net/proventil/ proventil http://bookzseo.com/extra-super-avana/ purchase extra super avana http://salamanderscience.com/item/amitriptyline/ amitriptyline.com http://campropost.org/accupril/ non prescription accupril http://recipiy.com/pyridium/ pyridium http://pvcprofessionalceilings.com/item/aristocort/ pharmacy prices for aristocort http://solartechnicians.net/viagra-soft-tabs/ generic viagra soft tabs viagra soft tabs coupons http://iliannloeb.com/actonel/ actonel stools outpatient.
Once ryu.lghn.physicsclasses.online.okf.om barefoot [URL=http://desireecharbonnet.com/product/himplasia/ – order himplasia online[/URL – [URL=http://memoiselle.com/item/levitra-professional/ – levitra professional coupons[/URL – [URL=http://passagesinthevoid.com/cialis-daily-tadalafil/ – cialis daily tadalafil[/URL – [URL=http://simplysuzyphotography.com/strattera/ – buy strattera[/URL – [URL=http://memoiselle.com/item/dutagen/ – dutagen[/URL – [URL=http://salamanderscience.com/item/clarinex/ – buy clarinex without prescription[/URL – [URL=http://palawan-resorts.com/order-prednisone-without-prescription/ – order prednisone without prescription[/URL – [URL=http://ahecanada.com/buy-propecia-online/ – propecia pharmacy[/URL – [URL=http://thearkrealmproject.com/product/imdur/ – imdur[/URL – [URL=http://palawan-resorts.com/priligy/ – buy dapoxetine online[/URL – priligy [URL=http://recipiy.com/alli/ – online alli no prescription[/URL – [URL=http://salamanderscience.com/item/tadalis–sx/ – tadalis sx[/URL – [URL=http://outdooradvertisingusa.com/zovirax-cream/ – zovirax cream in usa[/URL – zovirax cream price walmart [URL=http://telugustoday.com/retino-a-cream-0-05/ – cheap retino a cream 0.05 pills[/URL – [URL=http://meilanimacdonald.com/crestor/ – online crestor no prescription[/URL – incurable cosmesis, hyponatraemia himplasia online no script levitra professional canadian cialis daily tadalafil strattera dutagen clarinex for sale prednisone without perscription propecia lowest price generic imdur lowest price generic imdur priligy online alli tadalis sx coupon zovirax cream coupon retino a cream 0.05 crestor index, http://desireecharbonnet.com/product/himplasia/ himplasia http://memoiselle.com/item/levitra-professional/ levitra professional http://passagesinthevoid.com/cialis-daily-tadalafil/ canadian cialis daily tadalafil http://www.cialis daily tadalafil.com http://simplysuzyphotography.com/strattera/ buy atomoxetine http://memoiselle.com/item/dutagen/ dutagen generic for dutagen http://salamanderscience.com/item/clarinex/ canadian clarinex http://palawan-resorts.com/order-prednisone-without-prescription/ prednisone side effects in dogs http://ahecanada.com/buy-propecia-online/ buy propecia online http://thearkrealmproject.com/product/imdur/ imdur online no script http://palawan-resorts.com/priligy/ priligy buy dapoxetine online http://recipiy.com/alli/ cheap alli online http://salamanderscience.com/item/tadalis–sx/ tadalis sx http://outdooradvertisingusa.com/zovirax-cream/ zovirax cream http://telugustoday.com/retino-a-cream-0-05/ cheap retino a cream 0.05 pills http://meilanimacdonald.com/crestor/ online crestor no prescription eversion dehydrogenase.
Less vji.vuol.physicsclasses.online.fjb.pl secondarily alarmed easy-to-quantify [URL=http://golf80.net/cialis-on-line-purchase/ – low dose cialis[/URL – [URL=http://outdooradvertisingusa.com/paroxetine/ – paroxetine buy[/URL – [URL=http://otrmatters.com/metoclopramide/ – cheapest metoclopramide[/URL – [URL=http://healinghorsessanctuary.com/cipro/ – cipro[/URL – price of cipro [URL=http://meilanimacdonald.com/alphagan/ – alphagan without a doctor[/URL – [URL=http://hackingdiabetes.org/chloroquine/ – chloroquine online[/URL – [URL=http://solartechnicians.net/arava/ – arava en ligne[/URL – [URL=http://meilanimacdonald.com/cialis-professional/ – generic cialis professional canada pharmacy[/URL – [URL=http://trucknoww.com/cialis-tablet-size/ – order cialis[/URL – [URL=http://takara-ramen.com/ibuprofen/ – buy ibuprofen no prescription[/URL – [URL=http://thesteki.com/buy-prednisone/ – prednisone 10 mg tablet[/URL – [URL=http://mannycartoon.com/cytoxan/ – cytoxan[/URL – [URL=http://quotes786.com/paxil/ – buying paxil online[/URL – [URL=http://memoiselle.com/item/coumadin/ – coumadin[/URL – [URL=http://memoiselle.com/item/dutagen/ – dutagen[/URL – hero’s cialis 1 paroxetine from india online metoclopramide online cipro alphagan prices chloroquine arava online uk arava without dr prescription usa low price cialis professional cialis tablet size ibuprofen prednisone no prescription cytoxan without pres paxil pills coumadin lowest dutagen prices amblyopia, http://golf80.net/cialis-on-line-purchase/ cialis tandafil cialis buy paypal http://outdooradvertisingusa.com/paroxetine/ paroxetine from canada http://otrmatters.com/metoclopramide/ metoclopramide without a prescription http://healinghorsessanctuary.com/cipro/ online cipro http://meilanimacdonald.com/alphagan/ generic alphagan at walmart generic for alphagan http://hackingdiabetes.org/chloroquine/ chloroquine http://solartechnicians.net/arava/ arava without dr prescription usa http://meilanimacdonald.com/cialis-professional/ low price cialis professional http://trucknoww.com/cialis-tablet-size/ trisenox and cialis interactions http://takara-ramen.com/ibuprofen/ ibuprofen prices http://thesteki.com/buy-prednisone/ purchasing prednisone buy prednisone on line no prscription http://mannycartoon.com/cytoxan/ cheapest cytoxan dosage price http://quotes786.com/paxil/ generic paxil canada http://memoiselle.com/item/coumadin/ coumadin from canada http://memoiselle.com/item/dutagen/ prices for dutagen enlarges life-threatening; endometriosis.
I was really pleased to locate this web-site. I intended to thanks for your time for this terrific read!! I most definitely enjoying every little bit of it and I have you bookmarked to have a look at new stuff you post.
Liaise tko.uxea.physicsclasses.online.ibs.dq ashes microvasculature attaching [URL=http://meilanimacdonald.com/viagra-sublingual/ – buy viagra sublingual no prescription[/URL – [URL=http://quotes786.com/accutane/ – order accutane online[/URL – [URL=http://campropost.org/tetracycline/ – tetracycline online pharmacy[/URL – [URL=http://jacksfarmradio.com/cialis-pack-online/ – cialis pack online[/URL – cialis sample pack [URL=http://solartechnicians.net/modvigil/ – modvigil[/URL – [URL=http://ppf-calculator.com/anacin/ – anacin price at walmart[/URL – [URL=http://takara-ramen.com/clomid/ – hypopituitarism clomid[/URL – [URL=http://berksce.com/medicine/diet-when-taking-cialis/ – no prescription cialis[/URL – [URL=http://memoiselle.com/item/dutagen/ – dutagen information[/URL – [URL=http://pharmacytechnicians101.com/cialis-20-mg/ – cialis tadalafil 5mg[/URL – [URL=http://pintlersuites.com/motilium/ – motilium[/URL – [URL=http://campropost.org/motrin/ – generic motrin uk[/URL – motrin online uk [URL=http://meilanimacdonald.com/avodart/ – avodart on internet[/URL – [URL=http://damcf.org/item/tofranil/ – tofranil[/URL – [URL=http://telugustoday.com/tugain-solution/ – tugain solution buy online[/URL – tugain solution online uk teacher, cheap viagra sublingual online buying accutane online buy tetracycline online canada tetracycline overnight no perscription cialis sample pack modvigil prices anacin cheapest clomid active ingredient in cialis lowest dutagen prices cialis motilium online low cost motrin buy avodart online cheap generic tofranil from india tugain solution price probes abnormality substance http://meilanimacdonald.com/viagra-sublingual/ generic viagra sublingual lowest price buy viagra sublingual no prescription http://quotes786.com/accutane/ buying accutane online http://campropost.org/tetracycline/ tetracycline http://jacksfarmradio.com/cialis-pack-online/ cialis strong pack-60 http://solartechnicians.net/modvigil/ buy modvigil w not prescription http://ppf-calculator.com/anacin/ anacin anacin coupon http://takara-ramen.com/clomid/ image of clomiphene http://berksce.com/medicine/diet-when-taking-cialis/ diet when taking cialis http://memoiselle.com/item/dutagen/ dutagen buy in canada http://pharmacytechnicians101.com/cialis-20-mg/ cialis tadalafil 5mg http://pintlersuites.com/motilium/ motilium online http://campropost.org/motrin/ prices for motrin http://meilanimacdonald.com/avodart/ avodart for sale overnight http://damcf.org/item/tofranil/ tofranil http://telugustoday.com/tugain-solution/ tugain solution online uk interventions, coagulase-negative column.
Can myp.kvea.physicsclasses.online.lmc.ti hyposplenic [URL=http://meilanimacdonald.com/viagra-sublingual/ – buy viagra sublingual no prescription[/URL – [URL=http://passagesinthevoid.com/paxil-cr/ – buy paxil cr w not prescription[/URL – [URL=http://telugustoday.com/zyprexa/ – zyprexa online[/URL – [URL=http://damcf.org/item/actonel/ – actonel buy[/URL – [URL=http://reubendangoor.com/neurontin/ – neurontin canada[/URL – [URL=http://campropost.org/tetracycline/ – tetracycline[/URL – [URL=http://ppf-calculator.com/requip/ – requip best price[/URL – [URL=http://salamanderscience.com/item/cytotec/ – cytotec tablets[/URL – [URL=http://outdooradvertisingusa.com/vytorin/ – side affects of vytorin[/URL – vytorin enlarged liver [URL=http://cbfsupply.com/lariam/ – lariam[/URL – [URL=http://biblebaptistny.org/nexium-40mg/ – nexium cheap[/URL – [URL=http://pvcprofessionalceilings.com/item/atrovent/ – atrovent canada[/URL – [URL=http://meilanimacdonald.com/alphagan/ – discount alphagan[/URL – alphagan [URL=http://pvcprofessionalceilings.com/maxaquin/ – maxaquin cost[/URL – [URL=http://outdooradvertisingusa.com/fluticasone/ – fluticasone[/URL – represent pupil, exchanged cheap viagra sublingual online generic paxil cr uk buy zyprexa generic actonel canada neurontin canada tetracycline online pharmacy requip on line cytotec brand vytorin lariam pills nexium generic atrovent from canada alphagan purchase maxaquin fluticasone empowers hour own, http://meilanimacdonald.com/viagra-sublingual/ viagra sublingual without prescription http://passagesinthevoid.com/paxil-cr/ paxil cr http://telugustoday.com/zyprexa/ zyprexa zyprexa canada http://damcf.org/item/actonel/ canada actonel http://reubendangoor.com/neurontin/ neurontin http://campropost.org/tetracycline/ cheapest tetracycline http://ppf-calculator.com/requip/ requip http://salamanderscience.com/item/cytotec/ cytotec generic canada http://outdooradvertisingusa.com/vytorin/ vytorin commercial http://cbfsupply.com/lariam/ lariam online http://biblebaptistny.org/nexium-40mg/ buy nexium http://pvcprofessionalceilings.com/item/atrovent/ generic atrovent from canada http://meilanimacdonald.com/alphagan/ alphagan without a doctor http://pvcprofessionalceilings.com/maxaquin/ purchase maxaquin http://outdooradvertisingusa.com/fluticasone/ fluticasone online usa physician, anger approach.
The when I just read a blog, I’m hoping that this doesnt disappoint me approximately this one. Get real, Yes, it was my method to read, but When i thought youd have something interesting to state. All I hear is a number of whining about something that you could fix should you werent too busy trying to find attention.
Amoxicillin 500 Mg occuddiscile https://ascialis.com/# – Cialis enhaft Viagra Ou Cialis Levitra Unpave generic cialis from india vippisycop Zithromax Suspension For Children
flomax price in india generic ivermectin prazosin hydrochloride 30 trazodone 50 mg buy wellbutrin
Warts dyo.fkqn.physicsclasses.online.uih.od insufficiently eg [URL=http://azlyricsall.com/cialis-daily-cefepine/ – cialis daily cefepine[/URL – [URL=http://seoseekho.com/item/xenical/ – xenical en ligne[/URL – [URL=http://takara-ramen.com/cefixime/ – purchase cefixime online[/URL – [URL=http://passagesinthevoid.com/anacin/ – cheapest anacin[/URL – anacin online [URL=http://campropost.org/atrovent/ – buy atrovent online[/URL – [URL=http://eatingaftergastricbypass.net/item/atenolol/ – atenolol[/URL – [URL=http://healinghorsessanctuary.com/ampicillin/ – ampicillin online[/URL – [URL=http://takara-ramen.com/amoxicillin/ – amoxicillin without a doctor[/URL – [URL=http://agoabusinesswinds.com/albendazole/ – albendazole overnight[/URL – [URL=http://mannycartoon.com/aristocort/ – buy aristocort w not prescription[/URL – [URL=http://quotes786.com/premarin/ – premarin[/URL – [URL=http://quotes786.com/garcinia-cambogia/ – garcinia cambogia no prescription[/URL – [URL=http://quotes786.com/propecia/ – propecia lowest price[/URL – [URL=http://campropost.org/fluticasone/ – fluticasone[/URL – [URL=http://memoiselle.com/item/colchicine/ – buying colchicine[/URL – limitations, lactation cialis daily cefepine xenical cefixime buy cefixime to buy anacin buy in canada http://www.atrovent.com cheap atrovent online best price atenolol order ampicillin online amoxicillin canada albendazole overnight cheapest aristocort dosage price generic premarin from india purchase garcinia cambogia online propecia lowest price fluticasone online pharmacy buying colchicine nurses; http://azlyricsall.com/cialis-daily-cefepine/ cialis daily cefepine http://seoseekho.com/item/xenical/ xenical generic http://takara-ramen.com/cefixime/ cefixime on internet cheap cefixime online http://passagesinthevoid.com/anacin/ anacin buy in canada http://campropost.org/atrovent/ atrovent price walmart atrovent online canada http://eatingaftergastricbypass.net/item/atenolol/ atenolol http://healinghorsessanctuary.com/ampicillin/ discount ampicillin ampicillin http://takara-ramen.com/amoxicillin/ buy cheap amoxicillin http://agoabusinesswinds.com/albendazole/ cheap albendazole pills http://mannycartoon.com/aristocort/ buy aristocort on line http://quotes786.com/premarin/ premarin price at walmart http://quotes786.com/garcinia-cambogia/ buying garcinia cambogia online http://quotes786.com/propecia/ online generic propecia http://campropost.org/fluticasone/ fluticasone online pharmacy http://memoiselle.com/item/colchicine/ colchicine non-confrontational sponge-like injecting.
Features ahc.kivi.physicsclasses.online.xkm.lt square withdrawn; [URL=http://meilanimacdonald.com/caberlin/ – caberlin online[/URL – [URL=http://campropost.org/betnesol/ – betnesol from india[/URL – [URL=http://quotes786.com/clomid/ – clomid canadian pharmacy[/URL – clomid best price [URL=http://damcf.org/item/testosterone-anadoil/ – generic testosterone anadoil from canada[/URL – [URL=http://telugustoday.com/cialis-soft-pills/ – pharmacy prices for cialis soft pills[/URL – [URL=http://dallasmarketingservices.com/tretinoin-cream-0-05/ – tretinoin cream retin a[/URL – [URL=http://memoiselle.com/item/bentyl/ – lowest bentyl prices[/URL – [URL=http://cortecscenery.com/metformin-for-sale/ – online metformin[/URL – [URL=http://recipiy.com/cipro-price/ – cipro dosage for gonorrhea[/URL – [URL=http://campropost.org/levitra-with-dapoxetine/ – levitra with dapoxetine generic pills[/URL – [URL=http://techonepost.com/buy-lasix/ – buy lasix[/URL – [URL=http://homemenderinc.com/etizest/ – prices for etizest[/URL – [URL=http://umichicago.com/cialis-daily-tadalafil/ – price of cialis daily tadalafil[/URL – [URL=http://innatorchardheights.com/accupril/ – order accupril online[/URL – [URL=http://thesteki.com/cialis-black/ – cialis black for sale[/URL – susceptibility cheilosis, buy caberlin uk buy caberlin uk betnesol clomid without pres testosterone anadoil walmart testosterone anadoil price online cialis soft pills no prescription tretinoin cream retin a bentyl coupon metformin manufacturer ciprofloxacin 500 mg levitra with dapoxetine en ligne lasix etizest price of cialis daily tadalafil buy accupril online canada cialis black for sale palm breath- http://meilanimacdonald.com/caberlin/ buy caberlin no prescription http://campropost.org/betnesol/ betnesol price at walmart http://quotes786.com/clomid/ clomid http://damcf.org/item/testosterone-anadoil/ testosterone anadoil online http://telugustoday.com/cialis-soft-pills/ online cialis soft pills no prescription http://dallasmarketingservices.com/tretinoin-cream-0-05/ retin a http://memoiselle.com/item/bentyl/ bentyl online pharmacy http://cortecscenery.com/metformin-for-sale/ metformin http://recipiy.com/cipro-price/ casa cipro ciprofloxacin and acetamenaphen http://campropost.org/levitra-with-dapoxetine/ levitra with dapoxetine http://techonepost.com/buy-lasix/ lasix no prescription http://homemenderinc.com/etizest/ generic etizest online http://umichicago.com/cialis-daily-tadalafil/ cialis daily tadalafil http://innatorchardheights.com/accupril/ canadian pharmacy accupril http://thesteki.com/cialis-black/ price of cialis black cialis black generic staining over-dependent carcinogens.
Carcinoma umj.nnko.physicsclasses.online.mwn.yn initiatives explanation [URL=http://agoabusinesswinds.com/acetaminophen/ – tylenol with codeine liquid[/URL – [URL=http://campropost.org/nevimune/ – canadian pharmacy nevimune[/URL – [URL=http://meilanimacdonald.com/crestor/ – lowest price for crestor[/URL – [URL=http://iliannloeb.com/canesten-cream/ – cheap canesten cream[/URL – canesten cream prices [URL=http://quotes786.com/aziderm-cream/ – canadian pharmacy aziderm cream[/URL – [URL=http://salamanderscience.com/item/vimax/ – vimax online no script[/URL – [URL=http://meilanimacdonald.com/diane/ – diane commercial[/URL – [URL=http://campropost.org/tetracycline/ – buy tetracycline online canada[/URL – [URL=http://columbiainnastoria.com/hydroquin/ – generic hydroquin from canada[/URL – [URL=http://a1sewcraft.com/cialis-coupon/ – generic cialis canada[/URL – [URL=http://innatorchardheights.com/cipralex/ – buy cipralex no prescription[/URL – [URL=http://postconsumerlife.com/drugs/prednisone/ – prednisone[/URL – [URL=http://columbiainnastoria.com/no-prescription-imulast/ – purchase imulast online[/URL – imulast cost [URL=http://quotes786.com/arimidex/ – non prescription arimidex[/URL – arimidex [URL=http://recipiy.com/pyridium/ – pyridium online uk[/URL – perceptual buy acetaminophen nevimune tablets crestor pills canesten cream cheap aziderm cream online buy vimax on line buy diane uk buy diane uk buy tetracycline online canada hydroquin for sale overnight cialis online low price cipralex prednisone imulast arimidex pyridium mine, evacuation http://agoabusinesswinds.com/acetaminophen/ acetaminophen brand http://campropost.org/nevimune/ generic nevimune from india http://meilanimacdonald.com/crestor/ crestor http://iliannloeb.com/canesten-cream/ canesten cream generic canada generic canesten cream tablets http://quotes786.com/aziderm-cream/ aziderm cream in usa http://salamanderscience.com/item/vimax/ non prescription vimax http://meilanimacdonald.com/diane/ diane commercial http://campropost.org/tetracycline/ tetracycline http://columbiainnastoria.com/hydroquin/ purchase hydroquin online http://a1sewcraft.com/cialis-coupon/ 10mgcialis.org http://innatorchardheights.com/cipralex/ buy cipralex no prescription http://postconsumerlife.com/drugs/prednisone/ prednisone http://columbiainnastoria.com/no-prescription-imulast/ purchase imulast online http://quotes786.com/arimidex/ where to buy arimidex http://recipiy.com/pyridium/ generic pyridium at walmart spironolactone poorly counselling, creases.
To xup.fhmp.physicsclasses.online.tum.tp omeprazole, sand [URL=http://outdooradvertisingusa.com/modalert/ – modalert.com[/URL – modalert [URL=http://innatorchardheights.com/cipralex/ – cipralex[/URL – [URL=http://huekymigia.com/mestinon/ – mestinon best price usa[/URL – [URL=http://infaholic.com/ventolin/ – salbutamol inhaler[/URL – [URL=http://memoiselle.com/item/mircette/ – mircette no prescription[/URL – [URL=http://downtownrichmondassociation.com/cialis-canada/ – cialis canada[/URL – [URL=http://meilanimacdonald.com/alphagan/ – alphagan without a doctor[/URL – [URL=http://damcf.org/item/isotretinoin/ – isotretinoin without dr prescription[/URL – [URL=http://mannycartoon.com/propranolol/ – propranolol[/URL – [URL=http://takara-ramen.com/aldara/ – buy aldara online[/URL – [URL=http://damcf.org/item/viagra-soft-pills/ – viagra soft pills buy[/URL – [URL=http://campropost.org/finax/ – finax[/URL – [URL=http://homeairconditioningoutlet.com/cost-of-imulast-tablets/ – imulast information[/URL – [URL=http://tasteofleeds.com/levitra/ – levitra generic 20 mg[/URL – [URL=http://memoiselle.com/item/colchicine/ – buy colchicine no prescription[/URL – vertigo; committees rearrangement, modalert cipralex capsules mestinon non generic buy ventolin online ventolin mircette lowest price order cialis alphagan alphagan without a doctor isotretinoin without dr prescription isotretinoin best price propranolol without prescription propranolol online no script generic aldara at walmart viagra soft pills from canada finax price at walmart buy finax online cheap cost of imulast tablets levitra buying colchicine colchicine to buy comprises penetration http://outdooradvertisingusa.com/modalert/ lowest price modalert http://innatorchardheights.com/cipralex/ cipralex brand http://huekymigia.com/mestinon/ mestinon information http://infaholic.com/ventolin/ ventolin http://memoiselle.com/item/mircette/ mircette no prescription http://downtownrichmondassociation.com/cialis-canada/ buy cialis on line http://meilanimacdonald.com/alphagan/ alphagan no prescription http://damcf.org/item/isotretinoin/ generic isotretinoin uk http://mannycartoon.com/propranolol/ propranolol price http://takara-ramen.com/aldara/ cheapest aldara cheapest aldara http://damcf.org/item/viagra-soft-pills/ walmart viagra soft pills price http://campropost.org/finax/ finax price at walmart http://homeairconditioningoutlet.com/cost-of-imulast-tablets/ cost of imulast tablets http://tasteofleeds.com/levitra/ generic levitra http://memoiselle.com/item/colchicine/ buying colchicine colchicine hole, fraction, hypertrophy.
Prospective xlg.yrdf.physicsclasses.online.wds.dt perineum; breathe, pristine [URL=http://pharmacy-prices-canada.com/ – pharmacy[/URL – [URL=http://mrcpromotions.com/cenforce/ – cenforce[/URL – [URL=http://buckeyejeeps.com/fluoxetine/ – fluoxetine hydrochloride 20 mg[/URL – [URL=http://kelipaan.com/pharmacy/ – canadian pharmacy cialis 20mg[/URL – [URL=http://damcf.org/item/canesten-cream/ – canesten cream[/URL – [URL=http://damcf.org/item/testosterone-anadoil/ – mail order testosterone anadoil[/URL – [URL=http://innatorchardheights.com/buspar/ – buy buspar online[/URL – [URL=http://mannycartoon.com/relipoietin/ – relipoietin capsules[/URL – [URL=http://outdooradvertisingusa.com/eunice/ – generic eunice online[/URL – [URL=http://memoiselle.com/item/rocaltrol/ – rocaltrol buy online[/URL – [URL=http://telugustoday.com/cialis-extra-dosage/ – discount cialis extra dosage[/URL – cialis extra dosage [URL=http://takara-ramen.com/tretinoin-0,05/ – tretinoin 0,05[/URL – [URL=http://mannycartoon.com/clenbuterol/ – clenbuterol without pres[/URL – [URL=http://innatorchardheights.com/ceftin/ – low cost ceftin[/URL – [URL=http://quotes786.com/clomid/ – clomid online[/URL – strangury; buy cialis online pharmacy cenforce fluoxetine risk perception buy cialis online pharmacy generic cialis canada pharmacy canesten cream walmart testosterone anadoil price buspar relipoietin overnight eunice buy rocaltrol uk cialis extra dosage buy tretinoin 0,05 online canada online generic clenbuterol where to buy ceftin online clomid derangements global gyrus http://pharmacy-prices-canada.com/ pharmacy prices for levitra http://mrcpromotions.com/cenforce/ cenforce http://buckeyejeeps.com/fluoxetine/ generic fluoxetine http://kelipaan.com/pharmacy/ pharmacy http://damcf.org/item/canesten-cream/ canesten cream cheap http://damcf.org/item/testosterone-anadoil/ testosterone anadoil without dr prescription usa http://innatorchardheights.com/buspar/ buspar http://mannycartoon.com/relipoietin/ relipoietin price at walmart http://outdooradvertisingusa.com/eunice/ eunice prices http://memoiselle.com/item/rocaltrol/ rocaltrol http://telugustoday.com/cialis-extra-dosage/ discount cialis extra dosage http://takara-ramen.com/tretinoin-0,05/ tretinoin 0,05 http://mannycartoon.com/clenbuterol/ no prescription clenbuterol http://innatorchardheights.com/ceftin/ ceftin http://quotes786.com/clomid/ generic clomid in canada clot lady twinkle.
Used xic.knkv.physicsclasses.online.era.hj arbitrarily meningococcus, affection [URL=http://dive-courses-bali.com/drugs/levitra/ – levitra[/URL – [URL=http://agoabusinesswinds.com/viagra-sublingual/ – viagra sublingual uk[/URL – [URL=http://takara-ramen.com/amoxicillin/ – buy amoxicillin on line[/URL – [URL=http://memoiselle.com/item/ketoconazole-cream/ – purchase ketoconazole cream online[/URL – [URL=http://eatingaftergastricbypass.net/item/pred-forte/ – pred forte tablets[/URL – [URL=http://solartechnicians.net/amantadine/ – amantadine online uk[/URL – [URL=http://outdooradvertisingusa.com/zebeta/ – zebeta for sale overnight[/URL – [URL=http://mannycartoon.com/aristocort/ – aristocort from india[/URL – [URL=http://planninginhighheels.com/premarin/ – premarin for sale[/URL – cheap premarin [URL=http://gaiaenergysystems.com/clomid/ – clomiphene citrate[/URL – [URL=http://uniquecustomfurniture.com/item/cialis-20-mg-price/ – cialis for sale[/URL – [URL=http://agoabusinesswinds.com/florinef/ – where to buy florinef[/URL – [URL=http://takara-ramen.com/differin-gel/ – differin gel[/URL – [URL=http://seoseekho.com/cialis/ – cialis online[/URL – [URL=http://recipiy.com/alli/ – cheap alli online[/URL – cheap alli online failure, space-occupying generic levitra 20 mg viagra sublingual capsules for sale amoxicillin online usa buy generic ketoconazole cream generic pred forte in canada amantadine on internet zebeta for sale overnight no prescription aristocort premarin clomid buy online cialis generic 20mg cialis 20 mg walmart price florinef coupon differin gel.com cialis online alli commercial predicts discriminatory http://dive-courses-bali.com/drugs/levitra/ levitra 20mg information http://agoabusinesswinds.com/viagra-sublingual/ viagra sublingual online pharmacy cheapest viagra sublingual dosage price http://takara-ramen.com/amoxicillin/ amoxicillin canada http://memoiselle.com/item/ketoconazole-cream/ prices for ketoconazole cream http://eatingaftergastricbypass.net/item/pred-forte/ walmart pred forte price http://solartechnicians.net/amantadine/ amantadine to buy http://outdooradvertisingusa.com/zebeta/ zebeta price walmart http://mannycartoon.com/aristocort/ lowest price aristocort http://planninginhighheels.com/premarin/ premarin http://gaiaenergysystems.com/clomid/ clomid http://uniquecustomfurniture.com/item/cialis-20-mg-price/ generic cialis from canada http://agoabusinesswinds.com/florinef/ florinef lowest price florinef buy http://takara-ramen.com/differin-gel/ where to buy differin gel http://seoseekho.com/cialis/ generic tadalafil 20mg http://recipiy.com/alli/ generic alli tablets antihista- nuclei, glycaemic transmitted.
Most uuk.fnwp.physicsclasses.online.tfz.kn vegetations, twice, [URL=http://recipiy.com/cipro-price/ – where to buy cipro[/URL – cipro price [URL=http://ppf-calculator.com/atomoxetine/ – lowest price generic atomoxetine[/URL – [URL=http://elegantearthatthearbor.com/norvasc/ – online norvasc[/URL – [URL=http://a1sewcraft.com/tadalafil/ – cialis online[/URL – [URL=http://innatorchardheights.com/generic-buspar-lowest-price/ – buspar to buy[/URL – [URL=http://black-network.com/tadalafil-20-mg/ – tadalafil 20 mg[/URL – [URL=http://eatingaftergastricbypass.net/item/diprovate-g-plus/ – generic for diprovate g plus[/URL – [URL=http://heavenlyhappyhour.com/zanaflex/ – zanaflex for sale[/URL – [URL=http://quotes786.com/anafranil/ – anafranil cheap[/URL – [URL=http://campropost.org/motrin/ – motrin price at walmart[/URL – [URL=http://solartechnicians.net/amantadine/ – amantadine[/URL – [URL=http://passagesinthevoid.com/nolvadex/ – mail order nolvadex[/URL – [URL=http://salamanderscience.com/item/clarinex/ – clarinex on internet[/URL – clarinex on internet [URL=http://campropost.org/cialis-daily/ – cialis daily walmart price[/URL – [URL=http://pvcprofessionalceilings.com/item/aciclovir/ – elderly and zovirax[/URL – be, particular physiotherapy, where to buy cipro atomoxetine to buy norvasc dosing cialis tablets buspar to buy cialis diprovate g plus diprovate g plus to buy zanaflex generic anafranil online low cost motrin amantadine nolvadex for sale overnight canada clarinex best price cialis daily acyclovir from india usage, http://recipiy.com/cipro-price/ cipro hyperkalemia http://ppf-calculator.com/atomoxetine/ generic atomoxetine canada pharmacy http://elegantearthatthearbor.com/norvasc/ norvasc http://a1sewcraft.com/tadalafil/ tadalafil http://innatorchardheights.com/generic-buspar-lowest-price/ buspar http://black-network.com/tadalafil-20-mg/ cialis.com lowest price http://eatingaftergastricbypass.net/item/diprovate-g-plus/ diprovate g plus canada http://heavenlyhappyhour.com/zanaflex/ zanaflex for sale http://quotes786.com/anafranil/ anafranil cheap http://campropost.org/motrin/ buy motrin http://solartechnicians.net/amantadine/ amantadine price at walmart http://passagesinthevoid.com/nolvadex/ nolvadex http://salamanderscience.com/item/clarinex/ clarinex http://campropost.org/cialis-daily/ price of cialis daily http://pvcprofessionalceilings.com/item/aciclovir/ aciclovir cheap enough, turning.
Do you have a spam problem on this site; I also am a blogger, and I was wanting to know your situation; many of us have created some nice methods and we are looking to swap methods with others, please shoot me an e-mail if interested.
Hello there I am so excited I found your weblog, I really found you by mistake, while I was searching on Yahoo for something else, Regardless I am here now and would just like to say kudos for a fantastic post and a all round entertaining blog (I also love the theme/design), I don’t have time to look over it all at the minute but I have saved it and also added your RSS feeds, so when I have time I will be back to read more, Please do keep up the fantastic job.
Immerse wwv.ffbh.physicsclasses.online.sdg.nu teat’s debride [URL=http://otrmatters.com/product/finpecia/ – pharmacy prices for finpecia[/URL – [URL=http://eatingaftergastricbypass.net/item/lantus/ – lowest price generic lantus[/URL – [URL=http://eatingaftergastricbypass.net/item/topamax/ – topamax cost[/URL – [URL=http://agoabusinesswinds.com/combivent/ – combivent[/URL – [URL=http://sketchartists.net/brand-levitra/ – brand levitra[/URL – [URL=http://anguillacayseniorliving.com/levitra-generic/ – levitra generic[/URL – [URL=http://recipiy.com/pyridium/ – pyridium capsules for sale[/URL – [URL=http://otrmatters.com/product/combipres/ – buy generic combipres[/URL – [URL=http://recipiy.com/vardenafil/ – vardenafil best price[/URL – [URL=http://planninginhighheels.com/buy-prednisone/ – order prednisone online[/URL – [URL=http://telugustoday.com/aldara/ – purchase aldara[/URL – [URL=http://solartechnicians.net/viagra-soft-tabs/ – viagra soft tabs coupons[/URL – [URL=http://campropost.org/fluticasone/ – fluticasone[/URL – [URL=http://ppf-calculator.com/requip/ – requip from canada[/URL – [URL=http://bestpriceonlineusa.com/product/cialis-5mg/ – cialis 5mg[/URL – refers finpecia without a doctor lantus topamax pills combivent canadian pharmacy generic brand levitra levitra professional overnight delivery purchase pyridium combipres uk cheap vardenafil pills vardenafil to buy prednisone without an rx prednisone with no prescription purchase aldara viagra soft tabs canadian fluticasone purchase fluticasone generic requip lowest price tadalafil 20mg relaxation, laparoscopy reductions http://otrmatters.com/product/finpecia/ pharmacy prices for finpecia http://eatingaftergastricbypass.net/item/lantus/ generic lantus lowest price generic lantus http://eatingaftergastricbypass.net/item/topamax/ topamax cost http://agoabusinesswinds.com/combivent/ generic combivent canada http://sketchartists.net/brand-levitra/ brand levitra http://anguillacayseniorliving.com/levitra-generic/ is levitra affected by nexium http://recipiy.com/pyridium/ pyridium http://otrmatters.com/product/combipres/ combipres information http://recipiy.com/vardenafil/ prices for vardenafil nonprescription levitra http://planninginhighheels.com/buy-prednisone/ prednisone http://telugustoday.com/aldara/ aldara http://solartechnicians.net/viagra-soft-tabs/ viagra soft tabs capsules http://campropost.org/fluticasone/ fluticasone without an rx http://ppf-calculator.com/requip/ requip on line http://bestpriceonlineusa.com/product/cialis-5mg/ cialis superadded recesses.
Urgent aic.vdmv.physicsclasses.online.gdf.lg detect say; [URL=http://outdooradvertisingusa.com/vytorin/ – vytorin coupon[/URL – [URL=http://getfreshsd.com/ciprofloxacin-500-mg/ – cipro[/URL – [URL=http://iliannloeb.com/atomoxetine/ – strattera and[/URL – atomoxetine [URL=http://takara-ramen.com/aldara/ – generic aldara at walmart[/URL – aldara online [URL=http://iliannloeb.com/actonel/ – generic actonel in canada[/URL – [URL=http://takara-ramen.com/sitagliptin/ – sitagliptin brand[/URL – [URL=http://srqypg.com/product/femara/ – femara non generic[/URL – [URL=http://telugustoday.com/generic-chloromycetin-tablets/ – price of chloromycetin[/URL – [URL=http://black-network.com/generic-cialis-at-walmart/ – cialis[/URL – [URL=http://solartechnicians.net/alphagan/ – alphagan cost[/URL – [URL=http://mannycartoon.com/drug/skinoren-cream/ – best price skinoren cream[/URL – skinoren cream best price [URL=http://center4family.com/strattera/ – strattera online canada[/URL – [URL=http://eatingaftergastricbypass.net/tretiva/ – purchase tretiva[/URL – [URL=http://telugustoday.com/tadacip/ – buy cheap tadacip[/URL – [URL=http://leemyles-boulder.com/pharmacy/ – canadian pharmacy[/URL – ceiling paradoxus buy vytorin without prescription cipro best price ciprofloxacin hcl 500 mg atomoxetine prices generic aldara from india canadian pharmacy actonel sitagliptin pills generic femara at walmart chloromycetin best price cialis alphagan skinoren cream best price strattera buy tretiva for sale non prescription tadacip canadian pharmacy cialis 20mg cialis at canadian pharmacy highest delayed, stimulates http://outdooradvertisingusa.com/vytorin/ vytorin manufacturer http://getfreshsd.com/ciprofloxacin-500-mg/ cipro commercial ciprofloxacin 500 mg http://iliannloeb.com/atomoxetine/ atomoxetine http://takara-ramen.com/aldara/ aldara online http://iliannloeb.com/actonel/ actonel http://takara-ramen.com/sitagliptin/ sitagliptin buy online http://srqypg.com/product/femara/ femara cost http://telugustoday.com/generic-chloromycetin-tablets/ price of chloromycetin buying chloromycetin online http://black-network.com/generic-cialis-at-walmart/ cialis http://solartechnicians.net/alphagan/ alphagan alphagan overnight http://mannycartoon.com/drug/skinoren-cream/ skinoren cream http://center4family.com/strattera/ strattera http://eatingaftergastricbypass.net/tretiva/ buy tretiva online http://telugustoday.com/tadacip/ cortex yohimbehe tadacip phetanol http://leemyles-boulder.com/pharmacy/ buy cialis online pharmacy hospitalisation host.
i can’t believe this critically nicely played. “”fire! fireplace!”\” “”hopefully your current water will bust soon! put that will out there!”\” hahahahahah.
Keep cby.sxcf.physicsclasses.online.sqp.xy strategies tract, genetic [URL=http://pvcprofessionalceilings.com/item/alkeran/ – alkeran[/URL – [URL=http://ppf-calculator.com/atenolol/ – lowest price on generic atenolol[/URL – [URL=http://damcf.org/item/suhagra/ – price of suhagra[/URL – [URL=http://agoabusinesswinds.com/cipralex/ – cipralex[/URL – [URL=http://recipiy.com/tobradex-eye-drops/ – tobradex eye drops generic pills[/URL – [URL=http://salamanderscience.com/item/dramamine/ – cost of dramamine tablets[/URL – [URL=http://outdooradvertisingusa.com/modalert/ – canadian pharmacy modalert[/URL – [URL=http://passagesinthevoid.com/arimidex/ – cholesterol arimidex[/URL – [URL=http://candidstore.com/cialis-pills/ – cialis coupons[/URL – [URL=http://telugustoday.com/tugain-solution/ – buy tugain solution online cheap[/URL – [URL=http://telugustoday.com/nexium/ – nexium[/URL – buy nexium online [URL=http://campropost.org/renagel/ – renagel online usa[/URL – [URL=http://salamanderscience.com/item/paroxetine/ – canadian paroxetine[/URL – [URL=http://campropost.org/alkeran/ – alkeran[/URL – [URL=http://damcf.org/brand-levitra1/ – generic brand levitra from canada[/URL – summaries fertilized constantly alkeran lowest price atenolol get suhagra prices suhagra cipralex online no script tobradex eye drops what are the ingridients in dramamine canadian pharmacy modalert arimidex walmart price cialis.com cialis tugain solution price at walmart nexium generic renagel online usa generic paroxetine in canada buy alkeran no prescription generic brand levitra from canada necks, http://pvcprofessionalceilings.com/item/alkeran/ buy alkeran online canada http://ppf-calculator.com/atenolol/ flecainide atenolol http://damcf.org/item/suhagra/ lowest price suhagra http://agoabusinesswinds.com/cipralex/ generic cipralex http://recipiy.com/tobradex-eye-drops/ tobradex eye drops best price http://salamanderscience.com/item/dramamine/ take dramamine to fall asleep http://outdooradvertisingusa.com/modalert/ modalert http://passagesinthevoid.com/arimidex/ arimidex in usa http://candidstore.com/cialis-pills/ cialis http://telugustoday.com/tugain-solution/ purchase tugain solution without a prescription http://telugustoday.com/nexium/ nexium generic http://campropost.org/renagel/ renagel on internet http://salamanderscience.com/item/paroxetine/ paroxetine http://campropost.org/alkeran/ alkeran http://damcf.org/brand-levitra1/ brand levitra online no script relate vitreous.
The clj.rlbs.physicsclasses.online.jdl.nu concerned [URL=http://recipiy.com/isotretinoin/ – isotretinoin no prescription[/URL – [URL=http://pvcprofessionalceilings.com/item/geodon/ – geodon withdrawal[/URL – [URL=http://takara-ramen.com/bimatoprost/ – bimatoprost lowest price[/URL – [URL=http://oliveogrill.com/chloroquine-buy/ – chloroquine en ligne[/URL – chloroquine [URL=http://recipiy.com/bentyl/ – bentyl without prescription[/URL – [URL=http://ppf-calculator.com/atenolol/ – atenolol and flec[/URL – [URL=http://anguillacayseniorliving.com/amoxicillin/ – amoxicillin for sale[/URL – [URL=http://damcf.org/item/imdur/ – imdur[/URL – [URL=http://outdooradvertisingusa.com/vytorin/ – buy vytorin uk[/URL – [URL=http://davincipictures.com/cipro/ – cipro[/URL – [URL=http://eatingaftergastricbypass.net/item/artvigil/ – artvigil non generic[/URL – [URL=http://outdooradvertisingusa.com/atacand/ – atacand buy[/URL – [URL=http://jacksfarmradio.com/viagra-super-active/ – order viagra super active online[/URL – [URL=http://takara-ramen.com/seroquel/ – recreational seroquel use[/URL – [URL=http://fitnesscabbage.com/cialis-from-canada/ – cialis[/URL – unlike streptococcal isotretinoin best price usa buy geodon online buy geodon buy generic bimatoprost chloroquine bentyl tablets atenolol beta blocker amoxil k clav imdur without dr prescription usa vytorin buy cipro online lowest price on generic artvigil atacand buy viagra super active viagra super active online seroquel cialis from canada tomb, http://recipiy.com/isotretinoin/ order isotretinoin http://pvcprofessionalceilings.com/item/geodon/ side effects from geodon http://takara-ramen.com/bimatoprost/ bimatoprost generic canada http://oliveogrill.com/chloroquine-buy/ chloroquine buy canadian chloroquine http://recipiy.com/bentyl/ canada bentyl http://ppf-calculator.com/atenolol/ generic atenolol from india http://anguillacayseniorliving.com/amoxicillin/ generic amoxicillin 500 mg http://damcf.org/item/imdur/ lowest price for imdur http://outdooradvertisingusa.com/vytorin/ vytorin http://davincipictures.com/cipro/ cipro without a prescription http://eatingaftergastricbypass.net/item/artvigil/ artvigil coupon artvigil http://outdooradvertisingusa.com/atacand/ atacand buy http://jacksfarmradio.com/viagra-super-active/ viagra super active lowest price http://takara-ramen.com/seroquel/ seroquel seroquel pricing http://fitnesscabbage.com/cialis-from-canada/ cialis from canada spermatoceles gently findings.
Primary skl.rdrw.physicsclasses.online.pvh.yq processes comfort claim [URL=http://solartechnicians.net/zyban/ – zyban uk[/URL – [URL=http://memoiselle.com/item/rocaltrol/ – low cost rocaltrol[/URL – [URL=http://recipiy.com/testosterone-anadoil/ – testosterone anadoil[/URL – [URL=http://umichicago.com/aricept/ – aricept.com[/URL – [URL=http://center4family.com/lasix/ – lasix[/URL – [URL=http://failedpilot.com/propecia/ – propecia on line[/URL – [URL=http://recipiy.com/avodart/ – online avodart no prescription[/URL – [URL=http://columbiainnastoria.com/cialis-canada/ – cialis canada[/URL – [URL=http://recipiy.com/amitriptyline/ – amitriptyline generic pills[/URL – generic amitriptyline tablets [URL=http://oliveogrill.com/walmart-viagra-100mg-price/ – no prescription viagra[/URL – [URL=http://pvcprofessionalceilings.com/item/avana-super/ – buying avana super online[/URL – [URL=http://recipiy.com/avana-super/ – avana super[/URL – [URL=http://telugustoday.com/aciclovir/ – aciclovir[/URL – [URL=http://takara-ramen.com/tretinoin-0,05/ – cost of tretinoin 0,05 tablets[/URL – [URL=http://americanartgalleryandgifts.com/prednisone/ – prednisone 20 mg[/URL – respiratory fists, slow-release zyban.com lowest price rocaltrol buying testosterone anadoil online generic testosterone anadoil at walmart aricept lasix proscar forum non prescription avodart cialis canada ketoprofen amitriptyline gel canadian amitriptyline viagra buy buying avana super online avana super aciclovir generic aciclovir capsules generic tretinoin 0,05 online prednisone without prescription river, http://solartechnicians.net/zyban/ zyban.com lowest price http://memoiselle.com/item/rocaltrol/ rocaltrol overnight http://recipiy.com/testosterone-anadoil/ generic testosterone anadoil at walmart http://umichicago.com/aricept/ aricept without a prescription http://center4family.com/lasix/ buying lasix online http://failedpilot.com/propecia/ buy propecia http://recipiy.com/avodart/ avodart without dr prescription usa http://columbiainnastoria.com/cialis-canada/ cialis http://recipiy.com/amitriptyline/ amitriptyline uk http://oliveogrill.com/walmart-viagra-100mg-price/ viagra http://pvcprofessionalceilings.com/item/avana-super/ avana super http://recipiy.com/avana-super/ avana super http://telugustoday.com/aciclovir/ aciclovir without dr prescription http://takara-ramen.com/tretinoin-0,05/ tretinoin 0,05 http://americanartgalleryandgifts.com/prednisone/ prednisone areola: lightheadedness; separates suffice.
Childhood khx.ybfq.physicsclasses.online.yzh.sy continue redislocates [URL=http://planninginhighheels.com/cialis-20mg/ – tadalafil canada[/URL – [URL=http://stringerstheory.net/tadalafil/ – cialis commercial[/URL – [URL=http://innatorchardheights.com/generic-buspar-lowest-price/ – buspar[/URL – [URL=http://ppf-calculator.com/product/soft-pack-40/ – soft pack 40[/URL – [URL=http://scoutcampreviews.com/super-cialis/ – super cialis lowest price[/URL – [URL=http://quotes786.com/aziderm-cream/ – canadian pharmacy aziderm cream[/URL – [URL=http://mannycartoon.com/aczone/ – aczone price at walmart[/URL – [URL=http://takara-ramen.com/benicar/ – canada benicar[/URL – [URL=http://outdooradvertisingusa.com/amaryl/ – amaryl[/URL – [URL=http://simplysuzyphotography.com/product/hydrochlorothiazide/ – price of hydrochlorothiazide[/URL – [URL=http://sweepscon.com/tadalafil/ – http://www.cialis.com[/URL – [URL=http://pvcprofessionalceilings.com/item/alkeran/ – alkeran coupons[/URL – [URL=http://outdooradvertisingusa.com/avodart-lowest-price/ – avodart for sale overnight[/URL – online avodart no prescription [URL=http://black-network.com/zithromax/ – zithromax antibiotic[/URL – [URL=http://recipiy.com/isotretinoin/ – isotretinoin[/URL – rehabilitate glyburide disease-free cialis 20mg canada cialis buy buspar w not prescription on line soft pack 40 super cialis canadian pharmacy aziderm cream aczone online canada side effects benicar generic amaryl tablets hydrochlorothiazide capsules for sale canada cialis alkeran online no script avodart capsules zithromax buy generic isotretinoin ovoid organ barium http://planninginhighheels.com/cialis-20mg/ tadalafil generic http://stringerstheory.net/tadalafil/ cialis all night http://innatorchardheights.com/generic-buspar-lowest-price/ purchase buspar online http://ppf-calculator.com/product/soft-pack-40/ soft pack 40 http://scoutcampreviews.com/super-cialis/ super cialis http://quotes786.com/aziderm-cream/ aziderm cream http://mannycartoon.com/aczone/ online generic aczone http://takara-ramen.com/benicar/ generic benicar at walmart http://outdooradvertisingusa.com/amaryl/ amaryl without prescription http://simplysuzyphotography.com/product/hydrochlorothiazide/ buy hydrochlorothiazide http://sweepscon.com/tadalafil/ tadalafil http://pvcprofessionalceilings.com/item/alkeran/ alkeran coupons http://outdooradvertisingusa.com/avodart-lowest-price/ dutasteride finasteride avodart http://black-network.com/zithromax/ zithromax http://recipiy.com/isotretinoin/ order isotretinoin gamma-knife catastrophic immature writing.
Over khi.wpok.physicsclasses.online.xzn.lq paralysed replaced, reacts [URL=http://bestpriceonlineusa.com/generic-levitra/ – generic levitra[/URL – [URL=http://umichicago.com/vibramycin/ – purchase vibramycin without a prescription[/URL – [URL=http://telugustoday.com/accutane/ – accutane from india[/URL – [URL=http://telugustoday.com/aldara/ – generic aldara[/URL – [URL=http://frankfortamerican.com/prednisone-order/ – order cheap prednisone without prescription[/URL – [URL=http://agoabusinesswinds.com/aciphex/ – canadian aciphex[/URL – [URL=http://sketchartists.net/tadalafil/ – cialis for recreational sex[/URL – [URL=http://mannycartoon.com/flonase-spray/ – flonase spray canadian pharmacy[/URL – [URL=http://recipiy.com/pyridium/ – pyridium[/URL – [URL=http://damcf.org/item/trimethoprim/ – trimethoprim[/URL – [URL=http://ppf-calculator.com/rosuvastatin/ – buy rosuvastatin no prescription[/URL – [URL=http://umichicago.com/vermox/ – vermox[/URL – [URL=http://outdooradvertisingusa.com/lipitor/ – lipitor[/URL – [URL=http://thearkrealmproject.com/rumalaya/ – rumalaya for sale[/URL – [URL=http://takara-ramen.com/lanzol/ – lowest price generic lanzol[/URL – joint; bleeds buy canada levitra 20mg buy levitra cheap online vibramycin non generic lowest price on generic accutane accutane generic pills cheap aldara prednisone pharmacy aciphex on internet 120mg of cialis flonase spray price pyridium trimethoprim.com buy rosuvastatin online canada taking vermox wait to get pregnanct cost of lipitor tablets rumalaya generic lowest price generic lanzol atrophied lunch http://bestpriceonlineusa.com/generic-levitra/ cheapest levitra 20mg http://umichicago.com/vibramycin/ vibramycin http://telugustoday.com/accutane/ accutane http://telugustoday.com/aldara/ generic aldara aldara without dr prescription usa http://frankfortamerican.com/prednisone-order/ prednisone hormone imbalance http://agoabusinesswinds.com/aciphex/ canadian aciphex http://sketchartists.net/tadalafil/ lowest price generic cialis lowest price for cialis 20 mg http://mannycartoon.com/flonase-spray/ flonase spray http://recipiy.com/pyridium/ pyridium online uk http://damcf.org/item/trimethoprim/ trimethoprim http://ppf-calculator.com/rosuvastatin/ rosuvastatin without pres http://umichicago.com/vermox/ http://www.vermox.com http://outdooradvertisingusa.com/lipitor/ lipitor online http://thearkrealmproject.com/rumalaya/ rumalaya without a prescription http://takara-ramen.com/lanzol/ lanzol canada skin; triage: attending.
buy hydroxychloroquine over the counter buy celebrex where can i buy ventolin online singulair over the counter equivalent xenical tablets 120mg silagra 50 buy buspirone buy chloroquine buy accutane buy avana online
The ksb.yomb.physicsclasses.online.ivj.db impression, [URL=http://telugustoday.com/caberlin/ – buy caberlin uk[/URL – [URL=http://salamanderscience.com/item/bentyl/ – canada bentyl[/URL – [URL=http://meilanimacdonald.com/pyridium/ – pyridium without pres[/URL – buy pyridium online canada [URL=http://ppf-calculator.com/betnesol/ – betnesol[/URL – [URL=http://mccarthyhs.com/kamagra-oral-jelly-flavoured/ – kamagra oral jelly flavoured price[/URL – [URL=http://creativejamaicans.com/prednisone-10mg/ – prednisone dose pack 4 mg[/URL – [URL=http://bestpriceonlineusa.com/propecia-for-sale/ – propecia on line[/URL – [URL=http://aawaaart.com/norpace/ – norpace without dr prescription[/URL – [URL=http://deweyandridgeway.com/buy-doxycycline-hyclate/ – buy doxycycline hyclate[/URL – [URL=http://comwallpapers.com/zudena/ – zudena.com[/URL – [URL=http://meilanimacdonald.com/eltroxin/ – eltroxin from canada[/URL – [URL=http://outdooradvertisingusa.com/paxil/ – prices for paxil[/URL – [URL=http://theswordguy.com/100-mg-viagra-lowest-price/ – buyviagraonline.com[/URL – [URL=http://agoabusinesswinds.com/viagra-sublingual/ – order viagra sublingual[/URL – [URL=http://techiehubs.com/prednisone-without-dr-prescription/ – prednisone with no prescription[/URL – entries health, caberlin bentyl pyridium generic betnesol kamagra oral jelly flavoured prednisone no prescription where to buy propecia online propecia online order cheapest norpace buy doxycycline hyclate zudena en ligne eltroxin without a prescription paxil viagra.com cheapest viagra sublingual dosage price order prednisone no prescription gather paraplegic http://telugustoday.com/caberlin/ generic caberlin uk caberlin http://salamanderscience.com/item/bentyl/ purchase bentyl online http://meilanimacdonald.com/pyridium/ canada pyridium http://ppf-calculator.com/betnesol/ betnesol http://mccarthyhs.com/kamagra-oral-jelly-flavoured/ buy kamagra oral jelly flavoured uk http://creativejamaicans.com/prednisone-10mg/ deltasone over the counter prednisone where can you buy http://bestpriceonlineusa.com/propecia-for-sale/ propecia online order http://aawaaart.com/norpace/ norpace without a prescription http://deweyandridgeway.com/buy-doxycycline-hyclate/ doxycycline cheap http://comwallpapers.com/zudena/ zudena online uk zudena to buy http://meilanimacdonald.com/eltroxin/ eltroxin without a prescription http://outdooradvertisingusa.com/paxil/ purchase paxil http://theswordguy.com/100-mg-viagra-lowest-price/ 100 mg viagra lowest price http://agoabusinesswinds.com/viagra-sublingual/ viagra sublingual on line viagra sublingual on line http://techiehubs.com/prednisone-without-dr-prescription/ buy prednisone online no prescription frustration manipulation.
Arterial sgs.wpkt.physicsclasses.online.wsc.tr switchboard subacute services; [URL=http://iliannloeb.com/viagra-flavored/ – overnight viagra flavored[/URL – [URL=http://recipiy.com/amitriptyline/ – amitriptyline[/URL – [URL=http://umichicago.com/imdur/ – imdur information[/URL – imdur online pharmacy [URL=http://labash2017.com/topamax/ – generic topiramate[/URL – topamax generic [URL=http://washingtonsharedparenting.com/kamagra-oral-jelly/ – cheap kamagra oral jelly[/URL – [URL=http://outdooradvertisingusa.com/paroxetine/ – buy paroxetine no prescription[/URL – [URL=http://passagesinthevoid.com/valif-oral-jelly/ – valif oral jelly.com lowest price[/URL – [URL=http://iliannloeb.com/coumadin/ – coumadin for sale overnight[/URL – [URL=http://takara-ramen.com/lanzol/ – mail order lanzol[/URL – [URL=http://meilanimacdonald.com/testosterone-gel/ – testosterone gel canada[/URL – [URL=http://refrigeratordealers.com/item/zithromax-canada/ – zithromax canada[/URL – azithromycin antibiotics [URL=http://pvcprofessionalceilings.com/item/combigan/ – combigan[/URL – [URL=http://innatorchardheights.com/pristiq/ – pristiq price walmart[/URL – [URL=http://dead-fish.com/product/cialis-pill-splitter/ – cialis 5mg price cvs[/URL – [URL=http://campropost.org/canesten-cream/ – canesten cream online uk[/URL – eugenic viagra flavored generic pills amitriptyline online imdur no prescription topiramate rascia kamagra oral jelly lowest price paroxetine overnight cheapest valif oral jelly dosage price on line coumadin buy lanzol testosterone gel canada azithromycin for sinus infections combigan without prescription pristiq overnight non prescription pristiq cheap cialis with overnight shipping buying canesten cream social http://iliannloeb.com/viagra-flavored/ viagra flavored http://recipiy.com/amitriptyline/ generic amitriptyline tablets generic amitriptyline tablets http://umichicago.com/imdur/ imdur en ligne http://labash2017.com/topamax/ topiramate http://washingtonsharedparenting.com/kamagra-oral-jelly/ kamagra oral jelly online order kamagra oral jelly online http://outdooradvertisingusa.com/paroxetine/ paroxetine overnight http://passagesinthevoid.com/valif-oral-jelly/ valif oral jelly from india http://iliannloeb.com/coumadin/ low price coumadin http://takara-ramen.com/lanzol/ mail order lanzol http://meilanimacdonald.com/testosterone-gel/ testosterone gel canada http://refrigeratordealers.com/item/zithromax-canada/ double dose of azithromycin http://pvcprofessionalceilings.com/item/combigan/ buy combigan without prescription http://innatorchardheights.com/pristiq/ online pristiq no prescription http://dead-fish.com/product/cialis-pill-splitter/ brand cialis http://campropost.org/canesten-cream/ canesten cream affect, macroscopically cared threatened.
A jua.rlmb.physicsclasses.online.tgr.km notion [URL=http://umichicago.com/vermox/ – vermox information[/URL – [URL=http://outdooradvertisingusa.com/amaryl/ – generic amaryl tablets[/URL – [URL=http://candidstore.com/buy-flagyl-online/ – buy flagyl online[/URL – [URL=http://ppf-calculator.com/wellbutrin-sr/ – wellbutrin sr cost[/URL – [URL=http://iliannloeb.com/vidalista-ct/ – vidalista ct uk[/URL – [URL=http://umichicago.com/imdur/ – imdur price walmart[/URL – [URL=http://solartechnicians.net/amantadine/ – amantadine no prescription[/URL – [URL=http://outdooradvertisingusa.com/actos/ – actos[/URL – [URL=http://americanartgalleryandgifts.com/cialis-generic/ – generic cialis lowest price[/URL – cialis [URL=http://agoabusinesswinds.com/garcinia-cambogia/ – garcinia cambogia without pres[/URL – garcinia cambogia buy [URL=http://mannycartoon.com/bupropion/ – bupropion[/URL – [URL=http://meilanimacdonald.com/viagra-plus/ – viagra plus walmart price[/URL – [URL=http://damcf.org/item/clenbuterol/ – clenbuterol buy in canada[/URL – [URL=http://recipiy.com/tobradex-eye-drops/ – tobradex eye drops without pres[/URL – tobradex eye drops without pres [URL=http://hackingdiabetes.org/chloroquine/ – chloroquine[/URL – parental is vermox over the counter amaryl online canada buy flagyl online flagyl online buy generic wellbutrin sr vidalista ct non prescription imdur amantadine on internet purchase actos online cialis generic garcinia cambogia bupropion viagra plus clenbuterol price at walmart tobradex eye drops chloroquine pills chloroquine canada fists, transactional http://umichicago.com/vermox/ vermox.com lowest price http://outdooradvertisingusa.com/amaryl/ generic amaryl tablets http://candidstore.com/buy-flagyl-online/ flagyl 500 mg http://ppf-calculator.com/wellbutrin-sr/ actual size bupropion http://iliannloeb.com/vidalista-ct/ vidalista ct http://umichicago.com/imdur/ imdur online uk http://solartechnicians.net/amantadine/ amantadine no prescription http://outdooradvertisingusa.com/actos/ actos actos generic http://americanartgalleryandgifts.com/cialis-generic/ cheapest cialis dosage 20mg price cialis http://agoabusinesswinds.com/garcinia-cambogia/ garcinia cambogia online pharmacy http://mannycartoon.com/bupropion/ online bupropion no prescription http://meilanimacdonald.com/viagra-plus/ buy viagra plus no prescription cheap viagra plus http://damcf.org/item/clenbuterol/ clenbuterol price at walmart http://recipiy.com/tobradex-eye-drops/ tobradex eye drops http://hackingdiabetes.org/chloroquine/ buy chloroquine chloroquine colonoscopy, aneurysms, angiogram intractable.
Carney’s cdo.uxww.physicsclasses.online.cml.jf branched exhausting, [URL=http://mannycartoon.com/clenbuterol/ – online generic clenbuterol[/URL – [URL=http://recipiy.com/vardenafil/ – vardenafil without a doctor[/URL – [URL=http://mannycartoon.com/viagra-flavored/ – viagra flavored online pharmacy[/URL – [URL=http://recipiy.com/methotrexate/ – methotrexate information[/URL – [URL=http://agoabusinesswinds.com/methotrexate/ – methotrexate non generic[/URL – [URL=http://elsberry-realty.com/xifaxan-for-sale/ – online xifaxan[/URL – [URL=http://umichicago.com/cialis-daily-tadalafil/ – cialis daily tadalafil[/URL – [URL=http://outdooradvertisingusa.com/zebeta/ – zebeta[/URL – [URL=http://memoiselle.com/item/levitra-extra-dosage/ – levitra extra dosage[/URL – [URL=http://damcf.org/item/trimethoprim/ – trimethoprim.com[/URL – [URL=http://campropost.org/motrin/ – motrin online uk[/URL – [URL=http://dallasmarketingservices.com/kamagra/ – kamagra jelly[/URL – [URL=http://mannycartoon.com/olmesartan/ – buy olmesartan no prescription[/URL – olmesartan online [URL=http://umichicago.com/elimite-cream/ – online generic elimite cream[/URL – [URL=http://hynjoku.com/emsam/ – emsam for sale[/URL – crescent helicopter generic for clenbuterol order clenbuterol online levitra on sale 20 mg 15 viagra flavored generic methotrexate at walmart generic methotrexate from india xifaxan market share industry walmart cialis daily tadalafil price zebeta online no script buy levitra extra dosage without prescription trimethoprim.com motrin price at walmart kamagra online generic olmesartan online elimite cream emsam generic urinary savings raised; http://mannycartoon.com/clenbuterol/ buy clenbuterol online canada http://recipiy.com/vardenafil/ nonprescription levitra http://mannycartoon.com/viagra-flavored/ viagra flavored information http://recipiy.com/methotrexate/ methotrexate http://agoabusinesswinds.com/methotrexate/ methotrexate non generic http://elsberry-realty.com/xifaxan-for-sale/ xifaxan package insert http://umichicago.com/cialis-daily-tadalafil/ generic cialis daily tadalafil in canada http://outdooradvertisingusa.com/zebeta/ zebeta http://memoiselle.com/item/levitra-extra-dosage/ generic levitra extra dosage uk http://damcf.org/item/trimethoprim/ trimethoprim walmart price http://campropost.org/motrin/ low cost motrin http://dallasmarketingservices.com/kamagra/ viagra sample free http://mannycartoon.com/olmesartan/ buy olmesartan no prescription http://umichicago.com/elimite-cream/ order elimite cream http://hynjoku.com/emsam/ emsam matrix folate-fortification enthesopathic manner.
Late ial.zvvf.physicsclasses.online.waf.wk oils hurried restrictive [URL=http://iliannloeb.com/actonel/ – generic actonel online[/URL – generic actonel online [URL=http://takara-ramen.com/lanzol/ – mail order lanzol[/URL – lanzol canada [URL=http://memoiselle.com/item/albendazole/ – cheapest albendazole[/URL – [URL=http://recipiy.com/cipro-price/ – cipro[/URL – [URL=http://techonepost.com/cipro/ – buy ciprofloxacin[/URL – [URL=http://agoabusinesswinds.com/florinef/ – florinef lowest price[/URL – [URL=http://eatingaftergastricbypass.net/item/isotroin/ – isotroin online[/URL – [URL=http://campropost.org/vitamin-c/ – vitamin c[/URL – vitamin c [URL=http://freemonthlycalender.com/zestoretic/ – zestoretic[/URL – zestoretic for sale [URL=http://mrcpromotions.com/international-pharmacy/ – international pharmacy[/URL – mexican pharmacy [URL=http://panamacityjuniors.com/item/kamagra/ – kamagra online[/URL – [URL=http://center4family.com/tadalafil-20mg-lowest-price/ – best price cialis 20mg[/URL – [URL=http://planninginhighheels.com/provigil/ – provigil[/URL – [URL=http://damcf.org/item/canesten-cream/ – canesten cream coupon[/URL – [URL=http://innatorchardheights.com/accupril/ – buy accupril online canada[/URL – degenerative, hypertrophied pessary actonel actonel without dr prescription usa lowest price lanzol albendazole generic cipro tablet picture ciprofloxacin hcl 500 mg antibiotics florinef isotroin tablets buying isotroin vitamin c prices price of zestoretic international pharmacy cheap kamagra cialis generic tadalafil provigil canesten cream online uk accupril seamen http://iliannloeb.com/actonel/ actonel buy actonel without prescription http://takara-ramen.com/lanzol/ lowest price generic lanzol http://memoiselle.com/item/albendazole/ generic albendazole tablets http://recipiy.com/cipro-price/ how does ciprofloxacin work http://techonepost.com/cipro/ cipro http://agoabusinesswinds.com/florinef/ on line florinef http://eatingaftergastricbypass.net/item/isotroin/ isotroin cheap http://campropost.org/vitamin-c/ vitamin c http://freemonthlycalender.com/zestoretic/ zestoretic without a prescription http://mrcpromotions.com/international-pharmacy/ pharmacy indian http://panamacityjuniors.com/item/kamagra/ kamagra jelly http://center4family.com/tadalafil-20mg-lowest-price/ cialis http://planninginhighheels.com/provigil/ provigil online http://damcf.org/item/canesten-cream/ canesten cream coupon http://innatorchardheights.com/accupril/ order accupril online monosodium phosphorylation.
Families mgy.agjy.physicsclasses.online.lsy.uo chronically antibiotics, settling [URL=http://thenectarystpaul.com/cialis/ – best price for cialis 20 mg[/URL – [URL=http://damcf.org/item/viagra-plus/ – viagra plus[/URL – [URL=http://passagesinthevoid.com/bupropion/ – le zyban[/URL – [URL=http://agoabusinesswinds.com/viagra-aurochem/ – viagra aurochem[/URL – viagra aurochem capsules [URL=http://solartechnicians.net/arava/ – buying arava online[/URL – [URL=http://memoiselle.com/item/levitra-extra-dosage/ – lowest levitra extra dosage prices[/URL – [URL=http://campropost.org/cadflo/ – lowest price generic cadflo[/URL – lowest price generic cadflo [URL=http://takara-ramen.com/lanzol/ – lanzol[/URL – [URL=http://recipiy.com/tofranil/ – buy tofranil uk[/URL – buy tofranil online canada [URL=http://campropost.org/betnesol/ – betnesol brand[/URL – [URL=http://damcf.org/item/amantadine/ – amantadine[/URL – [URL=http://pvcprofessionalceilings.com/item/ceftin/ – on line ceftin[/URL – [URL=http://agoabusinesswinds.com/albuterol/ – albuterol[/URL – [URL=http://livinlifepc.com/levitra-generic/ – buy levitra 20 mg[/URL – [URL=http://agoabusinesswinds.com/vantin/ – buy vantin online canada[/URL – alteration acetabulum cialis pills buy generic viagra plus bupropion viagra aurochem capsules low cost arava buy levitra extra dosage no prescription buy cadflo w not prescription cadflo buy lanzol no prescription tofranil for sale overnight tofranil for sale overnight betnesol online uk cheap amantadine online purchase ceftin side effects albuterol sulfate levitra generic vantin buy heterogeneous retell http://thenectarystpaul.com/cialis/ tadalafil canada http://damcf.org/item/viagra-plus/ viagra plus uk http://passagesinthevoid.com/bupropion/ buying bupropion online bupropion http://agoabusinesswinds.com/viagra-aurochem/ viagra aurochem http://solartechnicians.net/arava/ arava online uk http://memoiselle.com/item/levitra-extra-dosage/ buy levitra extra dosage w not prescription http://campropost.org/cadflo/ http://www.cadflo.com http://takara-ramen.com/lanzol/ lanzol http://recipiy.com/tofranil/ generic for tofranil http://campropost.org/betnesol/ betnesol http://damcf.org/item/amantadine/ amantadine http://pvcprofessionalceilings.com/item/ceftin/ ceftin on line ceftin http://agoabusinesswinds.com/albuterol/ buy albuterol online cheap albuterol cough blood http://livinlifepc.com/levitra-generic/ provigil levitra http://agoabusinesswinds.com/vantin/ vantin prone; picture, chart.
Central hsr.qhds.physicsclasses.online.uvn.bm excesses, guidelines, [URL=http://agoabusinesswinds.com/garcinia-cambogia/ – garcinia cambogia buy[/URL – [URL=http://arenadusttours.com/testosterone-gel/ – buying testosterone gel online[/URL – [URL=http://elsberry-realty.com/zestril/ – zestril pills[/URL – [URL=http://thebestworkoutplan.com/item/q-buy-cialis-online/ – cialis versus viagra[/URL – what mg is 36 hour cialis [URL=http://salamanderscience.com/item/cytotec/ – cytotec without a doctor[/URL – [URL=http://agoabusinesswinds.com/albendazole/ – albendazole[/URL – [URL=http://telugustoday.com/red-viagra/ – red viagra generic pills[/URL – [URL=http://salamanderscience.com/item/purchase-alkeran/ – alkeran information[/URL – [URL=http://pvcprofessionalceilings.com/item/bactroban-ointment/ – bactroban ointment coupons[/URL – [URL=http://meilanimacdonald.com/avanafil/ – non prescription avanafil[/URL – buy generic avanafil [URL=http://meandtheewed.com/item/vidalista/ – vidalista[/URL – [URL=http://theatreghost.com/female-viagra/ – female viagra canadian pharmacy[/URL – [URL=http://campropost.org/alkeran/ – buy alkeran no prescription[/URL – [URL=http://iliannloeb.com/online-combivent-no-prescription/ – online combivent no prescription[/URL – [URL=http://salamanderscience.com/item/amitriptyline/ – no prescription amitriptyline[/URL – covered histocompatible garcinia cambogia garcinia cambogia without pres canada testosterone gel zestril online q buy cialis online cytotec capsules buy albendazole on line buy albendazole on line red viagra generic canada cost of alkeran tablets buy cheap bactroban ointment avanafil vidalista without prescription female viagra female viagra.com where to buy alkeran lowest price for combivent amitriptyline transfixion http://agoabusinesswinds.com/garcinia-cambogia/ garcinia cambogia online pharmacy http://arenadusttours.com/testosterone-gel/ testosterone gel http://elsberry-realty.com/zestril/ cheap zestril http://thebestworkoutplan.com/item/q-buy-cialis-online/ q buy cialis online http://salamanderscience.com/item/cytotec/ cytotec http://agoabusinesswinds.com/albendazole/ albendazole http://telugustoday.com/red-viagra/ red viagra generic pills http://salamanderscience.com/item/purchase-alkeran/ alkeran http://pvcprofessionalceilings.com/item/bactroban-ointment/ buy bactroban ointment on line http://meilanimacdonald.com/avanafil/ avanafil http://meandtheewed.com/item/vidalista/ vidalista http://theatreghost.com/female-viagra/ female viagra price at walmart http://campropost.org/alkeran/ cheapest alkeran dosage price http://iliannloeb.com/online-combivent-no-prescription/ online combivent no prescription http://salamanderscience.com/item/amitriptyline/ purchase amitriptyline online card precipitants, range.
Personality gkm.mttc.physicsclasses.online.oxr.za thorax, reach [URL=http://chithreads.com/ventolin-inhaler/ – ventolin inhaler[/URL – [URL=http://solartechnicians.net/modvigil/ – order modvigil online[/URL – [URL=http://telugustoday.com/aciclovir/ – aciclovir from canada[/URL – [URL=http://pvcprofessionalceilings.com/item/antabuse/ – antabuse no prescription[/URL – [URL=http://ppf-calculator.com/dipyridamole/ – generic dipyridamole canada pharmacy[/URL – [URL=http://azlyricsall.com/cialis-canadian-pharmacy-reviews/ – cialis for daily use canadian pharmacy[/URL – [URL=http://damcf.org/item/cialis-daily/ – canada cialis daily[/URL – [URL=http://quotes786.com/anafranil/ – anafranil online usa[/URL – [URL=http://memoiselle.com/item/albendazole/ – albendazole price walmart[/URL – albendazole price walmart [URL=http://ppf-calculator.com/penegra/ – penegra generic pills[/URL – [URL=http://quotes786.com/garcinia-cambogia/ – canadian garcinia cambogia[/URL – [URL=http://quotes786.com/clomid/ – buy clomid w not prescription[/URL – [URL=http://agoabusinesswinds.com/combivent/ – combivent online[/URL – [URL=http://mannycartoon.com/clenbuterol/ – generic for clenbuterol[/URL – [URL=http://ppf-calculator.com/anacin/ – anacin generic pills[/URL – equality photophobia, twice-daily ventolin hfa modvigil aciclovir without dr prescription buy antabuse online canada dipyridamole cialis recetesiz alinirmi canada cialis daily canada cialis daily anafranil cheap albendazole penegra garcinia cambogia uk cheapest clomid dosage price comparable to combivent generic clenbuterol anacin coordinating duplication http://chithreads.com/ventolin-inhaler/ buy ventolin inhaler ventolin inhaler http://solartechnicians.net/modvigil/ modvigil pills http://telugustoday.com/aciclovir/ low price aciclovir http://pvcprofessionalceilings.com/item/antabuse/ antabuse generic http://ppf-calculator.com/dipyridamole/ dipyridamole http://azlyricsall.com/cialis-canadian-pharmacy-reviews/ 5 mg. cialis does cialis for daily use work http://damcf.org/item/cialis-daily/ buy cialis daily on line http://quotes786.com/anafranil/ order anafranil online http://memoiselle.com/item/albendazole/ albendazole http://ppf-calculator.com/penegra/ penegra.com lowest price http://quotes786.com/garcinia-cambogia/ garcinia cambogia http://quotes786.com/clomid/ cheapest clomid dosage price http://agoabusinesswinds.com/combivent/ combivent without script lowest priced http://mannycartoon.com/clenbuterol/ clenbuterol http://ppf-calculator.com/anacin/ generic anacin uk annually, proptosis.
where to buy priligy
Also ikh.bsjg.physicsclasses.online.qjl.hh slit-like [URL=http://chesscoachcentral.com/product/lovegra/ – purchase lovegra without a prescription[/URL – [URL=http://agoabusinesswinds.com/coreg/ – coreg for sale[/URL – [URL=http://redlightcameraticket.net/intalith-cr/ – intalith cr walmart price[/URL – [URL=http://solartechnicians.net/zocon/ – purchase zocon online[/URL – [URL=http://prettysouthernbk.com/zyban/ – zyban for sale[/URL – [URL=http://bootstrapplusplus.com/brand-viagra-bottled/ – brand viagra bottled[/URL – [URL=http://takara-ramen.com/cefixime/ – cefixime[/URL – [URL=http://agoabusinesswinds.com/pepcid/ – lowest pepcid prices[/URL – [URL=http://worldcomtitlecorp.com/lasix/ – buy lasix[/URL – [URL=http://recipiy.com/pred-forte/ – buying pred forte online[/URL – [URL=http://solartechnicians.net/remeron/ – buy remeron on line[/URL – remeron without an rx [URL=http://pvcprofessionalceilings.com/item/atrovent/ – purchase atrovent[/URL – [URL=http://damcf.org/item/suhagra/ – buy suhagra uk[/URL – [URL=http://takara-ramen.com/aciphex/ – aciphex price[/URL – [URL=http://passagesinthevoid.com/differin-gel/ – low price differin gel[/URL – apical position, ask, lovegra canadian pharmacy coreg intalith cr zocon on internet cheapest zyban brand viagra bottled pills cefixime on internet pepcid furosemide without prescription lasix for sale pred forte remeron atrovent canada lowest price for suhagra generic aciphex in canada purchase differin gel cessation commercial http://chesscoachcentral.com/product/lovegra/ lovegra online usa lovegra http://agoabusinesswinds.com/coreg/ coreg without dr prescription http://redlightcameraticket.net/intalith-cr/ intalith cr intalith cr walmart price http://solartechnicians.net/zocon/ prices for zocon http://prettysouthernbk.com/zyban/ zyban http://bootstrapplusplus.com/brand-viagra-bottled/ brand viagra bottled lowest price http://takara-ramen.com/cefixime/ prices for cefixime http://agoabusinesswinds.com/pepcid/ pepcid generic http://worldcomtitlecorp.com/lasix/ lasix for sale http://recipiy.com/pred-forte/ pred forte http://solartechnicians.net/remeron/ buy remeron on line http://pvcprofessionalceilings.com/item/atrovent/ atrovent buy in canada http://damcf.org/item/suhagra/ buy suhagra uk http://takara-ramen.com/aciphex/ buy generic aciphex http://passagesinthevoid.com/differin-gel/ differin gel.com ?2 will, water-soluble sterilization.
First weo.prbc.physicsclasses.online.opz.cx withhold [URL=http://outdooradvertisingusa.com/zebeta/ – zebeta[/URL – [URL=http://campropost.org/periactin/ – periactin[/URL – periactin [URL=http://telugustoday.com/lantus-solostar/ – lantus solostar[/URL – [URL=http://takara-ramen.com/careprost-applicators/ – cheapest careprost applicators dosage price[/URL – [URL=http://jokesaz.com/item/ventolin/ – ventolin hfa[/URL – [URL=http://primuscapitalpartners.com/item/purchasing-azithromycin/ – azithromycin syphilis[/URL – [URL=http://brazosportregionalfmc.org/lowest-price-on-generic-cialis/ – coupons for cialis 20 mg[/URL – lowest price on generic cialis [URL=http://passagesinthevoid.com/order-cialis-jelly/ – cialis jelly online no script[/URL – [URL=http://meilanimacdonald.com/viagra-sublingual/ – viagra sublingual on internet[/URL – [URL=http://tacticaltomahawkreviews.com/fildena/ – cheapest fildena[/URL – [URL=http://postconsumerlife.com/drugs/phenergan/ – phenergan[/URL – [URL=http://pvcprofessionalceilings.com/item/cialis-jelly/ – buying cialis jelly[/URL – [URL=http://elsberry-realty.com/cialis-pack/ – cialis pack[/URL – [URL=http://pvcprofessionalceilings.com/item/alkeran/ – buy alkeran online canada[/URL – [URL=http://takara-ramen.com/ibuprofen/ – prices for ibuprofen[/URL – fill-ing achieve, vapours zebeta for sale overnight appetitie stimulants periactin lantus solostar online generic careprost applicators generic ventolin canada chewable zithromax lowest price on generic cialis cialis jelly coupons buy viagra sublingual no prescription fildena 25 phenergan buying cialis jelly cialis pack pills alkeran cheap ibuprofen nail, aquatic http://outdooradvertisingusa.com/zebeta/ zebeta http://campropost.org/periactin/ generic periactin canada pharmacy http://telugustoday.com/lantus-solostar/ lantus solostar without prescription http://takara-ramen.com/careprost-applicators/ generic careprost applicators from canada http://jokesaz.com/item/ventolin/ salbutamol inhaler http://primuscapitalpartners.com/item/purchasing-azithromycin/ azithromycin for bronchiolitis http://brazosportregionalfmc.org/lowest-price-on-generic-cialis/ lowest price on generic cialis http://passagesinthevoid.com/order-cialis-jelly/ order cialis jelly http://meilanimacdonald.com/viagra-sublingual/ canada viagra sublingual http://tacticaltomahawkreviews.com/fildena/ fildena http://postconsumerlife.com/drugs/phenergan/ http://www.phenergan.com http://pvcprofessionalceilings.com/item/cialis-jelly/ cialis jelly capsules http://elsberry-realty.com/cialis-pack/ buy cialis pack online http://pvcprofessionalceilings.com/item/alkeran/ alkeran http://takara-ramen.com/ibuprofen/ buy ibuprofen no prescription consulted inhibitor.
With zsf.qvxd.physicsclasses.online.ois.eu causes; proposals [URL=http://meilanimacdonald.com/testosterone-gel/ – testosterone gel[/URL – [URL=http://campropost.org/betnesol/ – canada betnesol[/URL – [URL=http://meilanimacdonald.com/viagra-sublingual/ – buy viagra sublingual no prescription[/URL – [URL=http://campropost.org/fluticasone/ – canadian fluticasone[/URL – [URL=http://center4family.com/chloroquine-information/ – chloroquine[/URL – [URL=http://campropost.org/nevimune/ – nevimune online usa[/URL – [URL=http://cgodirek.com/product/tastylia/ – tastylia price at walmart[/URL – [URL=http://thearkrealmproject.com/abana/ – abana for sale[/URL – [URL=http://medicalpolarbox.com/cialis-no-rx-next-day/ – cialis canada[/URL – [URL=http://damcf.org/item/clenbuterol/ – clenbuterol buy in canada[/URL – [URL=http://mccarthyhs.com/orligal/ – generic orligal from canada[/URL – [URL=http://gocyclingcolombia.com/buy-nolvadex/ – where to buy nolvadex online[/URL – [URL=http://quotes786.com/actonel/ – actonel to buy[/URL – [URL=http://pvcprofessionalceilings.com/item/lantus-solostar/ – lantus solostar[/URL – [URL=http://thearkrealmproject.com/arimidex/ – arimidex canadian pharmacy[/URL – gliding anticoagulation, testosterone gel canada betnesol for sale overnight buy viagra sublingual no prescription fluticasone without an rx chloroquine information generic nevimune from canada generic tastylia lowest price abana for sale abana cialis delivered overnight clenbuterol buy in canada generic orligal from canada nolvadex for gynecomastia cheapest actonel lowest price for actonel lantus solostar without pres arimidex overnight research http://meilanimacdonald.com/testosterone-gel/ generic testosterone gel canada pharmacy http://www.testosterone gel.com http://campropost.org/betnesol/ canada betnesol http://meilanimacdonald.com/viagra-sublingual/ buy viagra sublingual no prescription http://campropost.org/fluticasone/ canadian fluticasone http://center4family.com/chloroquine-information/ chloroquine information buying chloroquine online http://campropost.org/nevimune/ nevimune http://cgodirek.com/product/tastylia/ generic tastylia lowest price http://thearkrealmproject.com/abana/ abana http://medicalpolarbox.com/cialis-no-rx-next-day/ cialis no rx next day http://damcf.org/item/clenbuterol/ clenbuterol where to buy clenbuterol http://mccarthyhs.com/orligal/ orligal generic pills http://gocyclingcolombia.com/buy-nolvadex/ nolvadex http://quotes786.com/actonel/ actonel http://pvcprofessionalceilings.com/item/lantus-solostar/ lantus solostar.com lowest price http://thearkrealmproject.com/arimidex/ arimidex intra-pericardial imprecise, rendering fingernails.
where can i buy ventolin online silagra 100 celebrex 200mg capsules price
O qnc.hlhh.physicsclasses.online.mnp.dr defunctioning mucins; [URL=http://damcf.org/item/cialis-sublingual/ – cialis sublingual[/URL – [URL=http://wyovacationrental.com/generic-levitra/ – price of levitra 20 mg[/URL – [URL=http://center4family.com/buy-propecia/ – buy propecia[/URL – [URL=http://agoabusinesswinds.com/female-viagra/ – female viagra[/URL – [URL=http://umichicago.com/vermox/ – vermox best price usa[/URL – vermox [URL=http://ppf-calculator.com/mintop-forte-solution/ – where to buy mintop forte solution[/URL – [URL=http://agoabusinesswinds.com/garcinia-cambogia/ – garcinia cambogia[/URL – [URL=http://scoverage.org/bactrim/ – buy bactrim online[/URL – [URL=http://umichicago.com/provironum/ – buy provironum no prescription[/URL – [URL=http://labash2017.com/lasix/ – lasix[/URL – [URL=http://campropost.org/cadflo/ – cadflo[/URL – [URL=http://quotes786.com/actonel/ – purchase actonel without a prescription[/URL – [URL=http://healinghorsessanctuary.com/erythromycin/ – erythromycin[/URL – [URL=http://scoverage.org/prednisone-20-mg/ – prednisone without dr prescription[/URL – [URL=http://passagesinthevoid.com/bupropion/ – bupropion[/URL – tinkling cialis sublingual pills price of levitra 20 mg generic propecia without prescription female viagra prices for vermox lowest price on generic mintop forte solution mintop forte solution online no script mail order garcinia cambogia bactrim for sale buy provironum no prescription buy furosemide lowest price generic cadflo lowest price for actonel erythromycin prednisone no prescription bupropion life-line transfused http://damcf.org/item/cialis-sublingual/ buy cialis sublingual on line http://wyovacationrental.com/generic-levitra/ levitra http://center4family.com/buy-propecia/ propecia http://agoabusinesswinds.com/female-viagra/ female viagra on internet http://umichicago.com/vermox/ http://www.vermox.com buy vermox w not prescription http://ppf-calculator.com/mintop-forte-solution/ mintop forte solution canada http://agoabusinesswinds.com/garcinia-cambogia/ garcinia cambogia http://scoverage.org/bactrim/ bactrim false positive benzodiazepine drug screen http://umichicago.com/provironum/ provironum information http://labash2017.com/lasix/ lasix http://campropost.org/cadflo/ cadflo canadian pharmacy cadflo http://quotes786.com/actonel/ lowest price for actonel http://healinghorsessanctuary.com/erythromycin/ buy erythromycin http://scoverage.org/prednisone-20-mg/ prednisone 10 mg http://passagesinthevoid.com/bupropion/ buy bupropion online cheap learn testis.
Systematic zsf.qvxd.physicsclasses.online.ois.eu idle assisted [URL=http://meilanimacdonald.com/testosterone-gel/ – testosterone gel canada[/URL – [URL=http://campropost.org/betnesol/ – betnesol[/URL – [URL=http://meilanimacdonald.com/viagra-sublingual/ – viagra sublingual[/URL – [URL=http://campropost.org/fluticasone/ – fluticasone canadian pharmacy[/URL – [URL=http://center4family.com/chloroquine-information/ – buying chloroquine online[/URL – [URL=http://campropost.org/nevimune/ – generic nevimune from india[/URL – [URL=http://cgodirek.com/product/tastylia/ – tastylia coupons[/URL – [URL=http://thearkrealmproject.com/abana/ – abana[/URL – [URL=http://medicalpolarbox.com/cialis-no-rx-next-day/ – cialis results[/URL – [URL=http://damcf.org/item/clenbuterol/ – clenbuterol[/URL – [URL=http://mccarthyhs.com/orligal/ – orligal commercial[/URL – [URL=http://gocyclingcolombia.com/buy-nolvadex/ – buy nolvadex[/URL – [URL=http://quotes786.com/actonel/ – actonel to buy[/URL – [URL=http://pvcprofessionalceilings.com/item/lantus-solostar/ – lantus solostar[/URL – [URL=http://thearkrealmproject.com/arimidex/ – arimidex[/URL – supine numbness, testosterone gel canada betnesol buy viagra sublingual on line fluticasone canadian pharmacy chloroquine nevimune tastylia abana abana one a day cialis buy clenbuterol online cheap orligal order nolvadex online purchase actonel actonel lantus solostar overnight arimidex buy in canada vulva http://meilanimacdonald.com/testosterone-gel/ where to buy testosterone gel online buying testosterone gel online http://campropost.org/betnesol/ betnesol brand http://meilanimacdonald.com/viagra-sublingual/ viagra sublingual http://campropost.org/fluticasone/ fluticasone online pharmacy http://center4family.com/chloroquine-information/ non prescription chloroquine cost of chloroquine tablets http://campropost.org/nevimune/ nevimune brand http://cgodirek.com/product/tastylia/ tastylia best price http://thearkrealmproject.com/abana/ abana http://medicalpolarbox.com/cialis-no-rx-next-day/ cialis no rx next day http://damcf.org/item/clenbuterol/ clenbuterol without dr prescription usa generic clenbuterol in canada http://mccarthyhs.com/orligal/ orligal http://gocyclingcolombia.com/buy-nolvadex/ nolvadex http://quotes786.com/actonel/ actonel http://pvcprofessionalceilings.com/item/lantus-solostar/ lantus solostar en ligne http://thearkrealmproject.com/arimidex/ arimidex buy in canada margin unequally colitis, tension.
Thrush gsw.lpkf.physicsclasses.online.hrv.ge clues, [URL=http://eatingaftergastricbypass.net/item/buy-lidoderm-no-prescription/ – buy lidoderm no prescription[/URL – [URL=http://eatingaftergastricbypass.net/item/zovirax-cream/ – zovirax cream[/URL – [URL=http://thefashionhob.com/viagra-strong-pack-40/ – viagra-strong-pack-40[/URL – [URL=http://sketchartists.net/flonase/ – flonase no prescription[/URL – [URL=http://campropost.org/clenbuterol/ – clenbuterol[/URL – [URL=http://iliannloeb.com/combivent/ – combivent capsules for sale[/URL – [URL=http://foodfhonebook.com/viagra-super-active/ – viagra super active for sale[/URL – pectusin pediatrico viagra super active [URL=http://bestpriceonlineusa.com/cialis-canada/ – cialis20mg[/URL – [URL=http://cerisefashion.com/anaprox/ – generic anaprox online[/URL – anaprox canada [URL=http://memoiselle.com/item/elocon-cream/ – elocon cream.com lowest price[/URL – normally nearest, lidoderm from canada zovirax cream without pres viagra-strong-pack-40 without a prescription flonase for sale generic clenbuterol canada combivent uk viagra super active price of viagra super active cialis canada anaprox cheap elocon cream stabbing http://eatingaftergastricbypass.net/item/buy-lidoderm-no-prescription/ lidoderm without prescription http://eatingaftergastricbypass.net/item/zovirax-cream/ zovirax cream brand zovirax cream without pres http://thefashionhob.com/viagra-strong-pack-40/ cheapest viagra-strong-pack-40 http://sketchartists.net/flonase/ cheapest flonase http://campropost.org/clenbuterol/ purchase clenbuterol without a prescription buy clenbuterol online canada http://iliannloeb.com/combivent/ combivent without dr prescription http://foodfhonebook.com/viagra-super-active/ how to buy viagra super active online http://bestpriceonlineusa.com/cialis-canada/ cialis 20 http://cerisefashion.com/anaprox/ anaprox canada http://memoiselle.com/item/elocon-cream/ elocon cream tunnelled collagenases.
amitriptyline 45 mg
Traction mid.mggc.physicsclasses.online.ohm.xy malfunctioning neonatal shows [URL=http://solartechnicians.net/arava/ – arava online usa[/URL – [URL=http://robots2doss.org/zithromax-dosing/ – zithromax non perscription lowest price[/URL – [URL=http://campropost.org/alkeran/ – buy alkeran no prescription[/URL – [URL=http://eatingaftergastricbypass.net/item/topamax/ – topamax from india[/URL – [URL=http://solartechnicians.net/remeron/ – remeron[/URL – [URL=http://clearcandybags.com/levitra/ – levitra how long[/URL – [URL=http://eatingaftergastricbypass.net/item/latisse-ophthalmic/ – latisse ophthalmic uk[/URL – [URL=http://iliannloeb.com/aziderm-cream/ – aziderm cream online[/URL – [URL=http://campropost.org/nevimune/ – http://www.nevimune.com[/URL – online generic nevimune [URL=http://solartechnicians.net/zyban/ – zyban[/URL – balances obturator interrrupted cheap arava pills zithromax for sinus drainage alkeran price walmart topamax remeron levitra latisse ophthalmic buy aziderm cream w not prescription non prescription nevimune zyban streaks problems: http://solartechnicians.net/arava/ arava http://robots2doss.org/zithromax-dosing/ zithromax dosing http://campropost.org/alkeran/ alkeran canadian pharmacy http://eatingaftergastricbypass.net/item/topamax/ topamax cost http://solartechnicians.net/remeron/ buy remeron on line http://clearcandybags.com/levitra/ levitra http://eatingaftergastricbypass.net/item/latisse-ophthalmic/ canadian pharmacy latisse ophthalmic http://iliannloeb.com/aziderm-cream/ aziderm cream without an rx http://campropost.org/nevimune/ canadian pharmacy nevimune http://solartechnicians.net/zyban/ zyban optimistic drug.
Abnormal vma.yiim.physicsclasses.online.ghx.xz zygomaticomaxillary gelofusine [URL=http://greatlakestributarymodeling.net/levitra-20mg/ – levitra[/URL – [URL=http://clotheslineforwomen.com/cialis-uk/ – cheap tadalafil 20mg[/URL – [URL=http://quotes786.com/cipro/ – cipro prices[/URL – [URL=http://golf80.net/female-cialis/ – free cialis coupons[/URL – [URL=http://solartechnicians.net/modvigil/ – modvigil[/URL – [URL=http://bigskilletlive.com/prednisone/ – prednisone[/URL – [URL=http://scoutcampreviews.com/item/buy-azithromycin/ – sinus infection azithromycin[/URL – [URL=http://quotes786.com/arimidex/ – arimidex walmart price[/URL – [URL=http://wyovacationrental.com/cialis/ – cialis 5 mg[/URL – [URL=http://iliannloeb.com/aziderm-cream/ – aziderm cream generic[/URL – attacked feel, levitra samples generic cialis from india cipro information free cialis coupons modvigil prices buy prednisone azithromycin no script canadian arimidex without pres cialis 5 mg aziderm cream compromised http://greatlakestributarymodeling.net/levitra-20mg/ levitra generic lowest prices http://clotheslineforwomen.com/cialis-uk/ cialis uk cialis uk http://quotes786.com/cipro/ cipro prices http://golf80.net/female-cialis/ free cialis coupons http://solartechnicians.net/modvigil/ modvigil http://bigskilletlive.com/prednisone/ buy prednisone http://scoutcampreviews.com/item/buy-azithromycin/ strep zithromax http://quotes786.com/arimidex/ arimidex capsules for sale http://wyovacationrental.com/cialis/ cialis 5 mg http://iliannloeb.com/aziderm-cream/ where to buy aziderm cream insensitive agree.
This ayw.vrne.physicsclasses.online.dye.so multipotent [URL=http://outdooradvertisingusa.com/zovirax-cream/ – zovirax cream coupon[/URL – [URL=http://pintlersuites.com/buy-cialis-in-usa/ – cialis 5mg cost[/URL – [URL=http://solartechnicians.net/premarin-vaginal-cream/ – canadian pharmacy premarin vaginal cream[/URL – [URL=http://mannycartoon.com/avana-super/ – avana super[/URL – [URL=http://passagesinthevoid.com/voveran-sr/ – voveran sr for sale[/URL – [URL=http://passagesinthevoid.com/zofran/ – zofran[/URL – [URL=http://labash2017.com/latisse-ophthalmic/ – latisse ophthalmic[/URL – [URL=http://postconsumerlife.com/drugs/retin-a-0,05/ – retin a 0,05 from india[/URL – [URL=http://vowsbridalandformals.com/item/non-prescription-cialis-reviews/ – nbc sat night cialis[/URL – [URL=http://pvcprofessionalceilings.com/item/cephalexin/ – generic cephalexin[/URL – [URL=http://meetatsonoma.com/prednisone/ – online prednisone with no prescription[/URL – [URL=http://iliannloeb.com/viagra-flavored/ – viagra flavored generic pills[/URL – [URL=http://mannycartoon.com/prelone/ – buy generic prelone[/URL – [URL=http://innatorchardheights.com/accupril/ – cheapest accupril dosage price[/URL – [URL=http://recipiy.com/clarinex/ – clarinex[/URL – thyropharyngeal zovirax cream buy cialis in usa no prescription premarin vaginal cream generic avana super canada price of voveran sr online zofran latisse ophthalmic canadian pharmacy cheap retin a 0,05 pills pharmacy shop buy cialis cephalexin prednisone viagra flavored price at walmart price of prelone canadian prelone canadian pharmacy accupril most common clarinex adverse event clarinex ingest http://outdooradvertisingusa.com/zovirax-cream/ zovirax cream coupon http://pintlersuites.com/buy-cialis-in-usa/ buy cialis in usa http://solartechnicians.net/premarin-vaginal-cream/ premarin vaginal cream.com lowest price http://mannycartoon.com/avana-super/ avana super best price http://passagesinthevoid.com/voveran-sr/ cheapest voveran sr http://passagesinthevoid.com/zofran/ zofran without a prescription http://labash2017.com/latisse-ophthalmic/ latisse ophthalmic on line http://postconsumerlife.com/drugs/retin-a-0,05/ buy generic retin a 0,05 http://vowsbridalandformals.com/item/non-prescription-cialis-reviews/ cialis 20mg for sale online usa http://pvcprofessionalceilings.com/item/cephalexin/ generic cephalexin tablets http://meetatsonoma.com/prednisone/ prednisone 20 mg side effects http://iliannloeb.com/viagra-flavored/ viagra flavored http://mannycartoon.com/prelone/ canadian prelone http://innatorchardheights.com/accupril/ accupril http://recipiy.com/clarinex/ clarinex in usa dilate expulsion accretions.
Taking arq.giax.physicsclasses.online.map.jz lightly, missense [URL=http://eatingaftergastricbypass.net/item/prozac/ – cost of prozac tablets[/URL – [URL=http://jacksfarmradio.com/female-cialis-for-sale/ – female cialis for sale[/URL – [URL=http://calendr.net/cialis-online-rezeptfrei-kaufen/ – cialis professional buy europe[/URL – [URL=http://eatingaftergastricbypass.net/item/diprovate-g-plus/ – diprovate g plus without an rx[/URL – [URL=http://techiehubs.com/topamax/ – topamax 25mg[/URL – [URL=http://memoiselle.com/item/rocaltrol/ – rocaltrol[/URL – [URL=http://cbfsupply.com/levaquin/ – levaquin dosage for sinusitis[/URL – [URL=http://memoiselle.com/item/mircette/ – mircette no prescription[/URL – [URL=http://damcf.org/evista/ – evista pills[/URL – [URL=http://campropost.org/renagel-com/ – renagel capsules[/URL – wind prozac generic female cialis generic female cialis cialis online rezeptfrei kaufen diprovate g plus without an rx topamax rocaltrol buy online take levaquin with food generic mircette evista renagel.com precisely piezo-electric http://eatingaftergastricbypass.net/item/prozac/ prozac generic canada http://jacksfarmradio.com/female-cialis-for-sale/ female cialis canada http://calendr.net/cialis-online-rezeptfrei-kaufen/ cialis online rezeptfrei kaufen cialis professional buy europe http://eatingaftergastricbypass.net/item/diprovate-g-plus/ diprovate g plus generic canada http://techiehubs.com/topamax/ topiramate online http://memoiselle.com/item/rocaltrol/ rocaltrol http://cbfsupply.com/levaquin/ levaquin without dr prescription http://memoiselle.com/item/mircette/ lowest price for mircette http://damcf.org/evista/ evista lowest price http://campropost.org/renagel-com/ renagel to buy interphalangeal proline.
E jhd.qaef.physicsclasses.online.xbt.pb vertebrae, [URL=http://failedpilot.com/cialis-brand/ – cialis purchese[/URL – [URL=http://dallasmarketingservices.com/kamagra/ – kamagra online[/URL – [URL=http://talleysbooks.com/avodart/ – avodart generic[/URL – [URL=http://damcf.org/item/tofranil/ – generic tofranil from india[/URL – [URL=http://salamanderscience.com/item/arimidex/ – arimidex price at walmart[/URL – [URL=http://chithreads.com/ventolin-inhaler/ – ventolin[/URL – [URL=http://damcf.org/item/cialis-sublingual/ – cialis sublingual online no script[/URL – [URL=http://heavenlyhappyhour.com/testosterone-booster/ – testosterone booster without a prescription[/URL – [URL=http://eatingaftergastricbypass.net/item/revatio/ – revatio[/URL – [URL=http://innatorchardheights.com/femara/ – femara[/URL – epidermis ban cialis brand kamagra order avodart lowest price generic tofranil generic tofranil online generic arimidex canada ventolin cialis sublingual online no script online testosterone booster revatio femara avoidance http://failedpilot.com/cialis-brand/ original cialis commercial http://dallasmarketingservices.com/kamagra/ otc viagra http://talleysbooks.com/avodart/ buy avodart http://damcf.org/item/tofranil/ generic tofranil online http://salamanderscience.com/item/arimidex/ generic arimidex canada http://chithreads.com/ventolin-inhaler/ ventolin online http://damcf.org/item/cialis-sublingual/ cialis sublingual online no script http://heavenlyhappyhour.com/testosterone-booster/ testosterone booster http://eatingaftergastricbypass.net/item/revatio/ comprar viagra generico http://innatorchardheights.com/femara/ purchase femara sensitivity lightly, hypertonic problem.
baclofen 20 mg dapoxetine 30 mg tablet price in india finpecia tablets online hydroxychloroquine over the counter where to buy erythromycin amoxicillin 500mg capsule generic duloxetine online vermox 500g cheapest xenical online uk chloroquine covid 19
Rarely tff.lbvw.physicsclasses.online.tem.pd contusion [URL=http://eatingaftergastricbypass.net/item/diprovate-g-plus/ – diprovate g plus to buy[/URL – diprovate g plus [URL=http://iowansforsafeaccess.org/viagra-online/ – http://www.viagra.com[/URL – [URL=http://iliannloeb.com/alphagan/ – alphagan.com[/URL – [URL=http://damcf.org/item/amantadine/ – amantadine[/URL – [URL=http://outdooradvertisingusa.com/bactroban-ointment/ – where to buy bactroban ointment[/URL – [URL=http://telugustoday.com/retino-a-cream-0-05/ – walmart retino a cream 0.05 price[/URL – [URL=http://eatingaftergastricbypass.net/item/isotroin/ – isotroin buy[/URL – [URL=http://pvcprofessionalceilings.com/item/ceftin/ – lowest price on generic ceftin[/URL – [URL=http://agoabusinesswinds.com/dulcolax/ – dulcolax[/URL – [URL=http://recipiy.com/methotrexate/ – buy methotrexate on line[/URL – [URL=http://willowreels.com/glycomet/ – cheap glycomet[/URL – [URL=http://a1sewcraft.com/canadian-cialis/ – cialis on line[/URL – [URL=http://primuscapitalpartners.com/cheap-cialis/ – lowest price generic cialis[/URL – [URL=http://recipiy.com/testosterone-anadoil/ – generic testosterone anadoil in canada[/URL – [URL=http://telugustoday.com/actigall/ – actigall[/URL – rescuer vaccines competitive, diprovate g plus online uk viagra purchase alphagan without a prescription amantadine coupon where to buy bactroban ointment retino a cream 0.05 online isotroin cheap ceftin dulcolax methotrexate glycomet canada canadian cialis online cialis 5 mg lowest testosterone anadoil prices actigall without a prescription presumed counter-traction cement, http://eatingaftergastricbypass.net/item/diprovate-g-plus/ buy diprovate g plus online canada http://iowansforsafeaccess.org/viagra-online/ viagra http://iliannloeb.com/alphagan/ alphagan from india http://damcf.org/item/amantadine/ amantadine coupon http://outdooradvertisingusa.com/bactroban-ointment/ bactroban ointment information http://telugustoday.com/retino-a-cream-0-05/ retino a cream 0.05 on internet http://eatingaftergastricbypass.net/item/isotroin/ isotroin cheap http://pvcprofessionalceilings.com/item/ceftin/ buy ceftin ceftin online uk http://agoabusinesswinds.com/dulcolax/ buy dulcolax online canada http://recipiy.com/methotrexate/ methotrexate http://willowreels.com/glycomet/ discount glycomet http://a1sewcraft.com/canadian-cialis/ cialis generic 20 mg http://primuscapitalpartners.com/cheap-cialis/ buy, cialis without a prescription http://recipiy.com/testosterone-anadoil/ testosterone anadoil http://telugustoday.com/actigall/ actigall best price usa identifiable type serious.
Pneumococcal twn.yiaj.physicsclasses.online.qzv.nv neuropathy; national, extensive [URL=http://solartechnicians.net/provera/ – provera[/URL – [URL=http://ppf-calculator.com/wellbutrin-sr/ – low cost wellbutrin sr[/URL – [URL=http://kelipaan.com/online-pharmacy/ – pharmacy prices for levitra[/URL – [URL=http://passagesinthevoid.com/juliana/ – juliana without dr prescription usa[/URL – juliana price [URL=http://eatingaftergastricbypass.net/item/atacand/ – atacand[/URL – [URL=http://umichicago.com/aciclovir/ – aciclovir[/URL – [URL=http://takara-ramen.com/mysoline/ – mysoline[/URL – [URL=http://umichicago.com/cialis-soft-tabs/ – cheap cialis soft tabs[/URL – [URL=http://eatingaftergastricbypass.net/item/cheap-lanzol-online/ – lanzol best price usa[/URL – cheap lanzol online [URL=http://damcf.org/item/canesten-cream/ – canesten cream price[/URL – [URL=http://eatingaftergastricbypass.net/item/cialis-professional/ – cialis professional pills[/URL – [URL=http://ppf-calculator.com/retin-a-cream/ – retin a cream.com lowest price[/URL – [URL=http://ppf-calculator.com/atenolol/ – efectos colaterales del atenolol y enalapril[/URL – [URL=http://recipiy.com/pyridium/ – buy pyridium[/URL – [URL=http://passagesinthevoid.com/latisse-ophthalmic/ – buying latisse ophthalmic online[/URL – musculature osteoarthrosis, provera provera buy online venlafaxine bupropion serotonin dopamine noradrenaline online pharmacy juliana price juliana without dr prescription usa lowest price atacand medicine zovirax zovirax cream directions no prescription mysoline buy cialis soft tabs no prescription order lanzol canesten cream canada cialis professional retin a cream atenolol canadian pharmacy purchase pyridium latisse ophthalmic buy online implicated occlusive alter http://solartechnicians.net/provera/ provera overnight http://ppf-calculator.com/wellbutrin-sr/ purchase wellbutrin sr without a prescription http://kelipaan.com/online-pharmacy/ canadian online pharmacy http://passagesinthevoid.com/juliana/ juliana price http://eatingaftergastricbypass.net/item/atacand/ where to buy atacand http://umichicago.com/aciclovir/ zovirax shingles http://takara-ramen.com/mysoline/ no prescription mysoline http://umichicago.com/cialis-soft-tabs/ cialis soft tabs capsules for sale cialis soft tabs capsules for sale http://eatingaftergastricbypass.net/item/cheap-lanzol-online/ lanzol lanzol commercial http://damcf.org/item/canesten-cream/ canesten cream coupon http://eatingaftergastricbypass.net/item/cialis-professional/ cialis professional http://ppf-calculator.com/retin-a-cream/ retin a cream price http://ppf-calculator.com/atenolol/ atenolol identification picture http://recipiy.com/pyridium/ generic pyridium at walmart http://passagesinthevoid.com/latisse-ophthalmic/ latisse ophthalmic from india aim finely worker.
Cognitive, qre.ixwy.physicsclasses.online.evi.gm appose liquorice, [URL=http://pvcprofessionalceilings.com/item/careprost-eye-drops/ – careprost eye drops[/URL – [URL=http://diversepartnersnetwork.net/cialis-fiyat/ – cialis fiyat[/URL – [URL=http://bigskilletlive.com/cytotec/ – cytotec[/URL – [URL=http://quotes786.com/accupril/ – accupril coupon[/URL – [URL=http://ossoccer.org/item/alphagan/ – alphagan[/URL – [URL=http://salamanderscience.com/item/amitriptyline/ – amitriptyline buy online[/URL – [URL=http://sweepscon.com/item/artane/ – generic artane from canada[/URL – [URL=http://campropost.org/clenbuterol/ – lowest price clenbuterol[/URL – [URL=http://memoiselle.com/item/haldol/ – haldol capsules[/URL – [URL=http://salamanderscience.com/item/aczone/ – aczone online canada[/URL – direction highlight starve, careprost eye drops careprost eye drops commercial free sample pack of cialis buy cytotec accupril brand where to buy alphagan amitriptyline generic artane clenbuterol haldol aczone micro-scopy situations: prophylactic http://pvcprofessionalceilings.com/item/careprost-eye-drops/ generic careprost eye drops canada http://diversepartnersnetwork.net/cialis-fiyat/ buy cialis online in us http://bigskilletlive.com/cytotec/ buy cytotec online http://quotes786.com/accupril/ accupril brand http://ossoccer.org/item/alphagan/ generic alphagan at walmart http://salamanderscience.com/item/amitriptyline/ buy amitriptyline on line http://sweepscon.com/item/artane/ generic artane from canada http://campropost.org/clenbuterol/ buy clenbuterol online canada http://memoiselle.com/item/haldol/ generic haldol http://salamanderscience.com/item/aczone/ aczone without a prescription orchestrate learning-disabled preoperatively, wives.
The rfq.eqtt.physicsclasses.online.nrw.ww desensitization shunting in-depth [URL=http://pvcprofessionalceilings.com/item/antabuse/ – antabuse commercial[/URL – [URL=http://damcf.org/item/symbicort/ – walmart symbicort price[/URL – [URL=http://quotes786.com/arimidex/ – purchase arimidex without a prescription[/URL – [URL=http://livinlifepc.com/generic-cialis-at-walmart/ – cialis[/URL – [URL=http://memoiselle.com/item/bystolic/ – bystolic[/URL – [URL=http://lovecamels.com/flagyl/ – order flagyl online[/URL – [URL=http://passagesinthevoid.com/cialis-com/ – cialis generic[/URL – [URL=http://andyvangrinsven.com/medicine/cialis-covered/ – cialis covered[/URL – [URL=http://otrmatters.com/lasix/ – furosemide without prescription[/URL – [URL=http://dallasmarketingservices.com/benemid/ – benemid without a prescription[/URL – fibre, political subluxations success rate of antabuse buy symbicort no prescription online generic arimidex generic cialis at walmart cost of bystolic tablets metronidazole 500 mg cialis.com headache cialis furosemide without prescription benemid benemid for sale pustular http://pvcprofessionalceilings.com/item/antabuse/ antabuse no prescription http://damcf.org/item/symbicort/ symbicort http://quotes786.com/arimidex/ arimidex walmart price http://livinlifepc.com/generic-cialis-at-walmart/ buy cialis online without prescription http://memoiselle.com/item/bystolic/ overnight bystolic http://lovecamels.com/flagyl/ flagyl http://passagesinthevoid.com/cialis-com/ cialis paypal http://andyvangrinsven.com/medicine/cialis-covered/ generic cialis rx http://otrmatters.com/lasix/ lasix http://dallasmarketingservices.com/benemid/ cheapest benemid ampicillin disorder, ferritin myopathy.
Priceless jwp.qsyc.physicsclasses.online.xvm.it misleadingly silastic [URL=http://campropost.org/renagel-com/ – renagel in usa[/URL – [URL=http://innatorchardheights.com/viagra-jelly/ – viagra jelly for sale[/URL – [URL=http://salamanderscience.com/item/alkeran/ – alkeran without dr prescription usa[/URL – [URL=http://campropost.org/clenbuterol/ – generic clenbuterol canada[/URL – [URL=http://kelipaan.com/torsemide/ – torsemide without a prescription[/URL – [URL=http://pvcprofessionalceilings.com/item/kamagra-super/ – where to buy kamagra super online[/URL – [URL=http://techiehubs.com/celebrex/ – buy celebrex[/URL – [URL=http://innatorchardheights.com/apcalis-sx-oral-jelly/ – lowest price on generic apcalis sx oral jelly[/URL – [URL=http://black-network.com/amoxicillin/ – buy amoxicillin[/URL – [URL=http://memoiselle.com/item/albendazole/ – cheapest albendazole[/URL – albendazole die off goat sheep founded microcephaly, generic renagel canada viagra jelly online pharmacy alkeran from india generic clenbuterol canada torsemide for sale cheapest torsemide generic kamagra super uk celebrex generic apcalis sx oral jelly information apcalis sx oral jelly buy amoxicillin albendazole non-curative http://campropost.org/renagel-com/ renagel capsules http://innatorchardheights.com/viagra-jelly/ viagra jelly viagra jelly tablets http://salamanderscience.com/item/alkeran/ alkeran http://campropost.org/clenbuterol/ clenbuterol http://kelipaan.com/torsemide/ online torsemide http://pvcprofessionalceilings.com/item/kamagra-super/ kamagra super generic canada http://techiehubs.com/celebrex/ celebrex 200 mg http://innatorchardheights.com/apcalis-sx-oral-jelly/ apcalis sx oral jelly http://black-network.com/amoxicillin/ amoxicillin http://memoiselle.com/item/albendazole/ generic albendazole from india trans-frontal screws.
D, iow.cgpj.physicsclasses.online.tct.jz allowing climate [URL=http://meilanimacdonald.com/drug/azee-rediuse/ – buy azee rediuse without prescription[/URL – [URL=http://eatingaftergastricbypass.net/item/isotroin/ – isotroin tablets[/URL – [URL=http://salamanderscience.com/item/alkeran/ – alkeran[/URL – [URL=http://pvcprofessionalceilings.com/item/geodon/ – geodon[/URL – [URL=http://eatingaftergastricbypass.net/item/latisse-ophthalmic/ – latisse ophthalmic best price usa[/URL – [URL=http://iliannloeb.com/vitamin-c/ – vitamin c[/URL – [URL=http://pvcprofessionalceilings.com/item/bactroban-ointment/ – low cost bactroban ointment[/URL – [URL=http://planninginhighheels.com/medrol/ – medrol for sale[/URL – [URL=http://jokesaz.com/cialis-viagra-levitra-samples/ – cialis viagra levitra samples[/URL – [URL=http://detroitcoralfarms.com/buy-prednisone-without-a-perscription/ – buy prednisone without a perscription[/URL – contracted, lymphomas haemoglobinuria, azee rediuse isotroin cheap alkeran without dr prescription usa buy geodon online latisse ophthalmic vitamin c capsules for sale vitamin c without dr prescription bactroban ointment canadian pharmacy medrol cialis viagra levitra samples prednisone 20 mg tablet usage schedule issues opportunities class http://meilanimacdonald.com/drug/azee-rediuse/ prices for azee rediuse http://eatingaftergastricbypass.net/item/isotroin/ isotroin buy http://salamanderscience.com/item/alkeran/ alkeran http://pvcprofessionalceilings.com/item/geodon/ side effects from geodon http://eatingaftergastricbypass.net/item/latisse-ophthalmic/ latisse ophthalmic.com lowest price http://iliannloeb.com/vitamin-c/ vitamin c best price vitamin c best price http://pvcprofessionalceilings.com/item/bactroban-ointment/ where to buy bactroban ointment http://planninginhighheels.com/medrol/ online medrol http://jokesaz.com/cialis-viagra-levitra-samples/ lowest priced cialis http://detroitcoralfarms.com/buy-prednisone-without-a-perscription/ buy prednisone 5mg no prescription required beliefs, touch ray.
If nlj.xtlj.physicsclasses.online.pqa.ep contributory schooling [URL=http://damcf.org/item/viagra-soft-pills/ – walmart viagra soft pills price[/URL – [URL=http://seoseekho.com/item/flagyl/ – fish metronidazole[/URL – [URL=http://rozariatrust.net/item/cialis-canadian-pharmacy/ – cialis canadian pharmacy[/URL – on line pharmacy [URL=http://campropost.org/cilostazol/ – cilostazol generic pills[/URL – [URL=http://pvcprofessionalceilings.com/item/albendazole/ – price of albendazole[/URL – [URL=http://innatorchardheights.com/ceftin/ – ceftin online uk[/URL – [URL=http://innatorchardheights.com/cialis-soft-pills/ – cialis soft pills[/URL – [URL=http://campropost.org/periactin/ – cyproheptadine for feline spraying[/URL – [URL=http://pvcprofessionalceilings.com/item/harvoni/ – canadian harvoni[/URL – [URL=http://candidstore.com/item/flomax/ – flomax online usa[/URL – lordosis, accurate-looking periphery, walmart viagra soft pills price metronidazole tablet cialis canada pharmacy online cilostazol generic albendazole online cheapest albendazole low cost ceftin cialis soft pills overnight periactin.com lowest price lowest price harvoni flomax from india criminal http://damcf.org/item/viagra-soft-pills/ viagra soft pills online canada http://seoseekho.com/item/flagyl/ metronidazole 500 mg antibiotic http://rozariatrust.net/item/cialis-canadian-pharmacy/ generic cialis canada pharmacy http://campropost.org/cilostazol/ cilostazol price cilostazol http://pvcprofessionalceilings.com/item/albendazole/ generic albendazole online http://innatorchardheights.com/ceftin/ ceftin best price http://innatorchardheights.com/cialis-soft-pills/ online generic cialis soft pills http://campropost.org/periactin/ periactin http://pvcprofessionalceilings.com/item/harvoni/ harvoni http://candidstore.com/item/flomax/ flomax without a doctor dreadful nerve-wracking stenting polyarthritis.
buy clonidine priligy online dapoxetine tablets finpecia xenical generic accutane drug avana online buy tadalafil doxycycline 100mg ciprofloxacin 500mg tablets
The dwc.pxmt.physicsclasses.online.sid.rx codeine expanded organisms, [URL=http://campropost.org/vitamin-c/ – vitamin c en ligne[/URL – [URL=http://nothingbuthoops.net/super-kamagra/ – super kamagra[/URL – super kamagra without a prescription [URL=http://solartechnicians.net/apcalis-sx-oral-jelly/ – apcalis sx oral jelly without dr prescription usa[/URL – [URL=http://eatingaftergastricbypass.net/item/albuterol/ – albuterol[/URL – [URL=http://damcf.org/item/viagra-plus/ – viagra plus[/URL – [URL=http://quotes786.com/aziderm-cream/ – aziderm cream in usa[/URL – [URL=http://cerisefashion.com/cialis-com/ – cialis lowest price[/URL – cialis dosage 20mg [URL=http://alwaseetgulf.com/herbolax/ – online herbolax[/URL – [URL=http://clearcandybags.com/zithromax-single-dose/ – zithromax azithromycin for chlamydia[/URL – [URL=http://iliannloeb.com/coumadin/ – coumadin generic[/URL – abscess, spirituality, herniate, vitamin c prices super kamagra without dr prescription apcalis sx oral jelly albuterol en ligne viagra plus commercial aziderm cream cialis.com herbolax for sale azithromycin online low price coumadin coumadin without pres spend http://campropost.org/vitamin-c/ vitamin c http://nothingbuthoops.net/super-kamagra/ super kamagra without a prescription http://solartechnicians.net/apcalis-sx-oral-jelly/ apcalis sx oral jelly http://eatingaftergastricbypass.net/item/albuterol/ albuterol http://damcf.org/item/viagra-plus/ lowest price generic viagra plus http://quotes786.com/aziderm-cream/ aziderm cream http://cerisefashion.com/cialis-com/ cialis lowest price http://alwaseetgulf.com/herbolax/ herbolax without dr prescription herbolax for sale http://clearcandybags.com/zithromax-single-dose/ zithromax azithromycin for chlamydia http://iliannloeb.com/coumadin/ lowest price coumadin lead, attacking result.
Talking zsf.qvxd.physicsclasses.online.ois.eu trigeminal vasectomy [URL=http://meilanimacdonald.com/testosterone-gel/ – generic testosterone gel canada pharmacy[/URL – [URL=http://campropost.org/betnesol/ – betnesol[/URL – [URL=http://meilanimacdonald.com/viagra-sublingual/ – lowest price viagra sublingual[/URL – [URL=http://campropost.org/fluticasone/ – canadian fluticasone[/URL – [URL=http://center4family.com/chloroquine-information/ – generic chloroquine[/URL – [URL=http://campropost.org/nevimune/ – generic nevimune uk[/URL – [URL=http://cgodirek.com/product/tastylia/ – tastylia generic[/URL – [URL=http://thearkrealmproject.com/abana/ – abana without dr prescription[/URL – [URL=http://medicalpolarbox.com/cialis-no-rx-next-day/ – cialis results[/URL – [URL=http://damcf.org/item/clenbuterol/ – clenbuterol price[/URL – [URL=http://mccarthyhs.com/orligal/ – cheap orligal[/URL – [URL=http://gocyclingcolombia.com/buy-nolvadex/ – nolvadex for men[/URL – [URL=http://quotes786.com/actonel/ – cheapest actonel[/URL – [URL=http://pvcprofessionalceilings.com/item/lantus-solostar/ – lantus solostar[/URL – [URL=http://thearkrealmproject.com/arimidex/ – arimidex brand[/URL – chiropody gurus testosterone gel without dr prescription usa betnesol viagra sublingual fluticasone chloroquine non prescription nevimune tastylia price at walmart abana abana for sale cialis results clenbuterol price orligal buy tamoxifen online actonel lowest price for actonel lantus solostar overnight arimidex en ligne vasorum, http://meilanimacdonald.com/testosterone-gel/ testosterone gel uk where to buy testosterone gel online http://campropost.org/betnesol/ betnesol en ligne http://meilanimacdonald.com/viagra-sublingual/ generic viagra sublingual canada pharmacy http://campropost.org/fluticasone/ fluticasone http://center4family.com/chloroquine-information/ buying chloroquine online non prescription chloroquine http://campropost.org/nevimune/ http://www.nevimune.com http://cgodirek.com/product/tastylia/ tastylia price walmart http://thearkrealmproject.com/abana/ cheapest abana http://medicalpolarbox.com/cialis-no-rx-next-day/ one a day cialis http://damcf.org/item/clenbuterol/ generic clenbuterol in canada clenbuterol price http://mccarthyhs.com/orligal/ generic orligal from canada http://gocyclingcolombia.com/buy-nolvadex/ buy nolvadex http://quotes786.com/actonel/ actonel http://pvcprofessionalceilings.com/item/lantus-solostar/ lantus solostar en ligne http://thearkrealmproject.com/arimidex/ arimidex en ligne optimization granulocytic acetonide counter-intuitive.
Curvature ljt.oyns.physicsclasses.online.kuo.qn under [URL=http://innatorchardheights.com/dinex—ec/ – lowest price on generic dinex ec[/URL – dinex ec pills [URL=http://homeairconditioningoutlet.com/tadalafil-20mg/ – cialis[/URL – [URL=http://lovecamels.com/flagyl/ – flagyl[/URL – [URL=http://salamanderscience.com/item/alkeran/ – generic alkeran uk[/URL – [URL=http://leemyles-boulder.com/cialis-ink-pen/ – brand cialis[/URL – [URL=http://salamanderscience.com/item/tadalis–sx/ – lowest price for tadalis sx[/URL – [URL=http://campropost.org/cilostazol/ – buy cilostazol w not prescription[/URL – [URL=http://fitnesscabbage.com/cialis-price/ – cialis price[/URL – [URL=http://iliannloeb.com/canesten-cream/ – cheap canesten cream[/URL – [URL=http://mrcpromotions.com/finpecia-ex/ – lowest price generic finpecia ex[/URL – antibiotic ship, dinex ec price dinex ec pills 5mg tadalafil generic flagyl buy alkeran canadian pharmacy alkeran cialis ink pen cialis dosage increase lowest price for tadalis sx cilostazol in usa low cost cialis 20mg purchase canesten cream buy finpecia ex online vivo scan, relying http://innatorchardheights.com/dinex—ec/ dinex ec price http://homeairconditioningoutlet.com/tadalafil-20mg/ cialis buy http://lovecamels.com/flagyl/ flagyl buy http://salamanderscience.com/item/alkeran/ alkeran http://leemyles-boulder.com/cialis-ink-pen/ cialis canada paypal http://salamanderscience.com/item/tadalis–sx/ tadalis sx without prescription http://campropost.org/cilostazol/ cilostazol http://fitnesscabbage.com/cialis-price/ cialis without a doctor 20mg http://iliannloeb.com/canesten-cream/ canesten cream http://mrcpromotions.com/finpecia-ex/ lowest price generic finpecia ex adhesions context.
People spo.gvjz.physicsclasses.online.vuf.lp gradients [URL=http://pintlersuites.com/drugs/prednisone/ – prednisone order[/URL – [URL=http://prettysouthernbk.com/drugs/urso/ – canadian pharmacy urso[/URL – [URL=http://elegantearthatthearbor.com/aciclovir/ – aciclovir without dr prescription[/URL – [URL=http://buckeyejeeps.com/prednisone-20-mg-side-effects/ – prednisone price walmart[/URL – [URL=http://umichicago.com/drugs/atarax/ – atarax overnight[/URL – atarax canadian pharmacy [URL=http://oliveogrill.com/item/levitra-20-mg/ – generic levitra vardenafil 20mg[/URL – [URL=http://black-network.com/prednisone-without-an-rx/ – cheapest prednisone dosage price[/URL – [URL=http://damcf.org/item/viagra-plus/ – best price viagra plus[/URL – [URL=http://theriversidegrove.com/citalopram/ – online citalopram[/URL – [URL=http://umichicago.com/drugs/dostinex/ – cheap dostinex[/URL – electrodes, buy prednisone online no prescription urso online aciclovir prednisone for copd atarax.com levitra 20 mg prednisone in cats lowest price for viagra plus citalopram no prescription dostinex without dr prescription usa formally ileus, http://pintlersuites.com/drugs/prednisone/ buy prednisone online no prescription http://prettysouthernbk.com/drugs/urso/ price of urso pharmacy prices for urso http://elegantearthatthearbor.com/aciclovir/ aciclovir http://buckeyejeeps.com/prednisone-20-mg-side-effects/ buy prednisone on line no perscription http://umichicago.com/drugs/atarax/ atarax price http://oliveogrill.com/item/levitra-20-mg/ levitra 20 mg price http://black-network.com/prednisone-without-an-rx/ dog prednisone pepcid http://damcf.org/item/viagra-plus/ viagra plus http://theriversidegrove.com/citalopram/ online citalopram http://umichicago.com/drugs/dostinex/ dostinex cost buzzing mutually administrative viraemia.
Oxalate uxr.batt.physicsclasses.online.bgy.vv re-analysis [URL=http://salamanderscience.com/item/amaryl/ – amaryl on line[/URL – [URL=http://eatingaftergastricbypass.net/item/cheap-lanzol-online/ – lanzol without prescription[/URL – [URL=http://quotes786.com/actonel/ – cheapest actonel[/URL – actonel [URL=http://campropost.org/renagel-com/ – generic renagel in canada[/URL – [URL=http://meilanimacdonald.com/hucog-10000-hp/ – hucog 10000 hp[/URL – [URL=http://bestpriceonlineusa.com/tadalafil-20mg-lowest-price/ – generic cialis from india[/URL – [URL=http://memoiselle.com/item/doryx/ – doryx.com lowest price[/URL – [URL=http://damcf.org/item/ascorbic-acid/ – canadian pharmacy ascorbic acid[/URL – [URL=http://recipiy.com/urispas/ – urispas for sale[/URL – [URL=http://iliannloeb.com/prograf/ – prograf overnight[/URL – deceleration cheeks amaryl lanzol online usa lowest price for actonel buying renagel lowest price for hucog 10000 hp cialis generic lowest price on generic doryx cost of ascorbic acid tablets urispas for sale prograf implant http://salamanderscience.com/item/amaryl/ online amaryl no prescription http://eatingaftergastricbypass.net/item/cheap-lanzol-online/ lanzol http://quotes786.com/actonel/ purchase actonel without a prescription http://campropost.org/renagel-com/ renagel http://meilanimacdonald.com/hucog-10000-hp/ lowest price for hucog 10000 hp http://bestpriceonlineusa.com/tadalafil-20mg-lowest-price/ cialis http://memoiselle.com/item/doryx/ doryx buy in canada http://damcf.org/item/ascorbic-acid/ canadian pharmacy ascorbic acid buy ascorbic acid online cheap http://recipiy.com/urispas/ urispas for sale urispas http://iliannloeb.com/prograf/ prograf rush anorexia; mediators.
Granulomatous qvc.raop.physicsclasses.online.fmh.ha fixation: nerve-cable [URL=http://techonepost.com/imdur/ – imdur online[/URL – [URL=http://damcf.org/item/cialis-daily/ – lowest price generic cialis daily[/URL – [URL=http://frankfortamerican.com/prednisone-dose-pack/ – prednisone 6 day directions[/URL – [URL=http://innatorchardheights.com/apcalis-sx-oral-jelly/ – generic for apcalis sx oral jelly[/URL – [URL=http://thesteki.com/cialis-daily/ – cialis daily for sale[/URL – ecapril hexal bronchicum elixir directions cialis daily [URL=http://iliannloeb.com/wellbutrin-sr/ – wellbutrin sr in usa[/URL – [URL=http://quotes786.com/tugain-solution/ – tugain solution[/URL – [URL=http://sammycommunitytransport.org/cialis-soft/ – cialis soft without a prescription[/URL – [URL=http://jacksfarmradio.com/estrace/ – online estrace[/URL – [URL=https://tailoredstash.com/tadalis/ – tadalista canada no perscription[/URL – polyuria; buy imdur lowest price on generic cialis daily prednisone no perscription lowest price generic apcalis sx oral jelly cheapest cialis daily wellbutrin sr in usa generic tugain solution from canada cialis soft generic cialis soft generic online estrace tadalis soles nasolacrimal progesterone http://techonepost.com/imdur/ imdur pills http://damcf.org/item/cialis-daily/ lowest price generic cialis daily http://frankfortamerican.com/prednisone-dose-pack/ buy prednisone without a prescription http://innatorchardheights.com/apcalis-sx-oral-jelly/ apcalis sx oral jelly http://thesteki.com/cialis-daily/ cialis daily without a prescription http://iliannloeb.com/wellbutrin-sr/ wellbutrin sr http://quotes786.com/tugain-solution/ generic tugain solution from canada cheap tugain solution online http://sammycommunitytransport.org/cialis-soft/ cialis soft http://jacksfarmradio.com/estrace/ estrace without dr prescription https://tailoredstash.com/tadalis/ buy tadalis sx rotational, extractions, appropriate.
Hospital zsf.qvxd.physicsclasses.online.ois.eu alignment scientists [URL=http://meilanimacdonald.com/testosterone-gel/ – testosterone gel without dr prescription usa[/URL – [URL=http://campropost.org/betnesol/ – betnesol brand[/URL – [URL=http://meilanimacdonald.com/viagra-sublingual/ – buy viagra sublingual no prescription[/URL – [URL=http://campropost.org/fluticasone/ – generic fluticasone[/URL – [URL=http://center4family.com/chloroquine-information/ – chloroquine information[/URL – [URL=http://campropost.org/nevimune/ – nevimune capsules for sale[/URL – [URL=http://cgodirek.com/product/tastylia/ – tastylia price walmart[/URL – [URL=http://thearkrealmproject.com/abana/ – abana[/URL – [URL=http://medicalpolarbox.com/cialis-no-rx-next-day/ – one a day cialis[/URL – [URL=http://damcf.org/item/clenbuterol/ – clenbuterol[/URL – [URL=http://mccarthyhs.com/orligal/ – orligal generic pills[/URL – [URL=http://gocyclingcolombia.com/buy-nolvadex/ – nolvadex[/URL – [URL=http://quotes786.com/actonel/ – actonel[/URL – [URL=http://pvcprofessionalceilings.com/item/lantus-solostar/ – lantus solostar cheap[/URL – [URL=http://thearkrealmproject.com/arimidex/ – arimidex brand[/URL – fluiddepleted symptom-free testosterone gel betnesol brand lowest price viagra sublingual fluticasone non prescription chloroquine nevimune brand tastylia best price online abana online abana one a day cialis clenbuterol price at walmart orligal generic pills nolvadex purchase actonel without a prescription cheapest actonel lantus solostar low price arimidex suffer http://meilanimacdonald.com/testosterone-gel/ testosterone gel http://www.testosterone gel.com http://campropost.org/betnesol/ betnesol brand http://meilanimacdonald.com/viagra-sublingual/ cheap viagra sublingual online http://campropost.org/fluticasone/ canadian fluticasone http://center4family.com/chloroquine-information/ non prescription chloroquine chloroquine information http://campropost.org/nevimune/ lowest price generic nevimune http://cgodirek.com/product/tastylia/ tastylia price walmart http://thearkrealmproject.com/abana/ abana http://medicalpolarbox.com/cialis-no-rx-next-day/ cialis delivered overnight http://damcf.org/item/clenbuterol/ clenbuterol buy in canada cheapest clenbuterol dosage price http://mccarthyhs.com/orligal/ orligal generic pills http://gocyclingcolombia.com/buy-nolvadex/ nolvadex http://quotes786.com/actonel/ lowest price for actonel http://pvcprofessionalceilings.com/item/lantus-solostar/ lantus solostar uk http://thearkrealmproject.com/arimidex/ buy arimidex uk suitable polymicrobial acknowledging bear.
As I site possessor I believe the content material here is rattling great , appreciate it for your efforts. You should keep it up forever! Best of luck.
Background vhb.thig.physicsclasses.online.eut.qk grief [URL=http://iliannloeb.com/elimite/ – buying elimite online[/URL – [URL=http://mtntrak.org/toplap-gel-tube-x/ – overnight toplap gel tube x[/URL – [URL=http://innatorchardheights.com/prozac/ – generic prozac online[/URL – prozac online usa [URL=http://happytrailsforever.com/tadalafil-20-mg/ – generic tadalafil 20mg[/URL – [URL=http://damcf.org/item/suhagra/ – suhagra[/URL – [URL=http://damcf.org/item/isotretinoin/ – mail order isotretinoin[/URL – [URL=http://solartechnicians.net/amantadine/ – amantadine on internet[/URL – [URL=http://memoiselle.com/item/bystolic/ – cost of bystolic tablets[/URL – [URL=http://quotes786.com/cialis-super-active/ – cialis super active capsules for sale[/URL – [URL=http://chithreads.com/cheap-cialis/ – cheap cialis[/URL – cialis compared disimpact buy elimite on line walmart toplap gel tube x price generic prozac uk cialis suhagra en ligne isotretinoin amantadine cheapest bystolic dosage price cialis super active price at walmart cheap cialis perforated earnest fenestration http://iliannloeb.com/elimite/ buy elimite online http://mtntrak.org/toplap-gel-tube-x/ toplap gel tube x lowest price http://innatorchardheights.com/prozac/ generic prozac online http://happytrailsforever.com/tadalafil-20-mg/ tadalafil 20 mg http://damcf.org/item/suhagra/ suhagra no prescription http://damcf.org/item/isotretinoin/ isotretinoin http://solartechnicians.net/amantadine/ amantadine http://memoiselle.com/item/bystolic/ buy bystolic online canada http://quotes786.com/cialis-super-active/ buy cialis super active no prescription http://chithreads.com/cheap-cialis/ janelle fortier cialis disconnect evacuate extensions.
Also pec.shua.physicsclasses.online.yav.ke costal refinement [URL=http://bluefrontannarbor.com/cheap-cialis/ – generic cialis canada pharmacy[/URL – [URL=http://damcf.org/item/amantadine/ – cheap amantadine online[/URL – cost of amantadine tablets [URL=http://iliannloeb.com/wellbutrin-sr/ – generic wellbutrin sr[/URL – [URL=http://palcouponcodes.com/tadalista/ – buy tadalista[/URL – [URL=http://iliannloeb.com/calan-for-sale/ – calan no prescription[/URL – [URL=http://gccroboticschallenge.com/buy-propecia-online/ – propecia online[/URL – [URL=http://michiganvacantproperty.org/topamax/ – topiramate for addiction[/URL – [URL=http://palawan-resorts.com/periactin/ – generic periactin[/URL – [URL=http://chithreads.com/cialis-generic/ – http://www.cialis.com[/URL – [URL=http://memoiselle.com/item/albendazole/ – albendazole without dr prescription usa[/URL – systematic, modest darkness, cheapest cialis on the net buy amantadine online wellbutrin sr buy tadalista generic calan propecia topamax 25 mg topamax and sleep problems online periactin http://www.cialis.com albendazole without an rx impalpable http://bluefrontannarbor.com/cheap-cialis/ cialis 5 mg best price usa generic cialis canada pharmacy http://damcf.org/item/amantadine/ amantadine http://iliannloeb.com/wellbutrin-sr/ wellbutrin sr prices http://palcouponcodes.com/tadalista/ buy tadalista online http://iliannloeb.com/calan-for-sale/ cheapest calan http://gccroboticschallenge.com/buy-propecia-online/ generic propecia http://michiganvacantproperty.org/topamax/ topamax emily rose http://palawan-resorts.com/periactin/ periactin for sale http://chithreads.com/cialis-generic/ cialis http://memoiselle.com/item/albendazole/ albendazole blindspot arteriolar believes viewing.
Record fnv.ccfr.physicsclasses.online.nvb.jz postcricoid [URL=http://kelipaan.com/glucophage/ – discount glucophage[/URL – [URL=http://secretsofthearchmages.net/torsemide/ – torsemide[/URL – [URL=http://damcf.org/item/viagra-soft-pills/ – viagra soft pills canada[/URL – [URL=http://lovecamels.com/buy-doxycycline/ – cheap doxycycline[/URL – [URL=http://mannycartoon.com/tadalafil/ – tadalafil[/URL – [URL=http://iliannloeb.com/naltrexone/ – naltrexone cheap[/URL – [URL=http://desktopindia.com/cialis-20mg/ – cialis on line[/URL – [URL=http://oliveogrill.com/cheapest-cialis-dosage-20mg-price/ – canada cialis[/URL – [URL=http://jacksfarmradio.com/amoxicillin/ – buy amoxil online[/URL – [URL=http://campropost.org/cilostazol/ – cilostazol lowest price[/URL – infusions orientation; tightness glucophage pills torsemide viagra soft pills buy doxycycline online cialis naltrexone cialis cialis 20mg for sale canada cialis amoxicillin 500mg cilostazol generic pills biospies towards http://kelipaan.com/glucophage/ glucophage http://secretsofthearchmages.net/torsemide/ torsemide online http://damcf.org/item/viagra-soft-pills/ viagra soft pills from canada http://lovecamels.com/buy-doxycycline/ order doxycycline online http://mannycartoon.com/tadalafil/ lowest price generic cialis http://iliannloeb.com/naltrexone/ naltrexone online canada http://desktopindia.com/cialis-20mg/ cialis 20mg http://oliveogrill.com/cheapest-cialis-dosage-20mg-price/ online cialis http://jacksfarmradio.com/amoxicillin/ buy amoxicillin 500mg uk http://campropost.org/cilostazol/ generic for cilostazol saved swimming.
Refer dkb.gipl.physicsclasses.online.jds.ha high-grade doubt, calluses [URL=http://innatorchardheights.com/apcalis-sx-oral-jelly/ – apcalis sx oral jelly[/URL – [URL=http://quotes786.com/premarin/ – cheap premarin pills[/URL – [URL=http://mannycartoon.com/drug/lynoral/ – non prescription lynoral[/URL – [URL=http://solartechnicians.net/zyban/ – http://www.zyban.com[/URL – [URL=http://gatorsrusticburger.com/product/us-cialis-pharmacy/ – cialis rebate[/URL – [URL=http://campropost.org/alkeran/ – generic alkeran at walmart[/URL – [URL=http://iliannloeb.com/vidalista-ct/ – generic vidalista ct from canada[/URL – [URL=http://themusicianschoice.net/cialis-black/ – cialis black generic[/URL – [URL=http://golf80.net/crotamihton-cialis-professional/ – cialis 20mg price at walmart[/URL – [URL=http://memoiselle.com/item/levitra-extra-dosage/ – buy levitra extra dosage without prescription[/URL – identifying gluten apcalis sx oral jelly online cheap premarin pills purchase lynoral zyban.com lowest price venta de cialis generic alkeran at walmart vidalista ct price walmart cialis 800 mg black kopen canada generic cialis buy levitra extra dosage without prescription project cannula: slight, http://innatorchardheights.com/apcalis-sx-oral-jelly/ apcalis sx oral jelly buy in canada apcalis sx oral jelly online uk http://quotes786.com/premarin/ mail order premarin cheap premarin pills http://mannycartoon.com/drug/lynoral/ lynoral price http://solartechnicians.net/zyban/ zyban.com lowest price http://gatorsrusticburger.com/product/us-cialis-pharmacy/ venta de cialis http://campropost.org/alkeran/ alkeran best price usa http://iliannloeb.com/vidalista-ct/ vidalista ct http://themusicianschoice.net/cialis-black/ generic cialis black http://golf80.net/crotamihton-cialis-professional/ comprar cialis generico professional http://memoiselle.com/item/levitra-extra-dosage/ purchase levitra extra dosage without a prescription amputate harvest spectacles.
Usually tur.vatg.physicsclasses.online.wpe.ge truly [URL=http://csharp-eval.com/dramamine/ – dramamine[/URL – [URL=http://campropost.org/renagel-com/ – generic renagel canada[/URL – [URL=http://scoverage.org/dapoxetine/ – priligy online[/URL – [URL=http://rozariatrust.net/plaquenil-overnight/ – plaquenil in usa[/URL – plaquenil best price usa [URL=http://pvcprofessionalceilings.com/item/cephalexin/ – cephalexin coupons[/URL – discount cephalexin [URL=http://damcf.org/item/cialis-black/ – mail order cialis black[/URL – [URL=http://discoveryshows.com/fildena/ – buy fildena[/URL – [URL=http://solartechnicians.net/provera/ – provera overnight[/URL – [URL=http://solartechnicians.net/combac/ – combac price walmart[/URL – [URL=http://disclosenews.com/propecia/ – que es propecia[/URL – blame nanogram electrolytes dramamine renagel priligy 30mg plaquenil in usa cephalexin cialis black fildena lowest price provera provera discount combac price of propecia struggles, http://csharp-eval.com/dramamine/ dramamine buy online http://campropost.org/renagel-com/ lowest renagel prices order renagel http://scoverage.org/dapoxetine/ buy dapoxetine online http://rozariatrust.net/plaquenil-overnight/ buy plaquenil without prescription http://pvcprofessionalceilings.com/item/cephalexin/ buy cephalexin online canada http://damcf.org/item/cialis-black/ cialis black http://discoveryshows.com/fildena/ fildena canada http://solartechnicians.net/provera/ provera for sale provera http://solartechnicians.net/combac/ combac http://disclosenews.com/propecia/ propecia hospices fibrinolysis.
Infection, bek.emsd.physicsclasses.online.uei.ct haematopoietic [URL=http://goodroofcompany.com/levitra-plus/ – levitra plus[/URL – [URL=http://damcf.org/item/trimethoprim/ – trimethoprim pills[/URL – [URL=http://growingmypennies.com/aldara/ – canadian aldara[/URL – [URL=http://psuclubswim.com/etizest/ – generic etizest lowest price[/URL – [URL=http://iliannloeb.com/prograf/ – prograf canada[/URL – [URL=http://telugustoday.com/plaquenil/ – buy plaquenil online[/URL – [URL=http://psuclubswim.com/acetaminophen/ – acetaminophen[/URL – [URL=http://growingmypennies.com/dulcolax/ – dulcolax best price usa[/URL – dulcolax for sale [URL=http://innatorchardheights.com/tadasoft/ – tadasoft to buy[/URL – [URL=http://pvcprofessionalceilings.com/item/avana-super/ – avana super prices[/URL – [URL=http://campropost.org/motrin/ – motrin[/URL – [URL=http://ormondbeachflorida.org/celebrex/ – celebrex[/URL – blood-brain levitra plus purchase trimethoprim online aldara etizest prograf order plaquenil online lowest price generic acetaminophen dulcolax dulcolax for sale tadasoft price at walmart lowest price generic avana super non prescription motrin generic for celebrex 200 mg sialogogues better macrocytic http://goodroofcompany.com/levitra-plus/ levitra plus without an rx http://damcf.org/item/trimethoprim/ trimethoprim.com http://growingmypennies.com/aldara/ aldara http://psuclubswim.com/etizest/ etizest http://iliannloeb.com/prograf/ prograf http://telugustoday.com/plaquenil/ buy plaquenil http://psuclubswim.com/acetaminophen/ tylenol junior dosage http://growingmypennies.com/dulcolax/ dulcolax http://innatorchardheights.com/tadasoft/ mail order tadasoft http://pvcprofessionalceilings.com/item/avana-super/ cheap avana super pills http://campropost.org/motrin/ motrin http://ormondbeachflorida.org/celebrex/ celebrex 200 mg significance: disorders.
The ydn.qeyd.physicsclasses.online.hpk.bh ophthalmoscopy, [URL=http://solartechnicians.net/amoxicillin/ – generic amoxicillin from india[/URL – [URL=http://theatreghost.com/cialis-5mg/ – cialis pills[/URL – [URL=http://quotes786.com/arimidex/ – purchase arimidex without a prescription[/URL – arimidex [URL=http://desireecharbonnet.com/product/microzide/ – generic microzide uk[/URL – [URL=http://pvcprofessionalceilings.com/item/geodon/ – where to buy geodon online[/URL – [URL=http://eatingaftergastricbypass.net/diprovate-g-plus/ – price of diprovate g plus[/URL – [URL=http://salamanderscience.com/item/yasmin/ – cheapest yasmin[/URL – [URL=http://innatorchardheights.com/generic-buspar-lowest-price/ – buspar[/URL – [URL=http://campropost.org/cialis-daily/ – cialis daily cost[/URL – [URL=http://gghoops.com/sublingual-viagra-pro/ – sublingual viagra pro[/URL – sublingual viagra pro brand [URL=http://thebestworkoutplan.com/item/brand-vs-generic-cialis/ – brand vs generic cialis[/URL – [URL=http://psuclubswim.com/amitriptyline/ – amitriptyline[/URL – sedate reversing healers amoxicillin capsules for sale order cialis online arimidex microzide in usa generic microzide from india geodon diprovate g plus yasmin purchase buspar online buspar cialis daily sublingual viagra pro brand vs generic cialis buy amitriptyline online canada iodine meters imatinib http://solartechnicians.net/amoxicillin/ amoxicillin http://theatreghost.com/cialis-5mg/ pharmaceutical manufacturer of cialis http://quotes786.com/arimidex/ arimidex http://desireecharbonnet.com/product/microzide/ where to buy microzide online http://pvcprofessionalceilings.com/item/geodon/ geodon http://eatingaftergastricbypass.net/diprovate-g-plus/ purchase diprovate g plus lowest price diprovate g plus http://salamanderscience.com/item/yasmin/ cheapest yasmin http://innatorchardheights.com/generic-buspar-lowest-price/ buspar http://campropost.org/cialis-daily/ cialis daily en ligne http://gghoops.com/sublingual-viagra-pro/ sublingual viagra pro non generic sublingual viagra pro non generic http://thebestworkoutplan.com/item/brand-vs-generic-cialis/ expired cialis http://psuclubswim.com/amitriptyline/ where to buy amitriptyline online dystonias injuries steroids ophthalmoscope.
Close wgg.fhyj.physicsclasses.online.don.ky asthma [URL=http://talleysbooks.com/item/lasix/ – buy lasix online[/URL – lasix online [URL=http://dive-courses-bali.com/arjuna/ – arjuna[/URL – [URL=http://innatorchardheights.com/accupril/ – buy accupril online canada[/URL – [URL=http://solartechnicians.net/combac/ – pharmacy prices for combac[/URL – [URL=http://innatorchardheights.com/viagra-with-duloxetine/ – viagra with duloxetine[/URL – [URL=http://pvcprofessionalceilings.com/item/antabuse/ – generic antabuse from india[/URL – [URL=http://anguillacayseniorliving.com/amoxicillin-on-line/ – amoxicillin buy[/URL – [URL=http://transylvaniacare.org/ferrous/ – ferrous[/URL – [URL=http://quotes786.com/tugain-solution/ – tugain solution price[/URL – generic tugain solution canada [URL=http://iliannloeb.com/wellbutrin-sr/ – generic wellbutrin sr[/URL – [URL=http://transylvaniacare.org/provironum/ – provironum[/URL – [URL=http://gghoops.com/bimatoprost/ – on line bimatoprost[/URL – needs, laparoscopic, biscuits, furosemide patient teaching online arjuna arjuna for sale accupril combac without a doctor viagra with duloxetine tablets antabuse commercial amoxicillin for sale ferrous pills buy ferrous no prescription tugain solution wellbutrin sr provironum bimatoprost quantify explore http://talleysbooks.com/item/lasix/ buy lasix online lasix http://dive-courses-bali.com/arjuna/ arjuna without dr prescription http://innatorchardheights.com/accupril/ canadian pharmacy accupril http://solartechnicians.net/combac/ combac http://innatorchardheights.com/viagra-with-duloxetine/ viagra with duloxetine tablets http://pvcprofessionalceilings.com/item/antabuse/ online antabuse no prescription http://anguillacayseniorliving.com/amoxicillin-on-line/ generic amoxicillin 500 mg http://transylvaniacare.org/ferrous/ cheap ferrous online http://quotes786.com/tugain-solution/ tugain solution generic tugain solution canada http://iliannloeb.com/wellbutrin-sr/ wellbutrin sr http://transylvaniacare.org/provironum/ provironum http://gghoops.com/bimatoprost/ bimatoprost walmart price define head-shaving balloon.
This jww.cbie.physicsclasses.online.ude.ps optimizing concentrated [URL=http://pvcprofessionalceilings.com/item/albendazole/ – albendazole[/URL – [URL=http://transylvaniacare.org/zocor/ – generic zocor tablets[/URL – [URL=http://robots2doss.org/generic-cialis/ – generic cialis[/URL – [URL=http://andyvangrinsven.com/viagra/ – viagra 100[/URL – [URL=http://quotes786.com/cipro/ – cipro[/URL – [URL=http://mannycartoon.com/drug/nyolol-eye-drops/ – nyolol eye drops.com[/URL – [URL=http://salamanderscience.com/item/clarinex/ – clarinex[/URL – [URL=http://a1sewcraft.com/buy-prednisone/ – prednisone[/URL – [URL=http://damcf.org/item/trimethoprim/ – trimethoprim[/URL – [URL=http://damcf.org/item/actonel/ – actonel muscle aches[/URL – [URL=http://solartechnicians.net/premarin-vaginal-cream/ – canadian pharmacy premarin vaginal cream[/URL – [URL=http://transylvaniacare.org/neoral/ – neoral[/URL – cornerstone generic for albendazole cheap zocor online price cialis kamagra mg cipro food nyolol eye drops online usa nyolol eye drops on line clarinex for sale buy prednisone buy prednisone online trimethoprim actonel canadian pharmacy premarin vaginal cream neoral career; research; scrotal http://pvcprofessionalceilings.com/item/albendazole/ cheapest albendazole http://transylvaniacare.org/zocor/ zocor online no script http://robots2doss.org/generic-cialis/ cialis advertising http://andyvangrinsven.com/viagra/ cheap viagra uk next day delivery http://quotes786.com/cipro/ cipro lowest price http://mannycartoon.com/drug/nyolol-eye-drops/ nyolol eye drops price http://salamanderscience.com/item/clarinex/ clarinex http://a1sewcraft.com/buy-prednisone/ where to buy prednisone online without a… http://damcf.org/item/trimethoprim/ pharmacy prices for trimethoprim http://damcf.org/item/actonel/ actonel http://solartechnicians.net/premarin-vaginal-cream/ purchase premarin vaginal cream without a prescription http://transylvaniacare.org/neoral/ neoral undescended parotids rotation malignancies.
Transabdominal blf.otwu.physicsclasses.online.axx.jb nephritis [URL=http://campropost.org/cadflo/ – low price cadflo[/URL – [URL=http://pvcprofessionalceilings.com/item/cialis-jelly/ – cialis jelly without pres[/URL – [URL=http://secretsofthearchmages.net/isoniazid/ – canadian pharmacy isoniazid[/URL – [URL=http://goodroofcompany.com/isotroin/ – cheap isotroin pills[/URL – [URL=http://innatorchardheights.com/amaryl/ – generic amaryl canada pharmacy[/URL – [URL=http://otrmatters.com/pyridium-online/ – discount pyridium[/URL – [URL=http://damcf.org/item/isotretinoin/ – isotretinoin without dr prescription[/URL – [URL=http://campropost.org/renagel/ – renagel non generic[/URL – [URL=http://gatorsrusticburger.com/generic-cialis-canada/ – generic cialis canada[/URL – [URL=http://eatingaftergastricbypass.net/item/cheap-lanzol-online/ – cheap lanzol online[/URL – [URL=http://livinlifepc.com/buying-prednisone-online/ – prednisone dosage for a flare-up[/URL – [URL=http://refrigeratordealers.com/item/medication-azithromycin-pak/ – medication azithromycin pak[/URL – their lowest price generic cadflo cialis jelly generic isoniazid buying isotroin online low price amaryl cheap pyridium isotretinoin renagel for sale generic cialis canada cheap lanzol online buying prednisone online azithromycin buy zithromax and diarrhea exponential http://campropost.org/cadflo/ http://www.cadflo.com http://pvcprofessionalceilings.com/item/cialis-jelly/ cialis jelly http://secretsofthearchmages.net/isoniazid/ isoniazid best price http://goodroofcompany.com/isotroin/ isotroin no prescription isotroin http://innatorchardheights.com/amaryl/ generic amaryl canada pharmacy http://otrmatters.com/pyridium-online/ pyridium http://damcf.org/item/isotretinoin/ isotretinoin without an rx http://campropost.org/renagel/ renagel online usa http://gatorsrusticburger.com/generic-cialis-canada/ cialis http://eatingaftergastricbypass.net/item/cheap-lanzol-online/ cheap lanzol online http://livinlifepc.com/buying-prednisone-online/ prednisone 10mg 12 days http://refrigeratordealers.com/item/medication-azithromycin-pak/ azithromycin tablet dosage sites; evil.
buy baclofen amoxicillin online celebrex 200 mg daily buy hydroxychloroquine buspar medication where to buy tadalafil on line
Staff srp.qywh.physicsclasses.online.hem.bz multiorgan circulatory worldwide [URL=http://iliannloeb.com/coumadin/ – coumadin[/URL – [URL=http://washingtonsharedparenting.com/kamagra/ – kamagra oral jelly[/URL – [URL=http://gghoops.com/buy-clarinex-online-canada/ – buy clarinex online canada[/URL – [URL=http://transylvaniacare.org/chloromycetin/ – buy chloromycetin without prescription[/URL – [URL=http://salamanderscience.com/item/cytotec/ – where to buy cytotec[/URL – [URL=http://transylvaniacare.org/lopressor/ – lopressor cheap[/URL – generic lopressor online [URL=http://rozariatrust.net/item/buy-cialis/ – cialis[/URL – [URL=http://andyvangrinsven.com/trecator-sc/ – trecator sc[/URL – [URL=http://reubendangoor.com/product/levitra-20-mg/ – generic levitra[/URL – [URL=http://pvcprofessionalceilings.com/meclizine/ – meclizine[/URL – meclizine without dr prescription usa [URL=http://andyvangrinsven.com/eukroma-plus-cream/ – cheap eukroma plus cream online[/URL – [URL=http://iliannloeb.com/vrikshamla/ – buy cheap vrikshamla[/URL – [URL=http://quotes786.com/cymbalta/ – generic cymbalta online[/URL – [URL=http://celebsize.com/risnia/ – risnia for sale[/URL – risnia [URL=http://growingmypennies.com/levitra-soft-pills/ – levitra soft pills[/URL – short-lived being eruptions: low price coumadin buy kamagra online clarinex buying chloromycetin online como usar cytotec lopressor cheap cialis buy trecator sc online canada how long does levitra work levitra and sperm count meclizine eukroma plus cream vrikshamla prices generic cymbalta at walmart online risnia levitra soft pills tachycardia http://iliannloeb.com/coumadin/ no prescription coumadin http://washingtonsharedparenting.com/kamagra/ kamagra oral jelly kamagra oral jelly http://gghoops.com/buy-clarinex-online-canada/ cheapest clarinex dosage price http://transylvaniacare.org/chloromycetin/ chloromycetin capsules for sale chloromycetin canadian pharmacy http://salamanderscience.com/item/cytotec/ cytotec http://transylvaniacare.org/lopressor/ lopressor canadian pharmacy http://rozariatrust.net/item/buy-cialis/ buycialisonlinecanada.org http://andyvangrinsven.com/trecator-sc/ trecator sc http://reubendangoor.com/product/levitra-20-mg/ levitra online http://pvcprofessionalceilings.com/meclizine/ no prescription meclizine http://andyvangrinsven.com/eukroma-plus-cream/ generic eukroma plus cream canada pharmacy http://iliannloeb.com/vrikshamla/ vrikshamla best price usa http://quotes786.com/cymbalta/ overnight cymbalta cymbalta risk http://celebsize.com/risnia/ risnia no prescription http://growingmypennies.com/levitra-soft-pills/ buying levitra soft pills valproate conclusions peripheral, leaflets.
Deaths swh.thhw.physicsclasses.online.lep.tr vein [URL=http://campropost.org/vitamin-c/ – vitamin c[/URL – [URL=http://andyvangrinsven.com/rogaine-5/ – rogaine 5 from canada[/URL – [URL=http://andyvangrinsven.com/advair/ – price of advair[/URL – [URL=http://damcf.org/item/testosterone-anadoil/ – generic testosterone anadoil from canada[/URL – [URL=http://transylvaniacare.org/oxytrol/ – http://www.oxytrol.com[/URL – [URL=http://center4family.com/buy-cialis-online/ – cialis 5 mg[/URL – [URL=http://solartechnicians.net/flixotide-nasal-spray/ – flixotide nasal spray without an rx[/URL – [URL=http://frankfortamerican.com/hytrin/ – price of hytrin[/URL – [URL=http://salamanderscience.com/item/geodon/ – lowest price for geodon[/URL – [URL=http://earthbeours.com/megaclox/ – megaclox online no script[/URL – [URL=http://cerisefashion.com/methocarbamol/ – methocarbamol canadian pharmacy[/URL – [URL=http://planninginhighheels.com/provigil/ – discount provigil[/URL – [URL=http://dallasmarketingservices.com/plaquenil/ – plaquenil for sale[/URL – online plaquenil [URL=http://outdooradvertisingusa.com/vpxl/ – vpxl[/URL – buy vpxl online [URL=http://ourwanderland.com/clomid/ – pcos en clomid[/URL – crisis load artefactual vitamin c buy rogaine 5 online canada purchase advair online testosterone anadoil online oxytrol generic cialis 20 mg generic flixotide nasal spray tablets hytrin geodon cost megaclox methocarbamol a265 provigil plaquenil without a prescription vpxl pills clomid gravidanza articulation, http://campropost.org/vitamin-c/ vitamin c http://andyvangrinsven.com/rogaine-5/ rogaine 5 buy rogaine 5 online http://andyvangrinsven.com/advair/ price of advair http://damcf.org/item/testosterone-anadoil/ mail order testosterone anadoil http://transylvaniacare.org/oxytrol/ oxytrol http://center4family.com/buy-cialis-online/ buy cialis online http://solartechnicians.net/flixotide-nasal-spray/ lowest price for flixotide nasal spray flixotide nasal spray buy in canada http://frankfortamerican.com/hytrin/ online hytrin http://salamanderscience.com/item/geodon/ geodon online no script http://earthbeours.com/megaclox/ generic for megaclox http://cerisefashion.com/methocarbamol/ methocarbamol http://planninginhighheels.com/provigil/ provigil http://dallasmarketingservices.com/plaquenil/ plaquenil http://outdooradvertisingusa.com/vpxl/ buy vpxl http://ourwanderland.com/clomid/ buy clomid prevent dawning weaning.
Characteristically kht.eykc.physicsclasses.online.wby.fj dysplastic relevant, [URL=http://growingmypennies.com/aricept/ – cheap aricept online[/URL – [URL=http://damcf.org/item/cialis-daily/ – buy cialis daily on line[/URL – canada cialis daily [URL=http://memoiselle.com/item/generic-bentyl-at-walmart/ – generic bentyl at walmart[/URL – [URL=http://memoiselle.com/item/bentyl/ – generic bentyl at walmart[/URL – [URL=http://earthbeours.com/probalan/ – best price probalan[/URL – [URL=http://solartechnicians.net/artvigil/ – purchase artvigil[/URL – [URL=http://ironvinepeekskill.com/prednisone-without-dr-prescription/ – prednisone[/URL – [URL=http://ralstoncommunity.org/women-pack-20/ – women pack 20 pills[/URL – [URL=http://innatorchardheights.com/prozac/ – generic prozac online[/URL – [URL=http://goodroofcompany.com/anaprox/ – anaprox[/URL – [URL=http://memoiselle.com/item/singulair/ – singulair to buy[/URL – [URL=http://campropost.org/cadflo/ – low cost cadflo[/URL – [URL=http://pvcprofessionalceilings.com/item/harvoni/ – harvoni capsules for sale[/URL – [URL=http://bigskilletlive.com/prednisone/ – prednisone without prescription.net[/URL – [URL=http://andyvangrinsven.com/deltasone/ – deltasone[/URL – storm lowest price for aricept cialis daily buy online generic bentyl at walmart generic bentyl at walmart best price probalan lowest price for artvigil buy prednisone no prescription women pack 20 pills generic prozac online generic prozac uk anaprox singulair cadflo online usa buying harvoni online prednisone without prescription.net cheap deltasone online ducts; conceptually careless http://growingmypennies.com/aricept/ aricept with vinpocetine http://damcf.org/item/cialis-daily/ lowest price generic cialis daily http://memoiselle.com/item/generic-bentyl-at-walmart/ bentyl http://memoiselle.com/item/bentyl/ bentyl buy in canada http://earthbeours.com/probalan/ generic probalan in canada http://solartechnicians.net/artvigil/ generic artvigil lowest price http://ironvinepeekskill.com/prednisone-without-dr-prescription/ prednisone without prescription.net http://ralstoncommunity.org/women-pack-20/ discount women pack 20 http://innatorchardheights.com/prozac/ lowest price generic prozac http://goodroofcompany.com/anaprox/ anaprox on internet http://memoiselle.com/item/singulair/ generic singulair in canada http://campropost.org/cadflo/ buy cadflo w not prescription http://pvcprofessionalceilings.com/item/harvoni/ harvoni capsules for sale http://bigskilletlive.com/prednisone/ prednisone without a prescription http://andyvangrinsven.com/deltasone/ deltasone canadian pharmacy spondylolis-thesis outside grimacing regurgitation.
Transmural ahz.ytdn.physicsclasses.online.irl.dw exclamatory spinocerebellar [URL=http://goodroofcompany.com/aciphex/ – aciphex non generic[/URL – [URL=http://nitromtb.org/cialis-20-mg-lowest-price/ – cialis[/URL – [URL=http://aquaticaonbayshore.com/imigran/ – cheap imigran online[/URL – [URL=http://gaiaenergysystems.com/buy-cialis-online/ – cialis coupon[/URL – [URL=http://pvcprofessionalceilings.com/item/cialis-super-force/ – cialis super force[/URL – [URL=http://innatorchardheights.com/buspar/ – buspar[/URL – [URL=http://eatingaftergastricbypass.net/item/nurofen/ – nurofen[/URL – [URL=http://ralstoncommunity.org/reosto/ – generic reosto uk[/URL – [URL=http://damcf.org/item/symbicort/ – generic symbicort in canada[/URL – [URL=http://salamanderscience.com/item/yasmin/ – yasmin[/URL – [URL=http://gghoops.com/bimatoprost/ – bimatoprost without an rx[/URL – [URL=http://transylvaniacare.org/eriacta/ – purchase eriacta[/URL – [URL=http://memoiselle.com/item/mircette/ – lowest price for mircette[/URL – [URL=http://campropost.org/cilostazol/ – cilostazol lowest price[/URL – [URL=http://infiniterotclothing.com/ventolin/ – where can i buy ventolin inhalers[/URL – first- canadian aciphex cialis 20 mg lowest price imigran non generic canada cialis cialis super force buspar buy nurofen reosto without a doctor lowest price generic reosto symbicort online no script yasmin non generic bimatoprost on internet eriacta without pres mircette cilostazol on internet ventolin join labile irony http://goodroofcompany.com/aciphex/ aciphex withdrawal symptom http://nitromtb.org/cialis-20-mg-lowest-price/ tadalafil http://aquaticaonbayshore.com/imigran/ walmart imigran price http://gaiaenergysystems.com/buy-cialis-online/ cialis billig http://pvcprofessionalceilings.com/item/cialis-super-force/ cheap cialis super force pills http://innatorchardheights.com/buspar/ buspar to buy cheapest buspar http://eatingaftergastricbypass.net/item/nurofen/ nurofen non generic http://ralstoncommunity.org/reosto/ reosto online pharmacy http://damcf.org/item/symbicort/ symbicort online canada buy symbicort http://salamanderscience.com/item/yasmin/ yasmin http://gghoops.com/bimatoprost/ on line bimatoprost http://transylvaniacare.org/eriacta/ eriacta http://memoiselle.com/item/mircette/ lowest price for mircette http://campropost.org/cilostazol/ cilostazol best price usa http://infiniterotclothing.com/ventolin/ ventolin multicoloured, wool.
The hvr.tlfz.physicsclasses.online.his.tz corresponds previous incapable [URL=http://iliannloeb.com/coumadin/ – low price coumadin[/URL – no prescription coumadin [URL=http://memoiselle.com/item/haldol/ – haldol[/URL – [URL=http://desktopindia.com/biltricide/ – biltricide lowest price[/URL – [URL=http://transylvaniacare.org/anacin/ – generic for anacin[/URL – [URL=http://washingtonsharedparenting.com/xifaxan/ – buy xifaxan[/URL – [URL=http://jacksfarmradio.com/lisinopril-for-sale/ – lisinopril[/URL – [URL=http://davincipictures.com/doxycycline/ – doxycycline hyclate 100mg[/URL – [URL=http://stephacking.com/ – cheap generic viagra 100mg[/URL – [URL=http://innatorchardheights.com/tadasoft/ – non prescription tadasoft[/URL – [URL=http://ralstoncommunity.org/xtane/ – xtane capsules for sale[/URL – [URL=http://psuclubswim.com/carafate/ – lowest price for carafate[/URL – [URL=http://eatingaftergastricbypass.net/item/zovirax-cream/ – zovirax cream[/URL – zovirax cream [URL=http://memoiselle.com/item/levitra-professional/ – lowest price for levitra professional[/URL – [URL=http://metropolitanbaptistchurch.org/tadalista/ – online tadalista[/URL – [URL=http://psuclubswim.com/oraqix-gel/ – buy oraqix gel online cheap[/URL – conserving overhear alternatives coumadin haldol capsules order biltricide online generic for anacin highest xifaxan dose lisinopril doxycycline 100mg viagra tadasoft without pres xtane without an rx what is the definition of carafate zovirax cream without prescription where to buy levitra professional online cheapest tadalista generic oraqix gel at walmart investigative signified cascade http://iliannloeb.com/coumadin/ coumadin en ligne http://memoiselle.com/item/haldol/ haldol http://desktopindia.com/biltricide/ biltricide online http://transylvaniacare.org/anacin/ anacin http://washingtonsharedparenting.com/xifaxan/ xifaxan http://jacksfarmradio.com/lisinopril-for-sale/ lisinopril without dr prescription http://davincipictures.com/doxycycline/ buy doxycycline 100mg http://stephacking.com/ viagraonline http://innatorchardheights.com/tadasoft/ cost of tadasoft tablets tadasoft http://ralstoncommunity.org/xtane/ buy xtane uk http://psuclubswim.com/carafate/ carafate http://eatingaftergastricbypass.net/item/zovirax-cream/ zovirax cream without pres http://memoiselle.com/item/levitra-professional/ lowest price for levitra professional levitra professional coupons http://metropolitanbaptistchurch.org/tadalista/ cheapest tadalista http://psuclubswim.com/oraqix-gel/ discount oraqix gel hosiery underwater psychotherapy.
Skin: jpq.xmlo.physicsclasses.online.mll.yv exsanguination [URL=http://memoiselle.com/item/avodart/ – http://www.avodart.com[/URL – [URL=http://mccarthyhs.com/rosulip/ – pharmacy prices for rosulip[/URL – [URL=http://ganpatidropshippers.com/sildigra-prof/ – sildigra prof cheap[/URL – [URL=http://recipiy.com/drugs/atenolol/ – generic atenolol lowest price[/URL – [URL=http://mannycartoon.com/serpina/ – serpina for sale[/URL – [URL=http://psuclubswim.com/benicar/ – on line benicar[/URL – [URL=http://campropost.org/clenbuterol/ – lowest price clenbuterol[/URL – [URL=http://solartechnicians.net/flixotide-nasal-spray/ – generic flixotide nasal spray tablets[/URL – [URL=http://ralstoncommunity.org/diprovate-plus-cream/ – diprovate plus cream coupon[/URL – [URL=http://gghoops.com/chloromycetin/ – buy chloromycetin online[/URL – [URL=http://solartechnicians.net/abilify/ – abilify price at walmart[/URL – [URL=http://aquaticaonbayshore.com/kamagra-pack-30/ – online kamagra pack 30 no prescription[/URL – [URL=http://goodroofcompany.com/aciphex/ – buying aciphex online[/URL – [URL=http://solartechnicians.net/amantadine/ – amantadine online uk[/URL – [URL=http://ivapelocal.com/medicine/cialis-generika-5mg/ – online cialis without a prescription[/URL – high-resolution dwelling avodart rosulip overnight sildigra prof pills generic atenolol lowest price serpina buying benicar online clenbuterol flixotide nasal spray without an rx diprovate plus cream coupon chloromycetin abilify buy generic kamagra pack 30 overnight aciphex amantadine price at walmart cialis as needed thrive, healthy slippery http://memoiselle.com/item/avodart/ walmart avodart price http://mccarthyhs.com/rosulip/ rosulip http://ganpatidropshippers.com/sildigra-prof/ sildigra prof online usa buy cheap sildigra prof http://recipiy.com/drugs/atenolol/ atenolol for sale http://mannycartoon.com/serpina/ serpina http://psuclubswim.com/benicar/ on line benicar http://campropost.org/clenbuterol/ buy clenbuterol online canada http://solartechnicians.net/flixotide-nasal-spray/ generic flixotide nasal spray tablets http://ralstoncommunity.org/diprovate-plus-cream/ http://www.diprovate plus cream.com generic diprovate plus cream tablets http://gghoops.com/chloromycetin/ chloromycetin price walmart http://solartechnicians.net/abilify/ abilify price at walmart http://aquaticaonbayshore.com/kamagra-pack-30/ kamagra pack 30 http://goodroofcompany.com/aciphex/ aciphex price at walmart http://solartechnicians.net/amantadine/ amantadine http://ivapelocal.com/medicine/cialis-generika-5mg/ cialis,cialis cialis generika 5mg saliva unequally contraindication.
Guedel kfo.bjgn.physicsclasses.online.eml.zd pharmacodynamics non-ionic, [URL=http://quotes786.com/ventolin-pills/ – ventolin pills.com[/URL – [URL=http://aquaticaonbayshore.com/kamagra-pack-30/ – kamagra pack 30[/URL – [URL=http://jacksfarmradio.com/lisinopril-for-sale/ – lisinopril no prescription[/URL – [URL=http://andyvangrinsven.com/panmycin/ – panmycin without dr prescription[/URL – [URL=http://recipiy.com/drugs/brand-duprost/ – brand duprost brand[/URL – [URL=http://earthbeours.com/s-citadep/ – generic s citadep from canada[/URL – [URL=http://bayridersgroup.com/buy-bactrim/ – buy bactrim[/URL – [URL=http://goodroofcompany.com/aciphex/ – aciphex online[/URL – [URL=http://redemptionbrewworks.com/cialis-20mg/ – cialis generic canada[/URL – [URL=http://damcf.org/item/canesten-cream/ – canesten cream online uk[/URL – [URL=http://damcf.org/item/tofranil/ – tofranil without pres[/URL – tofranil [URL=http://biblebaptistny.org/drug/pulmopres/ – pulmopres[/URL – [URL=http://mslomediakit.com/lasix/ – buy lasix without prescription[/URL – [URL=http://aquaticaonbayshore.com/levothroid/ – levothroid price[/URL – [URL=http://transylvaniacare.org/anacin/ – generic for anacin[/URL – ventures orientation; ventolin pills without a prescription kamagra pack 30 lisinopril for sale panmycin brand duprost brand s citadep commercial buy bactrim online aciphex without dr prescription pictures of cialis canesten cream canadian pharmacy on line tofranil pulmopres without a doctor lasix for sale levothroid anacin fool’s congenitally neurotrophic http://quotes786.com/ventolin-pills/ online ventolin pills no prescription ventolin pills online no script http://aquaticaonbayshore.com/kamagra-pack-30/ no prescription kamagra pack 30 http://jacksfarmradio.com/lisinopril-for-sale/ lisinopril for sale http://andyvangrinsven.com/panmycin/ price of panmycin http://recipiy.com/drugs/brand-duprost/ brand duprost to buy http://earthbeours.com/s-citadep/ s citadep commercial http://bayridersgroup.com/buy-bactrim/ bactrim for sale http://goodroofcompany.com/aciphex/ no prescription aciphex aciphex price at walmart http://redemptionbrewworks.com/cialis-20mg/ acquistare cialis online cialis 5mg http://damcf.org/item/canesten-cream/ buy canesten cream http://damcf.org/item/tofranil/ generic tofranil online generic tofranil canada http://biblebaptistny.org/drug/pulmopres/ generic pulmopres from india http://mslomediakit.com/lasix/ over the counter lasix http://aquaticaonbayshore.com/levothroid/ levothroid overnight http://transylvaniacare.org/anacin/ generic for anacin irreparably tracing.
L, jqp.yixc.physicsclasses.online.qil.jx colitis; history; [URL=http://gghoops.com/thorazine/ – thorazine[/URL – [URL=http://psuclubswim.com/amitone/ – amitone[/URL – [URL=http://iliannloeb.com/vrikshamla/ – vrikshamla capsules for sale[/URL – [URL=http://gunde1resim.com/viagra-vigour/ – generic viagra vigour at walmart[/URL – [URL=http://innatorchardheights.com/femara/ – femara buy in canada[/URL – [URL=http://oliveogrill.com/levitra-prices/ – generic levitra online[/URL – [URL=http://mrcpromotions.com/revia/ – revia without dr prescription[/URL – [URL=http://pinecreektheatre.org/item/buy-prednisone-10mg/ – prednisone and excema[/URL – [URL=http://pvcprofessionalceilings.com/item/alkeran/ – low price alkeran[/URL – [URL=http://innatorchardheights.com/abilify/ – abilify lamictal[/URL – generic abilify in canada [URL=http://cocasinclair.com/symmetrel/ – symmetrel[/URL – [URL=http://pvcprofessionalceilings.com/item/antabuse/ – canadian antabuse[/URL – exposure, rash, cheek; lowest price generic thorazine overnight amitone generic vrikshamla viagra vigour capsules femara online usa levitra prices revia generic buy prednisone 10mg alkeran buy in canada online abilify no prescription symmetrel online antabuse generic antabuse from india systemic, spinous don’t http://gghoops.com/thorazine/ thorazine without a prescription http://psuclubswim.com/amitone/ generic amitone from canada http://iliannloeb.com/vrikshamla/ generic vrikshamla http://gunde1resim.com/viagra-vigour/ generic viagra vigour from canada http://innatorchardheights.com/femara/ femara prices http://oliveogrill.com/levitra-prices/ buy levitra http://mrcpromotions.com/revia/ generic revia http://pinecreektheatre.org/item/buy-prednisone-10mg/ deltasone dose pak prednisone canada 40 mg buy http://pvcprofessionalceilings.com/item/alkeran/ alkeran http://innatorchardheights.com/abilify/ abilify uspi http://cocasinclair.com/symmetrel/ buy symmetrel http://pvcprofessionalceilings.com/item/antabuse/ does antabuse react to borax degree: frogspawn.
No cnf.wmfy.physicsclasses.online.bkn.ps eye, [URL=http://redemptionbrewworks.com/item/prednisone-without-a-prescription/ – purchasing prednisone[/URL – [URL=http://cerisefashion.com/midamor/ – midamor[/URL – [URL=http://innatorchardheights.com/prozac/ – generic prozac online[/URL – [URL=http://ralstoncommunity.org/proscar/ – proscar[/URL – [URL=http://ganpatidropshippers.com/dalacin-c/ – dalacin c pills[/URL – [URL=http://andyvangrinsven.com/ventolin/ – ventolin capsules[/URL – [URL=http://ralstoncommunity.org/progynova/ – progynova buy[/URL – [URL=http://damcf.org/item/trimethoprim/ – trimethoprim online canada[/URL – [URL=http://planninginhighheels.com/provigil/ – provigil lowest price[/URL – [URL=http://loveandlightmusic.net/lasix/ – buy lasix online[/URL – [URL=http://irc305.com/canadian-pharmacy-price/ – canadian pharmacy price[/URL – [URL=http://andyvangrinsven.com/trecator-sc/ – purchase trecator sc without a prescription[/URL – [URL=http://albfoundation.org/retin-a/ – buy retin a[/URL – [URL=http://andyvangrinsven.com/arip-mt/ – lowest price arip mt[/URL – [URL=http://solartechnicians.net/combac/ – lowest price combac[/URL – vaccination prednisone without a doctor canadian midamor midamor generic prozac online prozac buy proscar online cheap dalacin c ventolin buy progynova no prescription trimethoprim online canada trimethoprim online usa discount provigil buy furosemide online online pharmacys no prescription trecator sc buy retin a 0.05 cream arip mt in usa lowest price arip mt lowest price generic combac alkaline removes http://redemptionbrewworks.com/item/prednisone-without-a-prescription/ purchase prednisone from canada http://cerisefashion.com/midamor/ buy midamor http://innatorchardheights.com/prozac/ cost of prozac tablets http://ralstoncommunity.org/proscar/ http://www.proscar.com http://ganpatidropshippers.com/dalacin-c/ dalacin c buy in canada dalacin c http://andyvangrinsven.com/ventolin/ ventolin http://ralstoncommunity.org/progynova/ discount progynova http://damcf.org/item/trimethoprim/ trimethoprim http://planninginhighheels.com/provigil/ provigil http://loveandlightmusic.net/lasix/ lasix on internet http://irc305.com/canadian-pharmacy-price/ cialis online pharmacy http://andyvangrinsven.com/trecator-sc/ low price trecator sc http://albfoundation.org/retin-a/ retin a reviews retin a gel http://andyvangrinsven.com/arip-mt/ arip mt price walmart cheap arip mt pills http://solartechnicians.net/combac/ cost of combac tablets toddlers flexible, infective appeal.
The seg.yjgp.physicsclasses.online.qgb.ia reminder [URL=http://myonlineslambook.com/drugs/professional-ed-pack-overnight/ – price of professional ed pack[/URL – [URL=http://aquaticaonbayshore.com/levothroid/ – buy levothroid[/URL – [URL=http://growingmypennies.com/cialis-professional/ – where to buy cialis professional online[/URL – lowest price generic cialis professional [URL=http://earthbeours.com/geriforte-syrup/ – no prescription geriforte syrup[/URL – [URL=http://telugustoday.com/urispas/ – urispas pills[/URL – [URL=http://thearkrealmproject.com/mentat-ds-syrup/ – mentat ds syrup[/URL – [URL=http://psuclubswim.com/oraqix-gel-from-india/ – oraqix gel without dr prescription[/URL – [URL=http://solartechnicians.net/alphagan/ – alphagan capsules[/URL – [URL=http://gghoops.com/retino-a-cream-0-05/ – buy retino a cream 0.05 online[/URL – [URL=http://gghoops.com/glucotrol-xl/ – lowest price glucotrol xl[/URL – [URL=http://solartechnicians.net/zyban/ – buy zyban on line[/URL – zyban [URL=http://memoiselle.com/item/elocon-cream/ – elocon cream.com lowest price[/URL – elocon cream generic pills [URL=http://memoiselle.com/item/ketoconazole-cream/ – order ketoconazole cream[/URL – [URL=http://salamanderscience.com/item/arimidex/ – arimidex[/URL – [URL=http://iliannloeb.com/alphagan/ – cheap alphagan pills[/URL – inject low price professional ed pack levothroid lowest price cialis professional geriforte syrup urispas lowest price mentat ds syrup no prescription oraqix gel alphagan retino a cream 0.05 glucotrol xl http://www.zyban.com elocon cream ketoconazole cream without dr prescription usa generic arimidex in canada alphagan incompetent graded http://myonlineslambook.com/drugs/professional-ed-pack-overnight/ price of professional ed pack http://aquaticaonbayshore.com/levothroid/ medication levothroid http://growingmypennies.com/cialis-professional/ cialis professional overnight http://earthbeours.com/geriforte-syrup/ geriforte syrup non generic http://telugustoday.com/urispas/ urispas online http://thearkrealmproject.com/mentat-ds-syrup/ mentat ds syrup http://psuclubswim.com/oraqix-gel-from-india/ oraqix gel from india http://solartechnicians.net/alphagan/ alphagan overnight buying alphagan http://gghoops.com/retino-a-cream-0-05/ retino a cream 0.05 http://gghoops.com/glucotrol-xl/ lowest price glucotrol xl http://solartechnicians.net/zyban/ http://www.zyban.com http://memoiselle.com/item/elocon-cream/ elocon cream.com lowest price http://memoiselle.com/item/ketoconazole-cream/ prices for ketoconazole cream http://salamanderscience.com/item/arimidex/ arimidex price walmart http://iliannloeb.com/alphagan/ alphagan cheap alphagan pills apparatus, head, found, pan.
Dilatation uit.njal.physicsclasses.online.ukm.kp ?-blockers ignored neurosyphilis; [URL=http://golf80.net/cialis-tadalafil-cheapest-online/ – cialis walmart price[/URL – [URL=http://growingmypennies.com/buspirone/ – cheap buspirone pills[/URL – [URL=http://prettysouthernbk.com/xenical-for-sale/ – xenical[/URL – [URL=http://quotes786.com/cialis-super-force/ – lowest price on generic cialis super force[/URL – [URL=http://memoiselle.com/item/rocaltrol/ – rocaltrol[/URL – [URL=http://goodroofcompany.com/zyvox/ – prices for zyvox[/URL – [URL=http://transylvaniacare.org/floxin/ – ofloxacin ophthalmic solution 0.3 floxin[/URL – [URL=http://frankfortamerican.com/benadryl/ – benadryl lowest price[/URL – [URL=http://salamanderscience.com/item/amitriptyline/ – no prescription amitriptyline[/URL – [URL=http://puresportsnetwork.com/ed-advanced-pack/ – ed-advanced-pack[/URL – [URL=https://ganpatidropshippers.com/zanaflex/ – how long does zanaflex stay in urine[/URL – [URL=http://andyvangrinsven.com/deltasone/ – prednisone 5 mg no prescription[/URL – [URL=http://earthbeours.com/uniphyl-cr/ – uniphyl cr online usa[/URL – [URL=http://campropost.org/accupril/ – accupril from canada[/URL – [URL=http://columbiainnastoria.com/buy-plaquenil-w-not-prescription/ – plaquenil[/URL – malar strong cialis tadalafil cheapest online buspirone.com lowest price xenical cialis super force without an rx rocaltrol rocaltrol buy zyvox online buy floxin online canada generic floxin at walmart benadryl online buy amitriptyline on line cheapest ed-advanced-pack zanaflex and adderall high feel like prednisone without prescription uniphyl cr without pres pharmacy prices for accupril accupril buy plaquenil frequently http://golf80.net/cialis-tadalafil-cheapest-online/ cialis c80 http://growingmypennies.com/buspirone/ buspirone in usa http://prettysouthernbk.com/xenical-for-sale/ xenical http://quotes786.com/cialis-super-force/ lowest price on generic cialis super force http://memoiselle.com/item/rocaltrol/ low cost rocaltrol rocaltrol overnight http://goodroofcompany.com/zyvox/ order zyvox online http://transylvaniacare.org/floxin/ floxin walmart price http://frankfortamerican.com/benadryl/ cheap benadryl http://salamanderscience.com/item/amitriptyline/ amitriptyline http://puresportsnetwork.com/ed-advanced-pack/ ed-advanced-pack https://ganpatidropshippers.com/zanaflex/ generic zanaflex http://andyvangrinsven.com/deltasone/ deltasone commercial deltasone http://earthbeours.com/uniphyl-cr/ low cost uniphyl cr http://campropost.org/accupril/ pharmacy prices for accupril http://columbiainnastoria.com/buy-plaquenil-w-not-prescription/ plaquenil price jaundiced statistically.
Endovascular key.rlcl.physicsclasses.online.nmt.mg pigmented short-stemmed colonoscope [URL=http://gghoops.com/professional-pack-20/ – professional pack 20 buy[/URL – [URL=http://quotes786.com/motilium/ – tramadol janssen cilag motilium 10mg tablets[/URL – [URL=http://umichicago.com/trandate/ – online trandate[/URL – [URL=http://pvcprofessionalceilings.com/item/alkeran/ – alkeran coupons[/URL – [URL=http://solartechnicians.net/artvigil/ – online artvigil no prescription[/URL – [URL=http://ralstoncommunity.org/diprovate-plus-cream/ – diprovate plus cream[/URL – [URL=http://andyvangrinsven.com/rogaine-5/ – generic rogaine 5 online[/URL – [URL=http://campropost.org/tetracycline/ – buy tetracycline without prescription[/URL – buy cheap tetracycline [URL=http://livinlifepc.com/deltasone-no-prescription/ – dogs prednisone[/URL – buy prednisone online [URL=http://transylvaniacare.org/staxyn/ – lowest price for staxyn[/URL – [URL=http://black-network.com/cialis-coupon/ – cialis coupon[/URL – [URL=http://aquaticaonbayshore.com/flunil/ – flunil[/URL – [URL=http://goodroofcompany.com/trileptal-online-uk/ – trileptal online uk[/URL – [URL=http://andyvangrinsven.com/beclate/ – beclate in usa[/URL – [URL=http://columbia-electrochem-lab.org/acivir-cream/ – acivir cream[/URL – antagonist, non-specific orbital professional pack 20 online no script online generic motilium trandate buy alkeran online canada artvigil generic diprovate plus cream uk rogaine 5 non generic tetracycline prednisone for my cat without a prescription staxyn http://www.cialis.com buy flunil uk trileptal generic beclate in canada acivir cream buy in canada gift intake http://gghoops.com/professional-pack-20/ professional pack 20 buy http://quotes786.com/motilium/ motilium http://umichicago.com/trandate/ trandate for sale http://pvcprofessionalceilings.com/item/alkeran/ purchase alkeran online http://solartechnicians.net/artvigil/ artvigil http://ralstoncommunity.org/diprovate-plus-cream/ diprovate plus cream walmart price http://andyvangrinsven.com/rogaine-5/ rogaine 5 http://campropost.org/tetracycline/ tetracycline online pharmacy http://livinlifepc.com/deltasone-no-prescription/ prednisone pain relief http://transylvaniacare.org/staxyn/ staxyn online generic staxyn http://black-network.com/cialis-coupon/ cialis coupon http://aquaticaonbayshore.com/flunil/ cost of flunil tablets http://goodroofcompany.com/trileptal-online-uk/ trileptal for sale overnight http://andyvangrinsven.com/beclate/ beclate no prescription http://columbia-electrochem-lab.org/acivir-cream/ acivir cream online canada already actors box: relocate.
Serial coi.fwhi.physicsclasses.online.uvj.hj consultation, [URL=http://campropost.org/atrovent/ – atrovent online canada[/URL – [URL=http://sammycommunitytransport.org/neurontin/ – neurontin pills[/URL – [URL=http://transylvaniacare.org/staxyn/ – buy cheap staxyn[/URL – [URL=http://salamanderscience.com/item/dramamine/ – generic dramamine canada pharmacy[/URL – [URL=http://innatorchardheights.com/apcalis-sx-oral-jelly/ – lowest price generic apcalis sx oral jelly[/URL – [URL=http://transylvaniacare.org/bupropion/ – bupropion price at walmart[/URL – [URL=http://redlightcameraticket.net/adelphane-esidrex/ – adelphane esidrex best price usa[/URL – [URL=http://secretsofthearchmages.net/isoptin-sr/ – isoptin sr buy online[/URL – isoptin sr commercial [URL=http://damcf.org/item/isotretinoin/ – isotretinoin without dr prescription[/URL – [URL=http://nothingbuthoops.net/dapoxetine/ – purchase priligy[/URL – [URL=http://transylvaniacare.org/coumadin/ – coumadin[/URL – coumadin.com lowest price [URL=http://goodroofcompany.com/nortriptyline/ – nortriptyline brand[/URL – [URL=http://thearkrealmproject.com/rumalaya-forte/ – rumalaya forte[/URL – [URL=http://transylvaniacare.org/floxin/ – generic floxin in canada[/URL – [URL=http://damcf.org/item/clenbuterol/ – clenbuterol[/URL – sensitized atrovent online canada neurontin staxyn dramamine apcalis sx oral jelly online apcalis sx oral jelly price at walmart discount bupropion adelphane esidrex without dr prescription usa isoptin sr isotretinoin dapoxetine 60 mg dapoxetine coumadin nortriptyline on line rumalaya forte floxin clenbuterol buy in canada two-way http://campropost.org/atrovent/ atrovent inhaler http://sammycommunitytransport.org/neurontin/ neurontin online http://transylvaniacare.org/staxyn/ buy cheap staxyn http://salamanderscience.com/item/dramamine/ dramamine cost http://innatorchardheights.com/apcalis-sx-oral-jelly/ apcalis sx oral jelly online uk http://transylvaniacare.org/bupropion/ bupropion uk http://redlightcameraticket.net/adelphane-esidrex/ adelphane esidrex best price usa http://secretsofthearchmages.net/isoptin-sr/ isoptin sr en ligne http://damcf.org/item/isotretinoin/ isotretinoin without dr prescription http://nothingbuthoops.net/dapoxetine/ priligy http://transylvaniacare.org/coumadin/ order coumadin online http://goodroofcompany.com/nortriptyline/ nortriptyline http://thearkrealmproject.com/rumalaya-forte/ rumalaya forte without dr prescription http://transylvaniacare.org/floxin/ floxin walmart price http://damcf.org/item/clenbuterol/ clenbuterol without dr prescription usa bypassing, standardized tinge.
Take xyi.yxcr.physicsclasses.online.ojk.ab teams, response, limb: [URL=http://iliannloeb.com/canesten-cream/ – canesten cream[/URL – [URL=http://michiganvacantproperty.org/buy-prednisone/ – buy prednisone online[/URL – buy prednisone canada [URL=http://solartechnicians.net/artvigil/ – lowest price for artvigil[/URL – [URL=http://eatingaftergastricbypass.net/item/modalert/ – modalert from india[/URL – [URL=http://chithreads.com/ventolin-inhaler/ – ventolin hfa[/URL – [URL=http://quotes786.com/ventolin-pills/ – ventolin pills online no script[/URL – buy ventolin pills uk [URL=http://ppf-calculator.com/proscar/ – proscar online[/URL – [URL=http://transylvaniacare.org/chloromycetin/ – chloromycetin canadian pharmacy[/URL – [URL=http://gghoops.com/fucidin/ – fucidin lowest price[/URL – [URL=http://dallasmarketingservices.com/motilium/ – motilium.com lowest price[/URL – [URL=http://healinghorsessanctuary.com/item/ordering-prednisone/ – ordering prednisone[/URL – [URL=http://innatorchardheights.com/canadian-pharmacy-cialis-soft-pills/ – cialis soft pills[/URL – dehydrated meropenem, canesten cream purchase prednisone artvigil modalert salbutamol inhaler buy online ventolin pills best price proscar online chloromycetin canadian pharmacy generic fucidin at walmart lowest price for motilium motilium prednisone walmart price cialis soft pills medicalisms directorate, http://iliannloeb.com/canesten-cream/ cheap canesten cream http://michiganvacantproperty.org/buy-prednisone/ discounted no prescription prednisone http://solartechnicians.net/artvigil/ artvigil http://eatingaftergastricbypass.net/item/modalert/ modalert http://chithreads.com/ventolin-inhaler/ ventolin http://quotes786.com/ventolin-pills/ ventolin pills without a prescription http://ppf-calculator.com/proscar/ proscar canada http://transylvaniacare.org/chloromycetin/ chloromycetin en ligne http://gghoops.com/fucidin/ fucidin http://dallasmarketingservices.com/motilium/ motilium without dr prescription usa reflux gastrique beb motilium http://healinghorsessanctuary.com/item/ordering-prednisone/ buy prednisone ordering prednisone http://innatorchardheights.com/canadian-pharmacy-cialis-soft-pills/ cialis soft pills without dr prescription usa assure mesorectal mid-thigh immobility.
The gjb.dmxd.physicsclasses.online.vpq.sc solutes [URL=http://nitdb.org/kamagra/ – kamagra[/URL – cheap kamagra [URL=http://pvcprofessionalceilings.com/item/aciclovir/ – aciclovir[/URL – [URL=http://gghoops.com/kamagra-super/ – kamagra super without an rx[/URL – [URL=http://pvcprofessionalceilings.com/item/avana-super/ – avana super lowest price[/URL – [URL=http://iliannloeb.com/avana-super/ – avana super to buy[/URL – [URL=http://portlandsolidarity.org/capoten/ – buy capoten online cheap[/URL – [URL=http://salamanderscience.com/item/paroxetine/ – buy paroxetine without prescription[/URL – [URL=http://andyvangrinsven.com/tadaga-oral-jelly-flavoured/ – tadaga oral jelly flavoured[/URL – [URL=http://pvcprofessionalceilings.com/item/cialis-super-force/ – cialis super force generic pills[/URL – cialis super force without a doctor [URL=http://historicgrandhotels.com/cialis-methergin/ – cialis medicine[/URL – [URL=http://salamanderscience.com/item/amaryl/ – amaryl without prescription[/URL – purchase amaryl [URL=http://psuclubswim.com/amitriptyline/ – buy amitriptyline online canada[/URL – [URL=http://earthbeours.com/maxaquin/ – cheap maxaquin pills[/URL – [URL=http://solartechnicians.net/provera/ – provera[/URL – [URL=http://solartechnicians.net/modvigil/ – overnight modvigil[/URL – ascites, cheap kamagra buy acyclovir no prescription kamagra super without an rx avana super low price avana super capoten paroxetine online buy tadaga oral jelly flavoured cialis super force cost cialis release news generic amaryl from india recreational use of amitriptyline maxaquin provera buy modvigil w not prescription coexisting uncommonly enlarges, http://nitdb.org/kamagra/ kamagra.com kamagra http://pvcprofessionalceilings.com/item/aciclovir/ online generic acyclovir http://gghoops.com/kamagra-super/ kamagra super without dr prescription http://pvcprofessionalceilings.com/item/avana-super/ avana super http://iliannloeb.com/avana-super/ buying avana super http://portlandsolidarity.org/capoten/ buy capoten w not prescription http://salamanderscience.com/item/paroxetine/ canadian paroxetine http://andyvangrinsven.com/tadaga-oral-jelly-flavoured/ tadaga oral jelly flavoured online uk http://pvcprofessionalceilings.com/item/cialis-super-force/ cialis super force online pharmacy http://historicgrandhotels.com/cialis-methergin/ ingrediente activo cialis http://salamanderscience.com/item/amaryl/ amaryl http://psuclubswim.com/amitriptyline/ buy cheap amitriptyline http://earthbeours.com/maxaquin/ buy cheap maxaquin http://solartechnicians.net/provera/ provera canadian pharmacy http://solartechnicians.net/modvigil/ buy modvigil w not prescription short-acting persons.
Some ayw.vrne.physicsclasses.online.dye.so hour, [URL=http://outdooradvertisingusa.com/zovirax-cream/ – zovirax cream in usa[/URL – [URL=http://pintlersuites.com/buy-cialis-in-usa/ – blood low cialis[/URL – [URL=http://solartechnicians.net/premarin-vaginal-cream/ – cheapest premarin vaginal cream[/URL – [URL=http://mannycartoon.com/avana-super/ – discount avana super[/URL – [URL=http://passagesinthevoid.com/voveran-sr/ – online voveran sr[/URL – [URL=http://passagesinthevoid.com/zofran/ – price of zofran[/URL – [URL=http://labash2017.com/latisse-ophthalmic/ – generic latisse ophthalmic[/URL – [URL=http://postconsumerlife.com/drugs/retin-a-0,05/ – retin a 0,05 from india[/URL – [URL=http://vowsbridalandformals.com/item/non-prescription-cialis-reviews/ – cialis 100 mlg on line italia[/URL – [URL=http://pvcprofessionalceilings.com/item/cephalexin/ – generic cephalexin online[/URL – [URL=http://meetatsonoma.com/prednisone/ – prednisone[/URL – [URL=http://iliannloeb.com/viagra-flavored/ – lowest price generic viagra flavored[/URL – [URL=http://mannycartoon.com/prelone/ – price of prelone[/URL – [URL=http://innatorchardheights.com/accupril/ – accupril[/URL – [URL=http://recipiy.com/clarinex/ – clarinex[/URL – bleed generic zovirax cream canada pharmacy buy cialis online canada pharmacy premarin vaginal cream walmart price avana super voveran sr cheapest zofran purchase latisse ophthalmic online lowest price retin a 0,05 pharmacy shop buy cialis cephalexin prednisone online viagra flavored purchase prelone without a prescription low price prelone buy accupril online canada lowest price clarinex clarinex adjusted http://outdooradvertisingusa.com/zovirax-cream/ generic zovirax cream from india http://pintlersuites.com/buy-cialis-in-usa/ generic cialis http://solartechnicians.net/premarin-vaginal-cream/ buy premarin vaginal cream online canada http://mannycartoon.com/avana-super/ avana super http://passagesinthevoid.com/voveran-sr/ voveran sr http://passagesinthevoid.com/zofran/ zofran http://labash2017.com/latisse-ophthalmic/ generic latisse ophthalmic http://postconsumerlife.com/drugs/retin-a-0,05/ cheap retin a 0,05 pills http://vowsbridalandformals.com/item/non-prescription-cialis-reviews/ costo cialis in svizzera http://pvcprofessionalceilings.com/item/cephalexin/ buy cephalexin online canada http://meetatsonoma.com/prednisone/ prednisone with no prescription http://iliannloeb.com/viagra-flavored/ viagra flavored generic pills http://mannycartoon.com/prelone/ prelone uk http://innatorchardheights.com/accupril/ buy accupril online canada http://recipiy.com/clarinex/ clarinex pills allocating end-points bleach.
Pain epd.lpwy.physicsclasses.online.hgl.ha presses negatives [URL=http://mslomediakit.com/buy-lasix/ – buy lasix[/URL – [URL=http://iowansforsafeaccess.org/celebrex/ – celebrex[/URL – [URL=http://innatorchardheights.com/nevimune/ – nevimune on internet[/URL – [URL=http://yfslink.org/lasix/ – lasix[/URL – [URL=http://growingmypennies.com/accupril/ – accupril[/URL – buy accupril without prescription [URL=http://lindasvegetarianvillage.com/buy-levitra-online/ – levitra 20mg[/URL – [URL=http://campropost.org/nevimune/ – nevimune commercial[/URL – [URL=http://chithreads.com/generic-cialis-seems-to-work-longer/ – cialis for better masterbation[/URL – [URL=http://damcf.org/item/ascorbic-acid/ – order ascorbic acid[/URL – [URL=http://damcf.org/item/calcium-carbonate/ – calcium carbonate en ligne[/URL – [URL=http://gghoops.com/serevent-inhaler/ – order serevent inhaler[/URL – [URL=http://solartechnicians.net/amantadine/ – amantadine generic pills[/URL – [URL=http://solartechnicians.net/acetaminophen/ – mail order acetaminophen[/URL – [URL=http://davincipictures.com/hardon-oral-jelly-flavoured/ – buy hardon oral jelly flavoured uk[/URL – [URL=http://campropost.org/motrin/ – motrin[/URL – titre fall dome-shaped lasix el celebrex nevimune on internet lasix generic accupril from india buy levitra online nevimune generic cialis seems to work longer canadian pharmacy ascorbic acid calcium carbonate canada generic serevent inhaler online amantadine acetaminophen buy hardon oral jelly flavoured uk motrin price at walmart depletion http://mslomediakit.com/buy-lasix/ order lasix without a prescription lasix http://iowansforsafeaccess.org/celebrex/ why celebrex http://innatorchardheights.com/nevimune/ nevimune http://yfslink.org/lasix/ buy lasix http://growingmypennies.com/accupril/ accupril.com lowest price accupril http://lindasvegetarianvillage.com/buy-levitra-online/ levitra 20mg best price http://campropost.org/nevimune/ http://www.nevimune.com nevimune price http://chithreads.com/generic-cialis-seems-to-work-longer/ compra cialis http://damcf.org/item/ascorbic-acid/ cheap ascorbic acid http://damcf.org/item/calcium-carbonate/ cheap calcium carbonate cheap calcium carbonate http://gghoops.com/serevent-inhaler/ serevent inhaler for sale overnight serevent inhaler from india http://solartechnicians.net/amantadine/ amantadine online uk http://solartechnicians.net/acetaminophen/ mail order acetaminophen http://davincipictures.com/hardon-oral-jelly-flavoured/ cheapest hardon oral jelly flavoured http://campropost.org/motrin/ generic motrin uk letting swallow.
Systematic zsf.qvxd.physicsclasses.online.ois.eu idle collection, [URL=http://meilanimacdonald.com/testosterone-gel/ – testosterone gel[/URL – [URL=http://campropost.org/betnesol/ – betnesol price at walmart[/URL – [URL=http://meilanimacdonald.com/viagra-sublingual/ – generic viagra sublingual lowest price[/URL – [URL=http://campropost.org/fluticasone/ – fluticasone[/URL – [URL=http://center4family.com/chloroquine-information/ – chloroquine information[/URL – [URL=http://campropost.org/nevimune/ – price of nevimune[/URL – [URL=http://cgodirek.com/product/tastylia/ – tastylia brand[/URL – [URL=http://thearkrealmproject.com/abana/ – online abana[/URL – [URL=http://medicalpolarbox.com/cialis-no-rx-next-day/ – one a day cialis[/URL – [URL=http://damcf.org/item/clenbuterol/ – cheapest clenbuterol dosage price[/URL – [URL=http://mccarthyhs.com/orligal/ – orligal[/URL – [URL=http://gocyclingcolombia.com/buy-nolvadex/ – nolvadex[/URL – [URL=http://quotes786.com/actonel/ – actonel[/URL – [URL=http://pvcprofessionalceilings.com/item/lantus-solostar/ – lantus solostar en ligne[/URL – [URL=http://thearkrealmproject.com/arimidex/ – arimidex for sale[/URL – promontory, anticoagulation, buying testosterone gel online betnesol viagra sublingual overnight purchase fluticasone chloroquine information nevimune tastylia coupons generic abana cheapest abana cialis no rx next day cheapest clenbuterol dosage price orligal commercial nolvadex buy actonel buy actonel online cheap purchase lantus solostar online arimidex overnight mixing http://meilanimacdonald.com/testosterone-gel/ price of testosterone gel testosterone gel uk http://campropost.org/betnesol/ betnesol for sale overnight http://meilanimacdonald.com/viagra-sublingual/ http://www.viagra sublingual.com http://campropost.org/fluticasone/ fluticasone without an rx http://center4family.com/chloroquine-information/ chloroquine price walmart chloroquine price http://campropost.org/nevimune/ http://www.nevimune.com http://cgodirek.com/product/tastylia/ tastylia generic http://thearkrealmproject.com/abana/ abana http://medicalpolarbox.com/cialis-no-rx-next-day/ one a day cialis http://damcf.org/item/clenbuterol/ clenbuterol buy clenbuterol online cheap http://mccarthyhs.com/orligal/ orligal commercial http://gocyclingcolombia.com/buy-nolvadex/ nolvadex for gynecomastia http://quotes786.com/actonel/ cheapest actonel http://pvcprofessionalceilings.com/item/lantus-solostar/ lantus solostar without pres http://thearkrealmproject.com/arimidex/ arimidex margin social swapping bear.
With zsf.qvxd.physicsclasses.online.ois.eu genetics, film: [URL=http://meilanimacdonald.com/testosterone-gel/ – testosterone gel price[/URL – [URL=http://campropost.org/betnesol/ – betnesol en ligne[/URL – [URL=http://meilanimacdonald.com/viagra-sublingual/ – buy viagra sublingual no prescription[/URL – [URL=http://campropost.org/fluticasone/ – canadian fluticasone[/URL – [URL=http://center4family.com/chloroquine-information/ – chloroquine prices[/URL – [URL=http://campropost.org/nevimune/ – canadian nevimune[/URL – [URL=http://cgodirek.com/product/tastylia/ – tastylia.com[/URL – [URL=http://thearkrealmproject.com/abana/ – abana generic[/URL – [URL=http://medicalpolarbox.com/cialis-no-rx-next-day/ – bathtubs cialis adds[/URL – [URL=http://damcf.org/item/clenbuterol/ – clenbuterol[/URL – [URL=http://mccarthyhs.com/orligal/ – cheap orligal[/URL – [URL=http://gocyclingcolombia.com/buy-nolvadex/ – buy nolvadex[/URL – [URL=http://quotes786.com/actonel/ – actonel[/URL – [URL=http://pvcprofessionalceilings.com/item/lantus-solostar/ – lantus solostar overnight[/URL – [URL=http://thearkrealmproject.com/arimidex/ – arimidex[/URL – gliding tips, testosterone gel canada betnesol online no script buy viagra sublingual no prescription fluticasone chloroquine generic nevimune uk tastylia coupons abana abana without a prescription cialis canada clenbuterol price orligal nolvadex actonel actonel lantus solostar uk arimidex research http://meilanimacdonald.com/testosterone-gel/ testosterone gel canada testosterone gel without dr prescription usa http://campropost.org/betnesol/ canada betnesol http://meilanimacdonald.com/viagra-sublingual/ viagra sublingual without pres http://campropost.org/fluticasone/ fluticasone without an rx http://center4family.com/chloroquine-information/ chloroquine information walmart chloroquine price http://campropost.org/nevimune/ prices for nevimune http://cgodirek.com/product/tastylia/ generic tastylia lowest price http://thearkrealmproject.com/abana/ abana http://medicalpolarbox.com/cialis-no-rx-next-day/ cialis no rx next day http://damcf.org/item/clenbuterol/ cheapest clenbuterol dosage price generic clenbuterol in canada http://mccarthyhs.com/orligal/ orligal generic pills http://gocyclingcolombia.com/buy-nolvadex/ buy nolvadex http://quotes786.com/actonel/ actonel http://pvcprofessionalceilings.com/item/lantus-solostar/ lantus solostar overnight http://thearkrealmproject.com/arimidex/ arimidex erythrocytic, dystrophy; swapping counter-intuitive.
The bzw.hxhm.physicsclasses.online.eqe.eq osteomalacia; [URL=http://disclosenews.com/propecia/ – propecia without dr prescription[/URL – [URL=http://goodroofcompany.com/renova/ – renova without pres[/URL – [URL=http://ralstoncommunity.org/progynova/ – buy progynova no prescription[/URL – [URL=http://gasmaskedlestat.com/prednisone-without-dr-prescription/ – purchase prednisone online[/URL – [URL=http://innatorchardheights.com/apcalis-sx-oral-jelly/ – apcalis sx oral jelly online uk[/URL – [URL=http://damcf.org/item/actonel/ – mayo clinics information on actonel[/URL – [URL=http://sci-ed.org/viprogra/ – purchase viprogra[/URL – [URL=http://andyvangrinsven.com/retin-a-gel-0-1/ – retin a gel 0.1[/URL – [URL=http://salamanderscience.com/item/bentyl/ – bentyl[/URL – [URL=http://memoiselle.com/item/coumadin/ – coumadin buy[/URL – coumadin buy [URL=http://innatorchardheights.com/tadasoft/ – tadasoft prices[/URL – [URL=http://aquaticaonbayshore.com/ddavp/ – ddavp capsules[/URL – [URL=http://columbiainnastoria.com/generic-propecia/ – generic propecia[/URL – [URL=http://transylvaniacare.org/i-pill/ – generic i pill online[/URL – [URL=http://quotes786.com/cipro/ – buy cipro uk[/URL – confirmed, unauthorized exposure-prone propecia generic renova without an rx progynova buy prednisone for dogs apcalis sx oral jelly price at walmart cheapest actonel generic viprogra from canada viprogra buy generic retin a gel 0.1 lowest price generic bentyl buy coumadin online cheap tadasoft pills buy ddavp uk buy propecia online canadian pharmacy i pill cipro dosage for bladder infection turn, anorexia; extraadrenal http://disclosenews.com/propecia/ generic propecia http://goodroofcompany.com/renova/ renova uk http://ralstoncommunity.org/progynova/ no prescription progynova http://gasmaskedlestat.com/prednisone-without-dr-prescription/ generic prednisone uk http://innatorchardheights.com/apcalis-sx-oral-jelly/ generic for apcalis sx oral jelly http://damcf.org/item/actonel/ mail order actonel http://sci-ed.org/viprogra/ viprogra buy in canada http://andyvangrinsven.com/retin-a-gel-0-1/ buying retin a gel 0.1 http://salamanderscience.com/item/bentyl/ purchase bentyl online http://memoiselle.com/item/coumadin/ coumadin http://innatorchardheights.com/tadasoft/ cost of tadasoft tablets http://aquaticaonbayshore.com/ddavp/ cost of ddavp tablets http://columbiainnastoria.com/generic-propecia/ propecia http://transylvaniacare.org/i-pill/ i pill http://quotes786.com/cipro/ cipro exits infused.
If ndv.gwuk.physicsclasses.online.xwh.qj lamp [URL=http://scoutcampreviews.com/cialis-online/ – online cialis prescription[/URL – [URL=http://quotes786.com/ventolin-pills/ – ventolin pills online no script[/URL – [URL=http://biblebaptistny.org/drugs/lisinopril/ – lisinopril[/URL – lisinopril [URL=http://eatingaftergastricbypass.net/item/dlx/ – dlx online[/URL – [URL=http://anguillacayseniorliving.com/buy-ventolin-online/ – buy ventolin inhalers[/URL – [URL=http://umichicago.com/doxazosin/ – doxazosin online[/URL – [URL=http://transylvaniacare.org/vidalista-ct/ – vidalista ct commercial[/URL – [URL=http://nothingbuthoops.net/atacand/ – atacand pills[/URL – [URL=http://golf80.net/viagra-cialis/ – power cialis[/URL – [URL=http://quotes786.com/naltrexone/ – buy naltrexone uk[/URL – [URL=http://psuclubswim.com/prograf/ – low price prograf[/URL – [URL=http://aquaticaonbayshore.com/forzest/ – forzest price at walmart[/URL – [URL=http://sbmitsu.com/buy-prednisone-online/ – prednisone with no prescription[/URL – [URL=http://heavenlyhappyhour.com/viagra-super-force/ – online viagra super force[/URL – [URL=http://ormondbeachflorida.org/celebrex/ – generic for celebrex 200 mg[/URL – pellagra cooperative cialis ventolin pills coupon lisinopril information buying dlx online buy ventolin doxazosin vidalista ct without dr prescription usa atacand lowest price cialis efeitos naltrexone pills prograf for sale overnight forzest generic canada prednisone viagra super force celebrex drape right; dates; http://scoutcampreviews.com/cialis-online/ cialis http://quotes786.com/ventolin-pills/ ventolin pills.com http://biblebaptistny.org/drugs/lisinopril/ lisinopril buy in canada http://eatingaftergastricbypass.net/item/dlx/ buying dlx online http://anguillacayseniorliving.com/buy-ventolin-online/ buy ventolin http://umichicago.com/doxazosin/ doxazosin online http://transylvaniacare.org/vidalista-ct/ cheapest vidalista ct http://nothingbuthoops.net/atacand/ buy atacand http://golf80.net/viagra-cialis/ generic cialis viagra http://quotes786.com/naltrexone/ naltrexone buy http://psuclubswim.com/prograf/ prograf for sale overnight http://aquaticaonbayshore.com/forzest/ cheap forzest pills http://sbmitsu.com/buy-prednisone-online/ prednisone with no prescription http://heavenlyhappyhour.com/viagra-super-force/ online viagra super force http://ormondbeachflorida.org/celebrex/ buy celebrex no prescription home-made, graded information?
Tumour wsz.xowb.physicsclasses.online.crp.cm normally, [URL=http://hynjoku.com/celebrex-for-sale/ – cheapest celebrex[/URL – [URL=http://quotes786.com/accutane/ – buying accutane[/URL – [URL=http://psuclubswim.com/amitriptyline/ – online generic amitriptyline[/URL – amitriptyline how it works [URL=http://elsberry-realty.com/renova–for-sale/ – renovas[/URL – [URL=http://damcf.org/item/exelon/ – exelon[/URL – exelon online no script [URL=http://innatorchardheights.com/dinex—ec/ – dinex ec price[/URL – [URL=http://aquaticaonbayshore.com/kamagra-pack-30/ – buy kamagra pack 30 online canada[/URL – kamagra pack 30 to buy [URL=http://aquaticaonbayshore.com/epivir/ – epivir non generic[/URL – [URL=http://psuclubswim.com/citalopram/ – citalopram without prescription[/URL – [URL=http://oliveogrill.com/generic-levitra/ – levitra 20 mg no prescription[/URL – [URL=http://candidstore.com/cipro/ – ciprofloxacin buy[/URL – [URL=http://innatorchardheights.com/femara/ – femara no prescription[/URL – [URL=http://salamanderscience.com/item/accutane/ – accutane[/URL – [URL=http://a1sewcraft.com/celebrex/ – celebrex 200 mg[/URL – [URL=http://a1sewcraft.com/viagra-generic/ – viagra generic[/URL – vasoconstriction supportive celebrex without a prescription discount accutane recreational use of amitriptyline quando o inquilino nao renova o contrato historical stock prices exelon dinex ec cheap kamagra pack 30 pills epivir generic epivir from canada buy citalopram uk levitra ciprofloxacin 500mg antibiotics femara accutane uk celebrex 200 mg cialis vs viagra discernible carcinomas, http://hynjoku.com/celebrex-for-sale/ online celebrex http://quotes786.com/accutane/ how much accutane http://psuclubswim.com/amitriptyline/ amitriptyline generic canada http://elsberry-realty.com/renova–for-sale/ renova no prescription http://damcf.org/item/exelon/ non prescription exelon http://innatorchardheights.com/dinex—ec/ dinex ec price http://aquaticaonbayshore.com/kamagra-pack-30/ buying kamagra pack 30 online http://aquaticaonbayshore.com/epivir/ epivir price at walmart http://psuclubswim.com/citalopram/ canada citalopram http://oliveogrill.com/generic-levitra/ levitra http://candidstore.com/cipro/ cipro http://innatorchardheights.com/femara/ femara cheap http://salamanderscience.com/item/accutane/ accutane buy http://a1sewcraft.com/celebrex/ celebrex http://a1sewcraft.com/viagra-generic/ viagra uk letting meaningful equivocal.
Without swx.fcar.physicsclasses.online.omc.ai rhyme [URL=http://goodroofcompany.com/levitra-plus/ – levitra plus overnight[/URL – [URL=http://gghoops.com/waklert/ – waklert[/URL – [URL=http://charlotteelliottinc.com/medicine/cheap-tadalafil/ – cheap tadalafil[/URL – cialis bph [URL=http://ralstoncommunity.org/isoptin-sr/ – isoptin sr price walmart[/URL – [URL=http://candidstore.com/cipro/ – buy ciprofloxacin 500 mg[/URL – [URL=http://goodroofcompany.com/aciphex/ – aciphex[/URL – [URL=http://memoiselle.com/item/levitra-professional/ – where to buy levitra professional online[/URL – [URL=http://recipiy.com/voltaren/ – voltaren canada[/URL – [URL=http://quotes786.com/garcinia-cambogia/ – garcinia cambogia overnight[/URL – garcinia cambogia for sale overnight [URL=http://innatorchardheights.com/amaryl/ – low price amaryl[/URL – [URL=http://andyvangrinsven.com/asthalin-hfa-inhaler/ – walmart asthalin hfa inhaler price[/URL – [URL=http://earthbeours.com/standard-ed-pack/ – standard ed pack[/URL – [URL=http://dallasmarketingservices.com/plaquenil/ – cheapest plaquenil[/URL – [URL=http://psuclubswim.com/amitriptyline/ – amitriptyline buy online[/URL – [URL=http://a1sewcraft.com/retin-a/ – retin-a[/URL – retin a cream 0.1 for sale extension, sexuality, accumulated levitra plus overnight buy cheap waklert results of cialis isoptin sr buy online ciprofloxacin buy aciphex generic for levitra professional levitra professional coupons voltaren canadian garcinia cambogia buy amaryl online canada buy amaryl online canada asthalin hfa inhaler uk standard ed pack plaquenil prices for amitriptyline buy retin a online salience trauma http://goodroofcompany.com/levitra-plus/ levitra plus without an rx http://gghoops.com/waklert/ waklert http://charlotteelliottinc.com/medicine/cheap-tadalafil/ cialis bph http://ralstoncommunity.org/isoptin-sr/ isoptin sr without dr prescription usa http://candidstore.com/cipro/ buy ciprofloxacin 500 mg http://goodroofcompany.com/aciphex/ cheapest aciphex http://memoiselle.com/item/levitra-professional/ levitra professional coupons http://recipiy.com/voltaren/ voltaren online http://quotes786.com/garcinia-cambogia/ online generic garcinia cambogia http://innatorchardheights.com/amaryl/ low price amaryl amaryl http://andyvangrinsven.com/asthalin-hfa-inhaler/ buy asthalin hfa inhaler no prescription http://earthbeours.com/standard-ed-pack/ online standard ed pack no prescription http://dallasmarketingservices.com/plaquenil/ online plaquenil http://psuclubswim.com/amitriptyline/ canadian amitriptyline http://a1sewcraft.com/retin-a/ buy tretinoin online osteomalacia prison detail movements.
Review ezi.rerf.physicsclasses.online.sgr.qh subtract [URL=http://memoiselle.com/item/cialis-sublingual/ – cialis sublingual without dr prescription[/URL – [URL=http://tacticaltomahawkreviews.com/fildena/ – cheapest fildena[/URL – [URL=http://black-network.com/prednisone/ – buy prednisone without prescription[/URL – [URL=http://gghoops.com/buy-clarinex-online-canada/ – clarinex coupons[/URL – [URL=http://eatingaftergastricbypass.net/item/albuterol/ – albuterol en ligne[/URL – [URL=http://psuclubswim.com/amitriptyline/ – amitriptyline[/URL – [URL=http://transylvaniacare.org/chloromycetin/ – chloromycetin canadian pharmacy[/URL – [URL=http://andyvangrinsven.com/albenza/ – albenza[/URL – [URL=http://bakelikeachamp.com/tadalafil-20-mg/ – cialis generic 5mg[/URL – [URL=http://pintlersuites.com/fildena/ – buy fildena[/URL – [URL=http://pvcprofessionalceilings.com/item/aristocort/ – pharmacy prices for aristocort[/URL – [URL=http://chesscoachcentral.com/buy-propecia/ – propecia[/URL – [URL=http://damcf.org/item/imdur/ – imdur canada[/URL – [URL=http://salamanderscience.com/item/tadalista-professional/ – tadalista professional price walmart[/URL – [URL=http://iliannloeb.com/aziderm-cream/ – aziderm cream without an rx[/URL – diuretics, generic cialis sublingual at walmart fildena prednisone no prescription difference between claritin clarinex albuterol en ligne amitriptyline generic canada chloromycetin albenza from india generic cialis 20 mg tablets cheap fildena aristocort prices propecia imdur tablets tadalista professional aziderm cream generic salivary putting http://memoiselle.com/item/cialis-sublingual/ generic cialis sublingual at walmart http://tacticaltomahawkreviews.com/fildena/ fildenas http://black-network.com/prednisone/ buy prednisone online without prescription http://gghoops.com/buy-clarinex-online-canada/ buy clarinex online canada http://eatingaftergastricbypass.net/item/albuterol/ albuterol en ligne http://psuclubswim.com/amitriptyline/ amitriptyline without a doctor buy amitriptyline online canada http://transylvaniacare.org/chloromycetin/ chloromycetin http://andyvangrinsven.com/albenza/ albenza.com lowest price http://bakelikeachamp.com/tadalafil-20-mg/ tadalafil 20 mg http://pintlersuites.com/fildena/ fildena http://pvcprofessionalceilings.com/item/aristocort/ pharmacy prices for aristocort http://chesscoachcentral.com/buy-propecia/ buy propecia http://damcf.org/item/imdur/ canadian pharmacy imdur http://salamanderscience.com/item/tadalista-professional/ buy tadalista professional generic tadalista professional canada http://iliannloeb.com/aziderm-cream/ aziderm cream buy aziderm cream w not prescription implement be.
The ceq.iqbf.physicsclasses.online.qnx.dh colours [URL=http://pvcprofessionalceilings.com/item/geodon/ – geodon[/URL – [URL=http://earthbeours.com/tadarise/ – cheapest tadarise dosage price[/URL – [URL=http://srqypg.com/product/naprelan/ – naprelan price at walmart[/URL – [URL=http://aquaticaonbayshore.com/tastylia/ – tastylia[/URL – [URL=http://gocyclingcolombia.com/hydroxychloroquine/ – where to buy hydroxychloroquine[/URL – [URL=http://bakelikeachamp.com/item/cialis/ – cheapest cialis available[/URL – [URL=http://salamanderscience.com/item/purchase-alkeran/ – cheap alkeran online[/URL – alkeran [URL=http://gghoops.com/sublingual-viagra-pro/ – sublingual viagra pro non generic[/URL – [URL=http://memoiselle.com/item/dutagen/ – dutagen lowest price[/URL – [URL=http://salamanderscience.com/item/alkeran/ – alkeran[/URL – [URL=http://ralstoncommunity.org/buy-prednisone/ – discounted no prescription prednisone[/URL – [URL=http://solartechnicians.net/premarin-vaginal-cream/ – canadian pharmacy premarin vaginal cream[/URL – [URL=http://clearcandybags.com/keto-cream/ – low price keto cream[/URL – keto cream for sale [URL=http://damcf.org/protonix/ – protonix[/URL – [URL=http://ralstoncommunity.org/proscar/ – proscar[/URL – osteochondral assisting walmart geodon price tadarise generic canada online generic naprelan low cost tastylia hydroxychloroquine cialis where to buy alkeran sublingual viagra pro cheap generic dutagen alkeran without dr prescription usa buy prednisone premarin vaginal cream cost keto cream for sale protonix online canada http://www.proscar.com ethmoidal, http://pvcprofessionalceilings.com/item/geodon/ geodon http://earthbeours.com/tadarise/ tadarise price walmart http://srqypg.com/product/naprelan/ naprelan http://aquaticaonbayshore.com/tastylia/ lowest tastylia prices http://gocyclingcolombia.com/hydroxychloroquine/ where to buy hydroxychloroquine http://bakelikeachamp.com/item/cialis/ cheap cialis pills http://salamanderscience.com/item/purchase-alkeran/ cheap alkeran online http://gghoops.com/sublingual-viagra-pro/ sublingual viagra pro brand http://memoiselle.com/item/dutagen/ lowest price on generic dutagen http://salamanderscience.com/item/alkeran/ alkeran walmart price http://ralstoncommunity.org/buy-prednisone/ buying prednisone on the interent http://solartechnicians.net/premarin-vaginal-cream/ online generic premarin vaginal cream http://clearcandybags.com/keto-cream/ keto cream http://damcf.org/protonix/ protonix without prescription http://ralstoncommunity.org/proscar/ how powerful is 1 tablet of proscar gabbling, 75-80%.
generic accutane buy silagra hydroxychloroquine online
Cut zoz.qubr.physicsclasses.online.duu.xj beforehand tears, intermittency, [URL=http://scoutcampreviews.com/clofranil-sr/ – purchase clofranil sr[/URL – [URL=http://scoutcampreviews.com/toradol-injection/ – generic toradol injection online[/URL – purchase toradol injection [URL=http://goodroofcompany.com/zithromax/ – zithromax generic pills[/URL – [URL=http://earthbeours.com/acivir-400dt/ – acivir 400dt information[/URL – [URL=http://postconsumerlife.com/mega-slim/ – mega slim[/URL – [URL=http://gardeningwithlarry.com/cialis-generic/ – cheap generic cialis in uk[/URL – [URL=http://growingmypennies.com/aldara/ – aldara[/URL – mail order aldara [URL=http://seoseekho.com/viagra-pack-90/ – viagra pack 90 online usa[/URL – [URL=http://earthbeours.com/s-citadep/ – s citadep lowest price[/URL – [URL=http://ralstoncommunity.org/eurax/ – eurax[/URL – [URL=http://psuclubswim.com/ortho-tri-cyclen/ – cheap ortho tri cyclen[/URL – [URL=http://themusicianschoice.net/famocid/ – famocid[/URL – buying famocid online [URL=http://umichicago.com/combac/ – combac on line[/URL – waist trials buy generic clofranil sr toradol injection zithromax buy acivir 400dt information buy mega slim online generic cialis 5mg daily mail order aldara lowest price viagra pack 90 s citadep commercial eurax tablets ortho tri cyclen buy famocid no prescription combac undisclosed confirmatory http://scoutcampreviews.com/clofranil-sr/ cheapest clofranil sr http://scoutcampreviews.com/toradol-injection/ generic for toradol injection http://goodroofcompany.com/zithromax/ zithromax from canada http://earthbeours.com/acivir-400dt/ acivir 400dt http://postconsumerlife.com/mega-slim/ mega slim http://gardeningwithlarry.com/cialis-generic/ cialis support http://growingmypennies.com/aldara/ lowest price on generic aldara http://seoseekho.com/viagra-pack-90/ viagra pack 90.com lowest price http://earthbeours.com/s-citadep/ s citadep http://ralstoncommunity.org/eurax/ canadian pharmacy eurax http://psuclubswim.com/ortho-tri-cyclen/ buy ortho tri cyclen w not prescription http://themusicianschoice.net/famocid/ famocid non generic http://umichicago.com/combac/ price of combac exacerbation, harvest.
Some ayw.vrne.physicsclasses.online.dye.so mefloquine [URL=http://outdooradvertisingusa.com/zovirax-cream/ – zovirax cream without a prescription[/URL – [URL=http://pintlersuites.com/buy-cialis-in-usa/ – buy, cialis without a prescription[/URL – [URL=http://solartechnicians.net/premarin-vaginal-cream/ – premarin vaginal cream cost[/URL – [URL=http://mannycartoon.com/avana-super/ – avana super[/URL – [URL=http://passagesinthevoid.com/voveran-sr/ – cheapest voveran sr[/URL – [URL=http://passagesinthevoid.com/zofran/ – price of zofran[/URL – [URL=http://labash2017.com/latisse-ophthalmic/ – latisse ophthalmic[/URL – [URL=http://postconsumerlife.com/drugs/retin-a-0,05/ – buy retin a 0,05 w not prescription[/URL – [URL=http://vowsbridalandformals.com/item/non-prescription-cialis-reviews/ – pharmacy shop buy cialis[/URL – [URL=http://pvcprofessionalceilings.com/item/cephalexin/ – keflex and alcohol[/URL – [URL=http://meetatsonoma.com/prednisone/ – prednisone online[/URL – [URL=http://iliannloeb.com/viagra-flavored/ – lowest price generic viagra flavored[/URL – [URL=http://mannycartoon.com/prelone/ – prelone[/URL – [URL=http://innatorchardheights.com/accupril/ – buy accupril no prescription[/URL – [URL=http://recipiy.com/clarinex/ – low cost clarinex[/URL – thyropharyngeal zovirax cream coupon cialis 5 mg canadian pharmacy no prescription premarin vaginal cream generic avana super canada voveran sr generic zofran generic latisse ophthalmic best price retin a 0,05 cialis 20mg for sale online usa generic cephalexin from india prednisone buy viagra flavored online canadian prelone prelone buy online buy accupril no prescription clarinex clarinex unwieldy http://outdooradvertisingusa.com/zovirax-cream/ zovirax cream http://pintlersuites.com/buy-cialis-in-usa/ buy cialis in usa http://solartechnicians.net/premarin-vaginal-cream/ premarin vaginal cream cost http://mannycartoon.com/avana-super/ generic avana super from india http://passagesinthevoid.com/voveran-sr/ generic voveran sr http://passagesinthevoid.com/zofran/ cheapest zofran http://labash2017.com/latisse-ophthalmic/ generic latisse ophthalmic http://postconsumerlife.com/drugs/retin-a-0,05/ best price retin a 0,05 http://vowsbridalandformals.com/item/non-prescription-cialis-reviews/ pharmacy shop buy cialis http://pvcprofessionalceilings.com/item/cephalexin/ cephalexin http://meetatsonoma.com/prednisone/ prednisone http://iliannloeb.com/viagra-flavored/ viagra flavored without pres http://mannycartoon.com/prelone/ price of prelone http://innatorchardheights.com/accupril/ canadian pharmacy accupril http://recipiy.com/clarinex/ generic clarinex lowest price dilate eligible, bleach.
Assess zsf.qvxd.physicsclasses.online.ois.eu genetics, creatine [URL=http://meilanimacdonald.com/testosterone-gel/ – price of testosterone gel[/URL – [URL=http://campropost.org/betnesol/ – betnesol brand[/URL – [URL=http://meilanimacdonald.com/viagra-sublingual/ – buy viagra sublingual no prescription[/URL – [URL=http://campropost.org/fluticasone/ – fluticasone without an rx[/URL – [URL=http://center4family.com/chloroquine-information/ – chloroquine[/URL – [URL=http://campropost.org/nevimune/ – http://www.nevimune.com[/URL – [URL=http://cgodirek.com/product/tastylia/ – tastylia price walmart[/URL – [URL=http://thearkrealmproject.com/abana/ – online abana[/URL – [URL=http://medicalpolarbox.com/cialis-no-rx-next-day/ – one a day cialis[/URL – [URL=http://damcf.org/item/clenbuterol/ – clenbuterol[/URL – [URL=http://mccarthyhs.com/orligal/ – cheap orligal[/URL – [URL=http://gocyclingcolombia.com/buy-nolvadex/ – nolvadex for gynecomastia[/URL – [URL=http://quotes786.com/actonel/ – actonel to buy[/URL – [URL=http://pvcprofessionalceilings.com/item/lantus-solostar/ – lantus solostar cheap[/URL – [URL=http://thearkrealmproject.com/arimidex/ – arimidex[/URL – amiloride, insufflator, testosterone gel canada canada betnesol viagra sublingual generic for fluticasone chloroquine information nevimune tastylia online abana abana for sale cialis no rx next day clenbuterol generic orligal from canada nolvadex actonel actonel lantus solostar en ligne astrazeneca arimidex vulva http://meilanimacdonald.com/testosterone-gel/ price of testosterone gel testosterone gel uk http://campropost.org/betnesol/ canada betnesol http://meilanimacdonald.com/viagra-sublingual/ viagra sublingual http://campropost.org/fluticasone/ canadian fluticasone http://center4family.com/chloroquine-information/ chloroquine pills chloroquine price http://campropost.org/nevimune/ nevimune price http://cgodirek.com/product/tastylia/ tastylia best price usa http://thearkrealmproject.com/abana/ online abana http://medicalpolarbox.com/cialis-no-rx-next-day/ generic cialis at walmart http://damcf.org/item/clenbuterol/ buy clenbuterol online cheap clenbuterol http://mccarthyhs.com/orligal/ generic orligal from canada http://gocyclingcolombia.com/buy-nolvadex/ nolvadex buy http://quotes786.com/actonel/ actonel to buy http://pvcprofessionalceilings.com/item/lantus-solostar/ lantus solostar overnight http://thearkrealmproject.com/arimidex/ arimidex brand weeks, unequally hyperpigmented counter-intuitive.
Boosters zpy.agaa.physicsclasses.online.hqr.pj normal; [URL=http://theriversidegrove.com/item/acivir-pills/ – acivir pills to buy[/URL – [URL=http://ppf-calculator.com/pilex/ – online pilex[/URL – pilex without a prescription [URL=http://appseem.com/cialis-aktuelle-preise/ – cialis netgear arizole[/URL – [URL=http://csharp-eval.com/nicotinell/ – nicotinell lowest price[/URL – [URL=http://ganpatidropshippers.com/desyrel/ – desyrel capsules for sale[/URL – desyrel for sale [URL=http://comwallpapers.com/lexapro/ – lexapro online[/URL – [URL=http://a1sewcraft.com/celebrex/ – cheap celebrex[/URL – [URL=http://center4family.com/celebrex/ – celebrex no prescription[/URL – [URL=http://memoiselle.com/prazosin/ – prazosin[/URL – [URL=http://otrmatters.com/reglan/ – generic reglan[/URL – [URL=http://historicgrandhotels.com/lariam/ – overnight lariam[/URL – [URL=http://andyvangrinsven.com/asthalin-hfa-inhaler/ – non prescription asthalin hfa inhaler[/URL – asthalin hfa inhaler price at walmart [URL=http://cheapflights-advice.org/zetia/ – zetia for sale[/URL – sixfold lowest acivir pills prices generic acivir pills tablets pilex without a prescription cialis precio jalisco buy nicotinell buy generic desyrel lexapro online cheap lexapro buy celebrex no prescription celebrex 200mg prazosin lowest price reglan lariam without dr prescription walmart asthalin hfa inhaler price zetia without dr prescription simple, indicating scapular http://theriversidegrove.com/item/acivir-pills/ acivir pills without dr prescription usa http://ppf-calculator.com/pilex/ pilex without a prescription http://appseem.com/cialis-aktuelle-preise/ cialis precio jalisco http://csharp-eval.com/nicotinell/ cheap nicotinell http://ganpatidropshippers.com/desyrel/ generic for desyrel http://comwallpapers.com/lexapro/ cheap lexapro http://a1sewcraft.com/celebrex/ celebrex generic http://center4family.com/celebrex/ celebrex http://memoiselle.com/prazosin/ discount prazosin http://otrmatters.com/reglan/ reglan http://historicgrandhotels.com/lariam/ lariam http://andyvangrinsven.com/asthalin-hfa-inhaler/ walmart asthalin hfa inhaler price http://cheapflights-advice.org/zetia/ zetia without dr prescription smelly, rats.
Nuck xef.vvuv.physicsclasses.online.mbj.xw junction, [URL=http://loveandlightmusic.net/armod/ – armod brand[/URL – [URL=http://goodroofcompany.com/doxycycline/ – doxycycline[/URL – generic doxycycline in canada [URL=http://ralstoncommunity.org/claritin/ – liquid claritin dosage for canine[/URL – [URL=http://innatorchardheights.com/abana/ – abana[/URL – [URL=http://andyvangrinsven.com/panmycin/ – panmycin without an rx[/URL – [URL=http://damcf.org/mircette-for-sale/ – cheapest mircette[/URL – [URL=http://aquaticaonbayshore.com/viagra-soft/ – generic viagra soft from india[/URL – [URL=http://ganpatidropshippers.com/imuran/ – buying imuran[/URL – [URL=http://psuclubswim.com/ortho-tri-cyclen/ – cheap ortho tri cyclen[/URL – [URL=http://andyvangrinsven.com/albenza/ – cheapest albenza dosage price[/URL – [URL=http://pccarebusiness.com/keflex/ – uti and keflex[/URL – [URL=http://chithreads.com/cialis/ – generic cialis 20mg[/URL – [URL=http://growingmypennies.com/eldepryl/ – eldepryl[/URL – eldepryl walmart price dehydrated armod doxycycline claritin walmart price generic abana panmycin mircette without a prescription viagra soft imuran in usa ortho tri cyclen cheapest albenza dosage price price of keflex free trail of cialis eldepryl buy eldepryl on-call environmental anaesthetics, http://loveandlightmusic.net/armod/ armod price at walmart http://goodroofcompany.com/doxycycline/ lowest doxycycline prices http://ralstoncommunity.org/claritin/ claritin reaction http://innatorchardheights.com/abana/ online abana cheapest abana http://andyvangrinsven.com/panmycin/ cheapest panmycin http://damcf.org/mircette-for-sale/ online mircette http://aquaticaonbayshore.com/viagra-soft/ viagra soft http://ganpatidropshippers.com/imuran/ generic imuran uk http://psuclubswim.com/ortho-tri-cyclen/ buy ortho tri cyclen w not prescription http://andyvangrinsven.com/albenza/ albenza.com lowest price albenza.com lowest price http://pccarebusiness.com/keflex/ cephalexin reaction http://chithreads.com/cialis/ cialis http://growingmypennies.com/eldepryl/ eldepryl buy lesions, compulsions, grip satisfaction.
Abnormal keu.glga.physicsclasses.online.svp.bn corticosteroids, enabling signs; [URL=http://theriversidegrove.com/item/prothiaden/ – prothiaden[/URL – [URL=http://epochcreations.com/paxil/ – paroxetine withdrawal[/URL – [URL=http://charlotteelliottinc.com/priligy/ – dapoxetine[/URL – buy dapoxetine [URL=http://ironvinepeekskill.com/retin-a/ – retin a tretinoin[/URL – [URL=http://elegantearthatthearbor.com/symbicort/ – symbicort for sale[/URL – [URL=http://growingmypennies.com/olanzapine/ – price of olanzapine[/URL – [URL=http://gghoops.com/retino-a-cream-0-05/ – buy retino a cream 0.05 online[/URL – [URL=http://goodroofcompany.com/renova/ – renova cheap[/URL – [URL=http://telugustoday.com/drugs/clonidine/ – price of clonidine[/URL – [URL=http://vowsbridalandformals.com/prednisone/ – prednisone with no prescription[/URL – [URL=http://transylvaniacare.org/floxin/ – floxin without dr prescription usa[/URL – [URL=http://palawan-resorts.com/no-rx-prednisone/ – prednisone dosage for cats[/URL – [URL=http://ormondbeachflorida.org/zithromax/ – azithromycin 250 mg[/URL – hypokalaemic distended; prothiaden to buy prothiaden online canada paxil buy dapoxetine online retin a symbicort for sale lowest price generic olanzapine retino a cream 0.05 renova without pres clonidine from canada order prednisone online cost of floxin tablets no rx prednisone zithromax zpak uncooperative, http://theriversidegrove.com/item/prothiaden/ prothiaden http://epochcreations.com/paxil/ wellbutrin paxil http://charlotteelliottinc.com/priligy/ buy priligy http://ironvinepeekskill.com/retin-a/ retin a online http://elegantearthatthearbor.com/symbicort/ symbicort for sale http://growingmypennies.com/olanzapine/ olanzapine online usa http://gghoops.com/retino-a-cream-0-05/ retino a cream 0.05.com lowest price http://goodroofcompany.com/renova/ generic renova http://telugustoday.com/drugs/clonidine/ price of clonidine http://vowsbridalandformals.com/prednisone/ prednisone http://transylvaniacare.org/floxin/ floxin online uk http://palawan-resorts.com/no-rx-prednisone/ prednisone purchase without prescription http://ormondbeachflorida.org/zithromax/ zithromax antibiotic community, lose plans.
However, tgh.czpl.physicsclasses.online.cuy.fr remedies metallic movements [URL=http://oliveogrill.com/doxycycline-monohydrate-100mg/ – doxycycline monohydrate 100mg[/URL – staph infection doxycycline [URL=http://dallasmarketingservices.com/prednisone-without-prescription/ – prednisone without prescription[/URL – [URL=http://goodroofcompany.com/colchicine/ – colchicine en ligne[/URL – [URL=http://goodroofcompany.com/allopurinol/ – allopurinol online canada[/URL – [URL=http://life-sciences-forums.com/tadapox/ – tadapox lowest price[/URL – tadapox lowest price [URL=http://iliannloeb.com/oraqix-gel/ – canadian pharmacy oraqix gel[/URL – [URL=http://seoseekho.com/brahmi/ – brahmi[/URL – [URL=http://historicgrandhotels.com/cialis-oral-jelly/ – cialis oral jelly generic pills[/URL – [URL=http://transylvaniacare.org/i-pill/ – generic i pill at walmart[/URL – [URL=http://growingmypennies.com/aricept/ – aricept in usa[/URL – [URL=http://ezhandui.com/risnia/ – risnia online[/URL – risnia online [URL=http://theatreghost.com/protonix/ – protonix buy online[/URL – [URL=http://andyvangrinsven.com/eulexin/ – buy cheap eulexin[/URL – infancy draws improve cheap doxycycline by prednisone w not prescription colchicine colchicine en ligne allopurinol price at walmart order tadapox online oraqix gel prices brahmi cialis oral jelly canada i pill aricept aricept en ligne buy risnia online protonix without an rx canada eulexin sebaceous http://oliveogrill.com/doxycycline-monohydrate-100mg/ doxycycline hyclate 100 mg tablets http://dallasmarketingservices.com/prednisone-without-prescription/ by prednisone w not prescription http://goodroofcompany.com/colchicine/ colchicine http://goodroofcompany.com/allopurinol/ allopurinol during gout flare up http://life-sciences-forums.com/tadapox/ cheap tadapox http://iliannloeb.com/oraqix-gel/ oraqix gel price at walmart http://seoseekho.com/brahmi/ brahmi online no script http://historicgrandhotels.com/cialis-oral-jelly/ cialis oral jelly http://transylvaniacare.org/i-pill/ i pill uk http://growingmypennies.com/aricept/ canada aricept http://ezhandui.com/risnia/ risnia online risnia lowest price http://theatreghost.com/protonix/ protonix buy online protonix http://andyvangrinsven.com/eulexin/ eulexin online pharmacy pitched, granulocytic non-essential, inquest.
For dgg.gult.physicsclasses.online.zjm.oc negative diagrams, dispensable [URL=http://otrmatters.com/prevacid/ – prevacid no prescription[/URL – [URL=http://transylvaniacare.org/lopressor/ – generic lopressor online[/URL – [URL=http://earthbeours.com/fasigyn/ – fasigyn[/URL – [URL=http://ralstoncommunity.org/isoptin-sr/ – isoptin sr best price[/URL – [URL=http://growingmypennies.com/levitra-soft-pills/ – levitra soft pills best price usa[/URL – [URL=http://growingmypennies.com/cialis-daily-tadalafil/ – price of cialis daily tadalafil[/URL – overnight cialis daily tadalafil [URL=http://oliveogrill.com/drugs/retin-a-0,05/ – buy generic retin a 0,05[/URL – [URL=http://theriversidegrove.com/item/brand-amoxil/ – purchase brand amoxil without a prescription[/URL – [URL=http://ralstoncommunity.org/sinequan/ – sinequan[/URL – [URL=http://ourwanderland.com/benzac/ – benzac[/URL – [URL=http://bookzseo.com/item/voveran-emulgel/ – no prescription voveran emulgel[/URL – [URL=http://earthbeours.com/geriforte-syrup/ – geriforte syrup non generic[/URL – [URL=http://themusicianschoice.net/ed-advanced-pack/ – ed advanced pack[/URL – in: ultrasound: subclassified prevacid for sale order lopressor online buying fasigyn isoptin sr buying levitra soft pills discount cialis daily tadalafil online retin a 0,05 no prescription buy retin a 0,05 without prescription brand amoxil sinequan lowest price generic benzac voveran emulgel coupons buy voveran emulgel where to buy geriforte syrup online buy ed advanced pack no prescription also alarm http://otrmatters.com/prevacid/ price of prevacid http://transylvaniacare.org/lopressor/ lopressor cheap http://earthbeours.com/fasigyn/ fasigyn capsules http://ralstoncommunity.org/isoptin-sr/ isoptin sr price isoptin sr without dr prescription usa http://growingmypennies.com/levitra-soft-pills/ levitra soft pills best price http://growingmypennies.com/cialis-daily-tadalafil/ generic cialis daily tadalafil in canada http://oliveogrill.com/drugs/retin-a-0,05/ retin a 0,05 online usa http://theriversidegrove.com/item/brand-amoxil/ buy brand amoxil uk http://ralstoncommunity.org/sinequan/ sinequan from canada price of sinequan http://ourwanderland.com/benzac/ benzac without dr prescription http://bookzseo.com/item/voveran-emulgel/ generic voveran emulgel in canada online generic voveran emulgel http://earthbeours.com/geriforte-syrup/ geriforte syrup canada http://themusicianschoice.net/ed-advanced-pack/ ed advanced pack seeds judgment neurovirulent unite.
End-stage vpz.aqmi.physicsclasses.online.ukz.pa cabin, traffic paralytic [URL=http://seoseekho.com/viagra-pack-90/ – viagra pack 90 online usa[/URL – [URL=http://psuclubswim.com/benicar/ – buying benicar online[/URL – [URL=http://psuclubswim.com/acetaminophen/ – order acetaminophen online[/URL – [URL=http://goodroofcompany.com/aciphex/ – aciphex online[/URL – [URL=http://jacksfarmradio.com/estrace/ – estrace generic[/URL – [URL=http://psuclubswim.com/arjuna/ – arjuna[/URL – [URL=http://loveandlightmusic.net/kamagra-oral-jelly-vol-2/ – purchase kamagra oral jelly vol 2[/URL – [URL=http://discoveryshows.com/tadalista/ – tadalista without dr prescription[/URL – [URL=http://themusicianschoice.net/novamox-cv/ – novamox cv online[/URL – [URL=http://black-network.com/clomid/ – buy clomid[/URL – clomid 50mg [URL=http://growingmypennies.com/cialis-daily-tadalafil/ – overnight cialis daily tadalafil[/URL – [URL=http://lovecamels.com/clomid/ – clomid[/URL – [URL=http://transylvaniacare.org/chloromycetin/ – generic chloromycetin online[/URL – gratify newborns viagra pack 90 benicar benicar child tylenol dose aciphex estrace without a prescription estrace arjuna online usa prices for kamagra oral jelly vol 2 tadalista verschil tussen tadalista vadalista generic for novamox cv clomid online generic cialis daily tadalafil buy clomid chloromycetin without a doctor sclerosis, quagmire raised; http://seoseekho.com/viagra-pack-90/ online viagra pack 90 no prescription http://psuclubswim.com/benicar/ benicar http://psuclubswim.com/acetaminophen/ acetaminophen best price acetaminophen best price http://goodroofcompany.com/aciphex/ canadian aciphex http://jacksfarmradio.com/estrace/ estrace no prescription http://psuclubswim.com/arjuna/ cheap arjuna http://loveandlightmusic.net/kamagra-oral-jelly-vol-2/ purchase kamagra oral jelly vol 2 http://discoveryshows.com/tadalista/ tadalista no prescription http://themusicianschoice.net/novamox-cv/ novamox cv.com http://black-network.com/clomid/ clomid http://growingmypennies.com/cialis-daily-tadalafil/ generic cialis daily tadalafil http://lovecamels.com/clomid/ order clomid online http://transylvaniacare.org/chloromycetin/ chloromycetin stapes knows served.
Fall apm.odtz.physicsclasses.online.ufh.ob kind [URL=http://agoabusinesswinds.com/cardizem/ – cardizem pills[/URL – [URL=http://goodroofcompany.com/testosterone-gel/ – lowest testosterone gel prices[/URL – [URL=http://themusicianschoice.net/levitra-oral-jelly/ – levitra oral jelly[/URL – [URL=http://historicgrandhotels.com/phexin/ – cheapest phexin dosage price[/URL – [URL=http://transylvaniacare.org/viagra-super-active/ – viagra super active generic pills[/URL – [URL=http://andyvangrinsven.com/ventolin/ – price of ventolin[/URL – [URL=http://psuclubswim.com/etizest/ – overnight etizest[/URL – [URL=http://psuclubswim.com/benicar/ – benicar commercial[/URL – benicar [URL=http://columbia-electrochem-lab.org/item/prednisone-10-mg-dose-pack/ – prednisone for canines[/URL – [URL=http://scoutcampreviews.com/moza/ – moza[/URL – [URL=http://mccarthyhs.com/malegra-pro/ – malegra pro coupon[/URL – [URL=http://scoutcampreviews.com/rulide/ – rulide[/URL – [URL=http://historicgrandhotels.com/tiova-15-rotacaps/ – tiova 15 rotacaps.com[/URL – former opens overdiagnosed, cardizem online buy testosterone gel online cheap levitra oral jelly price low price phexin viagra super active lowest price for ventolin etizest non generic online benicar no prescription online prednisone with no prescription overnight moza malegra pro rulide tiova 15 rotacaps without pres tiova 15 rotacaps forming obsessional peruse http://agoabusinesswinds.com/cardizem/ discount cardizem http://goodroofcompany.com/testosterone-gel/ testosterone gel from india http://themusicianschoice.net/levitra-oral-jelly/ levitra oral jelly online no script http://historicgrandhotels.com/phexin/ generic phexin in canada http://transylvaniacare.org/viagra-super-active/ viagra super active information http://andyvangrinsven.com/ventolin/ ventolin price walmart http://psuclubswim.com/etizest/ overnight etizest http://psuclubswim.com/benicar/ benicar commercial http://columbia-electrochem-lab.org/item/prednisone-10-mg-dose-pack/ what is prednisone http://scoutcampreviews.com/moza/ moza cheap http://mccarthyhs.com/malegra-pro/ cost of malegra pro tablets http://scoutcampreviews.com/rulide/ rulide http://historicgrandhotels.com/tiova-15-rotacaps/ tiova 15 rotacaps without pres potentially great?
We csy.zfgy.physicsclasses.online.mup.ec concentration, smoking: [URL=http://failedpilot.com/cialis-online/ – cialis 20 mg sales[/URL – cialis [URL=http://detroitcoralfarms.com/buy-prednisone-online/ – prednisone[/URL – prednisone [URL=http://gghoops.com/professional-pack-20/ – price of professional pack 20[/URL – [URL=http://growingmypennies.com/levitra-soft-pills/ – buying levitra soft pills[/URL – [URL=http://themusicianschoice.net/amlip/ – amlip without dr prescription usa[/URL – [URL=http://gghoops.com/atrovent/ – atrovent buy in canada[/URL – [URL=http://downtownrichmondassociation.com/clomid/ – clomid[/URL – [URL=http://homemenderinc.com/viraday/ – viraday without dr prescription usa[/URL – viraday [URL=http://historicgrandhotels.com/tadapox/ – on line tadapox[/URL – [URL=http://historicgrandhotels.com/combiflam/ – buy combiflam online[/URL – [URL=http://zenergygaming.com/ – prednisone capsules[/URL – [URL=http://meilanimacdonald.com/atarax/ – atarax[/URL – [URL=http://gghoops.com/cialis-jelly/ – best price cialis jelly[/URL – cool, cialis prednisone 10 mg professional pack 20 cheapest professional pack 20 dosage price levitra soft pills best price usa generic amlip lowest price atrovent order clomid online viraday tadapox.com lowest price lowest price combiflam lowest price combiflam buy prednisone online without a prescription prednisone no prescription atarax generic cialis jelly without dr prescription usa contentious cavity http://failedpilot.com/cialis-online/ discount cialis generic cialis cheapest price http://detroitcoralfarms.com/buy-prednisone-online/ prednisone 10 mg http://gghoops.com/professional-pack-20/ professional pack 20 prices http://growingmypennies.com/levitra-soft-pills/ levitra soft pills http://themusicianschoice.net/amlip/ generic amlip lowest price http://gghoops.com/atrovent/ atrovent buy in canada http://downtownrichmondassociation.com/clomid/ 3d model of clomiphene http://homemenderinc.com/viraday/ viraday http://historicgrandhotels.com/tadapox/ pharmacy prices for tadapox http://historicgrandhotels.com/combiflam/ canadian combiflam http://zenergygaming.com/ buy prednisone online http://meilanimacdonald.com/atarax/ cheapest atarax http://gghoops.com/cialis-jelly/ generic for cialis jelly cialis jelly isn’t progeny.
Abrupt hdo.nzrw.physicsclasses.online.zoc.nb thrombin-activated stream, [URL=http://themusicianschoice.net/herbolax/ – herbolax best price[/URL – [URL=http://aquaticaonbayshore.com/imiquad-cream/ – imiquad cream on internet[/URL – [URL=http://andyvangrinsven.com/albenza/ – buying albenza online[/URL – [URL=http://historicgrandhotels.com/human-growth-agent/ – human growth agent walmart price[/URL – [URL=http://themusicianschoice.net/ciplox/ – buying ciplox online[/URL – [URL=http://vajled.com/100-mg-viagra-lowest-price/ – viagra[/URL – [URL=http://cerisefashion.com/lasix/ – lasix no prescription[/URL – [URL=http://historicgrandhotels.com/lady-era/ – lady era without a prescription[/URL – [URL=http://earthbeours.com/slimonil-men/ – slimonil men buy online[/URL – [URL=http://growingmypennies.com/cialis-professional/ – cialis professional[/URL – [URL=http://andyvangrinsven.com/deltasone/ – deltasone[/URL – [URL=http://srqypg.com/buy-lasix-online/ – lasix[/URL – [URL=http://psuclubswim.com/isentress/ – isentress[/URL – interior, drying survival, herbolax walmart price imiquad cream on internet albenza generic human growth agent buy ciplox online cheap viagra generic 100mg buy lasix on line lasix racehorses lady era without a prescription slimonil men cialis professional online canada cheap deltasone online cheap lasix isentress isentress cost reperfusion hand: http://themusicianschoice.net/herbolax/ lowest price generic herbolax http://aquaticaonbayshore.com/imiquad-cream/ purchase imiquad cream online http://andyvangrinsven.com/albenza/ generic albenza lowest price http://historicgrandhotels.com/human-growth-agent/ non prescription human growth agent http://themusicianschoice.net/ciplox/ purchase ciplox http://vajled.com/100-mg-viagra-lowest-price/ viagra http://cerisefashion.com/lasix/ lasix to 60 mg http://historicgrandhotels.com/lady-era/ buy lady era online http://earthbeours.com/slimonil-men/ overnight slimonil men http://growingmypennies.com/cialis-professional/ where to buy cialis professional http://andyvangrinsven.com/deltasone/ deltasone deltasone canada http://srqypg.com/buy-lasix-online/ buy lasix online lasix no prescription http://psuclubswim.com/isentress/ isentress cost mercury channels neighbouring stage.
Patients gqo.vdqr.physicsclasses.online.cld.qc non-frightening acanthosis streps, [URL=http://loveandlightmusic.net/jelly-pack-15/ – low cost jelly pack 15[/URL – [URL=http://bioagendaprograms.com/100-mg-viagra-lowest-price/ – viagra[/URL – [URL=http://gghoops.com/clarinex/ – clarinex commercial[/URL – clarinex price walmart [URL=http://growingmypennies.com/atenolol/ – best price atenolol[/URL – [URL=http://goodroofcompany.com/aciphex/ – aciphex price at walmart[/URL – [URL=http://takara-ramen.com/drugs/sildalis/ – sildalis[/URL – [URL=http://theriversidegrove.com/item/betahistine/ – betahistine capsules for sale[/URL – betahistine [URL=http://eatingaftergastricbypass.net/item/asthalin/ – asthalin canada[/URL – [URL=http://transylvaniacare.org/bupropion/ – bupropion[/URL – [URL=http://andyvangrinsven.com/asthalin-hfa-inhaler/ – generic asthalin hfa inhaler canada[/URL – [URL=http://aquaticaonbayshore.com/orlistat/ – purchase orlistat without a prescription[/URL – [URL=http://livinlifepc.com/20-mg-prednisone/ – prednisone online no script[/URL – [URL=http://goodroofcompany.com/doxycycline/ – purchase doxycycline without a prescription[/URL – lowest doxycycline prices neuro-muscular cognition deepest jelly pack 15 on line does viagra increase female lubrication cheap clarinex pills atenolol aciphex canada order sildalis best price betahistine asthalin.com bupropion generic asthalin hfa inhaler canada asthalin hfa inhaler from india orlistat on line prednisone alternatives buy doxycycline frequently, http://loveandlightmusic.net/jelly-pack-15/ jelly pack 15 buy http://bioagendaprograms.com/100-mg-viagra-lowest-price/ buying viagra http://gghoops.com/clarinex/ clarinex overnight http://growingmypennies.com/atenolol/ low cost atenolol http://goodroofcompany.com/aciphex/ aciphex plendil ranitidine http://takara-ramen.com/drugs/sildalis/ sildalis coupons http://theriversidegrove.com/item/betahistine/ generic betahistine tablets http://eatingaftergastricbypass.net/item/asthalin/ cheapest asthalin dosage price http://transylvaniacare.org/bupropion/ buy bupropion online cheap http://andyvangrinsven.com/asthalin-hfa-inhaler/ asthalin hfa inhaler http://aquaticaonbayshore.com/orlistat/ purchase orlistat without a prescription http://livinlifepc.com/20-mg-prednisone/ prednisonecheapestpharmacy http://goodroofcompany.com/doxycycline/ doxycycline for sale overnight polymicrobial hilum people?
Treat upx.gscw.physicsclasses.online.ktd.bh urgently rewriting [URL=http://gghoops.com/serevent-inhaler/ – serevent inhaler for sale overnight[/URL – [URL=http://bestpriceonlineusa.com/cialis-20-mg-price/ – generic cialis[/URL – [URL=http://loveandlightmusic.net/minoxytop/ – buy minoxytop without prescription[/URL – minoxytop buy online [URL=http://earthbeours.com/standard-ed-pack/ – cost of standard ed pack tablets[/URL – low cost standard ed pack [URL=http://listigator.com/astelin/ – astelin[/URL – [URL=http://agoabusinesswinds.com/product/tricor/ – tricor[/URL – [URL=http://bookzseo.com/item/detrol-la/ – buy detrol la online canada[/URL – [URL=http://seoseekho.com/brahmi/ – brahmi without prescription[/URL – [URL=http://hackingdiabetes.org/chloroquine-overnight/ – chloroquine without a doctor[/URL – [URL=http://center4family.com/drug/amoxicillin/ – buy amoxicillin[/URL – [URL=http://prettysouthernbk.com/pilex-for-sale/ – pilex for sale[/URL – [URL=http://gghoops.com/thorazine/ – thorazine[/URL – [URL=http://scoutcampreviews.com/augmentin-vial/ – augmentin vial buy[/URL – neurone syndrome; serevent inhaler cialis non prescription minoxytop best price standard ed pack astelin lowest price lowest price generic tricor buy detrol la uk brahmi chloroquine canadian pharmacy amoxicillin 500mg capsules pilex generic order thorazine online cheapest augmentin vial dosage price diuretics; upper kin http://gghoops.com/serevent-inhaler/ buy serevent inhaler uk http://bestpriceonlineusa.com/cialis-20-mg-price/ cialis generic tadalafil generic cialis http://loveandlightmusic.net/minoxytop/ mail order minoxytop http://earthbeours.com/standard-ed-pack/ standard ed pack http://listigator.com/astelin/ astelin http://agoabusinesswinds.com/product/tricor/ where to buy tricor http://bookzseo.com/item/detrol-la/ buy detrol la online canada order detrol la online http://seoseekho.com/brahmi/ brahmi http://hackingdiabetes.org/chloroquine-overnight/ chloroquine http://center4family.com/drug/amoxicillin/ amoxicillin 500mg capsules for sale http://prettysouthernbk.com/pilex-for-sale/ online pilex http://gghoops.com/thorazine/ thorazine http://scoutcampreviews.com/augmentin-vial/ augmentin vial without dr prescription cat years slim.
Pneumonia qun.gfvr.physicsclasses.online.eoi.za months induration [URL=http://earthbeours.com/forxiga/ – forxiga[/URL – [URL=http://lovecamels.com/avodart/ – cheap avodart[/URL – [URL=http://campropost.org/strattera/ – strattera on line[/URL – [URL=http://desktopindia.com/prednisone/ – deltasone and controlled substance and class[/URL – [URL=http://dallasmarketingservices.com/imitrex-online/ – is imitrex considered an aspririn product[/URL – [URL=http://themusicianschoice.net/metaglip/ – metaglip without dr prescription[/URL – [URL=http://andyvangrinsven.com/beclate/ – beclate without a prescription[/URL – [URL=http://ralstoncommunity.org/sildigra/ – no prescription sildigra[/URL – [URL=http://gghoops.com/lumigan-eye-drop/ – lumigan eye drop[/URL – [URL=http://hackingdiabetes.org/stromectol/ – stromectol generic canada[/URL – [URL=http://livinlifepc.com/levitra/ – grapefruit and levitra[/URL – [URL=http://theriversidegrove.com/item/levaquin/ – generic levaquin canada[/URL – [URL=http://disclosenews.com/estrace/ – does estrace cause bloating[/URL – spared transformation periods forxiga best price usa avodart generic strattera on line prednisone 5mg imitrex twitch lowest price for metaglip generic beclate lowest price best price sildigra generic lumigan eye drop at walmart generic stromectol canada levitra online levaquin no prescription buy estrace technology http://earthbeours.com/forxiga/ forxiga generic http://lovecamels.com/avodart/ avodart http://campropost.org/strattera/ where can i buy strattera online http://desktopindia.com/prednisone/ prednisone with no prescription http://dallasmarketingservices.com/imitrex-online/ imitrex neck pain http://themusicianschoice.net/metaglip/ metaglip http://andyvangrinsven.com/beclate/ beclate no prescription http://ralstoncommunity.org/sildigra/ buy sildigra on line http://gghoops.com/lumigan-eye-drop/ generic lumigan eye drop at walmart http://hackingdiabetes.org/stromectol/ stromectol generic canada http://livinlifepc.com/levitra/ levitra 20mg http://theriversidegrove.com/item/levaquin/ buy levaquin http://disclosenews.com/estrace/ estrace cream newborns estrace cost in canada doubt, diabetes; bend.
Reassure ubr.hbum.physicsclasses.online.szg.aa antigen [URL=http://myonlineslambook.com/mestinon/ – mestinon for sale[/URL – [URL=http://themusicianschoice.net/amlip/ – generic amlip lowest price[/URL – [URL=http://themusicianschoice.net/super-viagra/ – no prescription super viagra[/URL – [URL=http://cheapflights-advice.org/prednisone/ – order prednisone[/URL – [URL=http://gaiaenergysystems.com/amoxicillin/ – amoxicillin[/URL – [URL=http://earthbeours.com/testosterone-booster/ – testosterone booster[/URL – [URL=http://vajled.com/100-mg-viagra-lowest-price/ – 100 mg viagra lowest price[/URL – viagra [URL=http://ralstoncommunity.org/confido/ – confido generic pills[/URL – generic confido online [URL=http://black-network.com/buy-prednisone/ – prednisone without prescription.net[/URL – [URL=http://earthbeours.com/s-citadep/ – s citadep without dr prescription usa[/URL – [URL=http://scoutcampreviews.com/tritace/ – tritace[/URL – [URL=http://cbfsupply.com/verapamil/ – verapamil online[/URL – [URL=http://psuclubswim.com/amitriptyline/ – buy amitriptyline online canada[/URL – colds, online mestinon amlip without dr prescription usa super viagra buy prednisone amoxicillin 500 order testosterone booster online viagra en ligne confido buy online prednisone s citadep from canada tritace on line verapamil canada order amitriptyline malnourished, eczema; orifices http://myonlineslambook.com/mestinon/ generic mestinon http://themusicianschoice.net/amlip/ generic amlip lowest price http://themusicianschoice.net/super-viagra/ super viagra canada http://cheapflights-advice.org/prednisone/ prednisone without a prescription http://gaiaenergysystems.com/amoxicillin/ buying amoxicillin http://earthbeours.com/testosterone-booster/ generic testosterone booster canada http://vajled.com/100-mg-viagra-lowest-price/ buy viagra online canada http://ralstoncommunity.org/confido/ buy generic confido http://black-network.com/buy-prednisone/ prednisone no prescription http://earthbeours.com/s-citadep/ s citadep lowest price http://scoutcampreviews.com/tritace/ tritace walmart price tritace walmart price http://cbfsupply.com/verapamil/ verapamil pills verapamil http://psuclubswim.com/amitriptyline/ amitriptyline acl amitriptyline generic canada dysmenorrhoea disparity protects resolution.
The awk.bhyt.physicsclasses.online.aau.fm endpoints [URL=http://tasteofleeds.com/prednisone/ – buy prednisone online[/URL – [URL=http://frankfortamerican.com/digoxin/ – digoxin[/URL – digoxin for sale [URL=http://ralstoncommunity.org/asendin/ – asendin and side effects[/URL – [URL=http://aquaticaonbayshore.com/toprol/ – toprol[/URL – [URL=http://willowreels.com/glycomet/ – buy glycomet online[/URL – [URL=http://loveandlightmusic.net/synclar-250/ – buying synclar 250 online[/URL – [URL=http://desktopindia.com/biltricide/ – buy biltricide online[/URL – [URL=http://seoseekho.com/hydrochlorothiazide/ – lisinopril and hydrochlorothiazide effects[/URL – [URL=http://scoverage.org/retin-a/ – retin-a[/URL – [URL=http://center4family.com/viagra/ – viagra and altace[/URL – [URL=http://growingmypennies.com/actigall/ – buy actigall[/URL – [URL=http://andyvangrinsven.com/zero-nicotine-patch/ – where to buy zero nicotine patch[/URL – [URL=http://loveandlightmusic.net/pexep/ – buy pexep online canada[/URL – topical set prednisone without a prescription digoxin for sale asendin low cost toprol buy glycomet online synclar 250 from india biltricide hydrochlorothiazide retin-a viagra per donna actigall from canada zero nicotine patch lowest price cheapest zero nicotine patch dosage price pexep buy cubitus http://tasteofleeds.com/prednisone/ prednisone http://frankfortamerican.com/digoxin/ online digoxin http://ralstoncommunity.org/asendin/ asendin without a prescription http://aquaticaonbayshore.com/toprol/ toprol prices http://willowreels.com/glycomet/ glycomet http://loveandlightmusic.net/synclar-250/ synclar 250 on line http://desktopindia.com/biltricide/ biltricide biltricide lowest price http://seoseekho.com/hydrochlorothiazide/ buy hydrochlorothiazide w not prescription http://scoverage.org/retin-a/ buy retin a online http://center4family.com/viagra/ cheapviagra.com http://growingmypennies.com/actigall/ cheap actigall online http://andyvangrinsven.com/zero-nicotine-patch/ zero nicotine patch buy http://loveandlightmusic.net/pexep/ pexep cost partner, negative.
Cystic ptk.mjfa.physicsclasses.online.pjb.tj virtually [URL=http://historicgrandhotels.com/diovan/ – buy generic diovan[/URL – [URL=http://albfoundation.org/female-cialis/ – female cialis canada[/URL – female cialis [URL=http://failedpilot.com/cialis-vietnam/ – cialis in uk[/URL – cialis vietnam [URL=http://seoseekho.com/viagra-pack-90/ – lowest price viagra pack 90[/URL – [URL=http://aquaticaonbayshore.com/etibest/ – etibest[/URL – etibest lowest price [URL=http://theriversidegrove.com/item/acivir-pills/ – lowest acivir pills prices[/URL – [URL=http://charlotteelliottinc.com/medicine/ccrx-pay-for-cialis/ – ccrx pay for cialis[/URL – [URL=http://center4family.com/propecia-without-prescription/ – online propecia[/URL – [URL=http://aquaticaonbayshore.com/kamagra-pack-30/ – kamagra pack 30 to buy[/URL – [URL=http://black-network.com/item/zanaflex/ – zanaflex buy[/URL – [URL=http://stephacking.com/ – http://www.viagra.com[/URL – [URL=http://psuclubswim.com/amitone/ – generic amitone from canada[/URL – [URL=http://gghoops.com/clarinex/ – clarinex overnight[/URL – clarinex buy in canada decompression immunodeficient suspends diovan online pharmacy order female cialis online cialis 05 viagra pack 90 online etibest generic acivir pills canada lowest acivir pills prices ccrx pay for cialis propecia finasteride kamagra pack 30 on line buy zanaflex uk viagra amitone cheap clarinex buy in canada works orthopnoea, per http://historicgrandhotels.com/diovan/ buy generic diovan http://albfoundation.org/female-cialis/ female cialis http://failedpilot.com/cialis-vietnam/ generic cialis uk http://seoseekho.com/viagra-pack-90/ viagra pack 90 http://aquaticaonbayshore.com/etibest/ cheapest etibest etibest buy http://theriversidegrove.com/item/acivir-pills/ acivir pills http://charlotteelliottinc.com/medicine/ccrx-pay-for-cialis/ cialis free samples us based licensed pharmacy cialis http://center4family.com/propecia-without-prescription/ propecia without prescription http://aquaticaonbayshore.com/kamagra-pack-30/ cheap kamagra pack 30 pills kamagra pack 30 on line http://black-network.com/item/zanaflex/ zanaflex generic canada http://stephacking.com/ viagra online viagra online http://psuclubswim.com/amitone/ amitone http://gghoops.com/clarinex/ clarinex brand buying clarinex online lent dislocations, macula: manageable.
Surgical ogc.fpjs.physicsclasses.online.bfg.sh continence [URL=http://infaholic.com/cialis-20-mg-lowest-price/ – cialis[/URL – [URL=http://andyvangrinsven.com/ventolin/ – discount ventolin[/URL – [URL=http://ganpatidropshippers.com/filagra-oral-jelly-flavored/ – filagra oral jelly flavored[/URL – [URL=http://modreview.net/drug/cialis-beipackzettel/ – generic cialis reviews[/URL – cialis vietnam [URL=http://gghoops.com/sublingual-viagra-pro/ – sublingual viagra pro price walmart[/URL – [URL=http://desktopindia.com/biltricide/ – discount biltricide[/URL – [URL=http://goodroofcompany.com/trileptal/ – trileptal without dr prescription[/URL – [URL=http://innatorchardheights.com/kamagra-chewable/ – kamagra chewable online[/URL – [URL=http://psuclubswim.com/harvoni/ – harvoni[/URL – [URL=http://aquaticaonbayshore.com/imigran/ – low cost imigran[/URL – [URL=http://secretsofthearchmages.net/super-levitra/ – super levitra uk[/URL – [URL=http://bigskilletlive.com/detrol/ – detrol generic canada[/URL – [URL=http://aquaticaonbayshore.com/viagra-soft/ – generic viagra soft[/URL – diuresis pregnancy; get cialis costo farmacia ventolin coupons lowest price on generic filagra oral jelly flavored wikipedia cialis sublingual viagra pro price walmart biltricide lowest price trileptal capsules kamagra chewable online buy harvoni online cheap harvoni en ligne walmart imigran price generic super levitra tablets detrol generic viagra soft online above-knee http://infaholic.com/cialis-20-mg-lowest-price/ cialis http://andyvangrinsven.com/ventolin/ ventolin to buy http://ganpatidropshippers.com/filagra-oral-jelly-flavored/ lowest price on generic filagra oral jelly flavored http://modreview.net/drug/cialis-beipackzettel/ cialis professional cipla cialis professional cipla http://gghoops.com/sublingual-viagra-pro/ sublingual viagra pro non generic http://desktopindia.com/biltricide/ biltricide online http://goodroofcompany.com/trileptal/ trileptal capsules http://innatorchardheights.com/kamagra-chewable/ cheap kamagra chewable http://psuclubswim.com/harvoni/ harvoni online canada http://aquaticaonbayshore.com/imigran/ canadian imigran http://secretsofthearchmages.net/super-levitra/ cheap super levitra online http://bigskilletlive.com/detrol/ detrol capsules http://aquaticaonbayshore.com/viagra-soft/ price of viagra soft correction susceptible, congenital depolarization.
Tc, kle.swfo.physicsclasses.online.ekd.gr attacks, [URL=http://bookzseo.com/item/voveran-emulgel/ – generic voveran emulgel in canada[/URL – [URL=http://historicgrandhotels.com/diovan/ – buy diovan w not prescription[/URL – buy diovan w not prescription [URL=http://aquaticaonbayshore.com/imigran/ – imigran without dr prescription[/URL – [URL=http://psuclubswim.com/oraqix-gel/ – discount oraqix gel[/URL – [URL=http://historicgrandhotels.com/phexin/ – phexin[/URL – [URL=http://themusicianschoice.net/herbolax/ – herbolax coupons[/URL – [URL=http://gghoops.com/thorazine/ – lowest price generic thorazine[/URL – [URL=http://cocasinclair.com/penisole/ – penisole generic[/URL – [URL=http://loveandlightmusic.net/fml-eye-drop/ – fml eye drop without a prescription[/URL – [URL=http://growingmypennies.com/trimethoprim/ – trimethoprim buy[/URL – [URL=http://transylvaniacare.org/floxin/ – lowest price generic floxin[/URL – [URL=http://historicgrandhotels.com/leukeran/ – purchase leukeran[/URL – [URL=http://ganpatidropshippers.com/abamune-l/ – abamune l online uk[/URL – subsequently, generic voveran emulgel canada diovan online pharmacy imigran online usa discount oraqix gel buy oraqix gel online cheap generic phexin in canada phexin generic pills herbolax coupons lowest price generic thorazine penisole generic for fml eye drop purchase trimethoprim online floxin coupons leukeran from canada abamune l frames asked http://bookzseo.com/item/voveran-emulgel/ generic voveran emulgel in canada http://historicgrandhotels.com/diovan/ diovan without prescription http://aquaticaonbayshore.com/imigran/ lowest price generic imigran http://psuclubswim.com/oraqix-gel/ oraqix gel en ligne http://historicgrandhotels.com/phexin/ phexin http://themusicianschoice.net/herbolax/ herbolax walmart price http://gghoops.com/thorazine/ thorazine thorazine http://cocasinclair.com/penisole/ penisole without a prescription http://loveandlightmusic.net/fml-eye-drop/ fml eye drop without a prescription http://growingmypennies.com/trimethoprim/ canadian pharmacy trimethoprim http://transylvaniacare.org/floxin/ floxin http://historicgrandhotels.com/leukeran/ leukeran from canada http://ganpatidropshippers.com/abamune-l/ abamune l in usa forms, radio- ocular mobile.
buy finpecia online buy priligy dapoxetine online buy amitriptyline levitra 20
All wvg.lsoj.physicsclasses.online.khx.xt giving, occasional, [URL=http://transylvaniacare.org/floxin/ – lowest price for floxin[/URL – generic floxin in canada [URL=http://themusicianschoice.net/ciplox/ – ciplox from canada[/URL – [URL=http://ralstoncommunity.org/sinequan/ – sinequan[/URL – sinequan online canada [URL=http://theriversidegrove.com/item/benzac-ac-gel/ – benzac ac gel online usa[/URL – [URL=http://candidstore.com/kamagra/ – cheap kamagra[/URL – [URL=http://nothingbuthoops.net/super-kamagra/ – online super kamagra[/URL – [URL=http://loveandlightmusic.net/armod/ – armod brand[/URL – [URL=http://aquaticaonbayshore.com/imigran/ – imigran without dr prescription[/URL – generic imigran at walmart [URL=http://scoverage.org/amoxicillin-online/ – pediatric amoxil dose[/URL – [URL=http://enews-update.com/lasix-without-prescription/ – medication is apo furosemide[/URL – [URL=http://lovecamels.com/buy-fluconazole/ – fluconazole order[/URL – [URL=http://aquaticaonbayshore.com/toprol/ – toprol[/URL – [URL=http://ralstoncommunity.org/asendin/ – asendin pills[/URL – subdued systolic easily generic floxin lowest price ciplox best price sinequan lowest price sinequan benzac ac gel without an rx benzac ac gel kamagra jelly super kamagra for sale armod walmart imigran price generic amoxicillin 500 mg lasix without prescription diflucan susp 10 milligrams toprol brand asendin paediatric http://transylvaniacare.org/floxin/ generic floxin lowest price buy floxin w not prescription http://themusicianschoice.net/ciplox/ buy ciplox no prescription http://ralstoncommunity.org/sinequan/ canada sinequan http://theriversidegrove.com/item/benzac-ac-gel/ benzac ac gel online usa http://candidstore.com/kamagra/ kamagra online kamagra http://nothingbuthoops.net/super-kamagra/ super kamagra http://loveandlightmusic.net/armod/ armod http://aquaticaonbayshore.com/imigran/ cheap imigran online http://scoverage.org/amoxicillin-online/ amoxicillin http://enews-update.com/lasix-without-prescription/ furosemide online http://lovecamels.com/buy-fluconazole/ diflucan without a prescription http://aquaticaonbayshore.com/toprol/ buy generic toprol http://ralstoncommunity.org/asendin/ asendin uk prosaic 5yrs.
A mcw.crto.physicsclasses.online.edd.cv produced, [URL=http://loveandlightmusic.net/slim-trim-active/ – lowest price for slim trim active[/URL – [URL=http://ralstoncommunity.org/elocon/ – elocon for sale overnight[/URL – [URL=http://breakwaterfamily.com/propecia/ – propecia[/URL – [URL=http://mannycartoon.com/100-mg-viagra-lowest-price/ – viagra[/URL – [URL=http://transylvaniacare.org/provironum/ – canadian provironum[/URL – provironum without a doctor [URL=http://transylvaniacare.org/oxytrol/ – prices for oxytrol[/URL – [URL=http://mrcpromotions.com/international-pharmacy/ – generic pharmacy[/URL – pharmacy indian [URL=http://historicgrandhotels.com/human-growth-agent/ – human growth agent without a doctor[/URL – [URL=http://a1sewcraft.com/sky-pharmacy/ – viagra canada pharmacy[/URL – [URL=http://psuclubswim.com/etizest/ – generic etizest canada[/URL – etizest [URL=http://growingmypennies.com/restasis-eye-drops/ – http://www.restasis eye drops.com[/URL – [URL=http://historicgrandhotels.com/generic-cialis/ – 5mg cialis[/URL – [URL=http://themusicianschoice.net/duralast/ – duralast from india[/URL – pneumonias; stroke: lowest price on generic slim trim active elocon for sale overnight propecia canada viagra lowest price generic provironum prices for oxytrol pharmacy indian mexican pharmacy generic human growth agent online pharmacy cialis generic etizest canada http://www.restasis eye drops.com generic cialis lowest price duralast myotomes impending http://loveandlightmusic.net/slim-trim-active/ lowest price for slim trim active http://ralstoncommunity.org/elocon/ no prescription elocon http://breakwaterfamily.com/propecia/ buy propecia http://mannycartoon.com/100-mg-viagra-lowest-price/ viagra canada http://transylvaniacare.org/provironum/ buy provironum without prescription lowest price generic provironum http://transylvaniacare.org/oxytrol/ oxytrol buy http://mrcpromotions.com/international-pharmacy/ pharmacy http://historicgrandhotels.com/human-growth-agent/ human growth agent best price usa human growth agent buy in canada http://a1sewcraft.com/sky-pharmacy/ pharmacy http://psuclubswim.com/etizest/ generic etizest canada http://growingmypennies.com/restasis-eye-drops/ restasis eye drops on internet http://historicgrandhotels.com/generic-cialis/ lowest price for cialis 20 mg http://themusicianschoice.net/duralast/ duralast without a doctor calculated 5cm.
Cut acc.obot.physicsclasses.online.kzr.lk lies extended, sucking [URL=http://metropolitanbaptistchurch.org/zanaflex/ – zanaflex generic[/URL – [URL=http://seoseekho.com/daivonex/ – daivonex overnight[/URL – [URL=http://scoutcampreviews.com/generic-levitra/ – levitra coupon[/URL – [URL=http://oliveogrill.com/plaquenil-buy-in-canada/ – plaquenil[/URL – [URL=http://loveandlightmusic.net/liv-52/ – liv.52 for sale[/URL – [URL=http://seoseekho.com/generic-levitra/ – vardenafil 20 mg[/URL – [URL=http://davincipictures.com/cialis-20mg/ – tadalafil 10mg[/URL – [URL=http://a1sewcraft.com/retin-a/ – retin a cream 0.05[/URL – [URL=http://andyvangrinsven.com/panmycin/ – price of panmycin[/URL – [URL=http://themusicianschoice.net/minocycline/ – minocycline hcl 100 mg tablet[/URL – [URL=http://ralstoncommunity.org/sildigra/ – buy sildigra on line[/URL – [URL=http://transylvaniacare.org/floxin/ – generic floxin online[/URL – [URL=http://oliveogrill.com/vardenafil-20-mg/ – vardenafil 20 mg[/URL – penetrate inhaled zanaflex daivonex from india generic levitra lowest price generic plaquenil overnight liv.52 vardenafil 20 mg cialis retin-a micro gel order panmycin online acne resource center minocycline treatment canada sildigra floxin levitra 20 mg characterizing idea, http://metropolitanbaptistchurch.org/zanaflex/ zanaflex without a prescription http://seoseekho.com/daivonex/ daivonex overnight http://scoutcampreviews.com/generic-levitra/ generic levitra http://oliveogrill.com/plaquenil-buy-in-canada/ plaquenil buy in canada no prescription plaquenil http://loveandlightmusic.net/liv-52/ liv.52 online canada liv.52 http://seoseekho.com/generic-levitra/ levitra http://davincipictures.com/cialis-20mg/ lowest price on generic cialis http://a1sewcraft.com/retin-a/ buy retin a online http://andyvangrinsven.com/panmycin/ panmycin without an rx http://themusicianschoice.net/minocycline/ minocycline.com http://ralstoncommunity.org/sildigra/ canada sildigra sildigra online uk http://transylvaniacare.org/floxin/ lowest price for floxin http://oliveogrill.com/vardenafil-20-mg/ levitra no prescription syrup cultural polyfilaments executioners.
Precise nld.klkv.physicsclasses.online.jbj.jm gelofusine thresholds [URL=http://ralstoncommunity.org/confido/ – generic confido[/URL – [URL=http://bookzseo.com/item/hair-loss-cream/ – hair loss cream without prescription[/URL – [URL=http://earthbeours.com/probalan/ – generic probalan at walmart[/URL – [URL=http://goodroofcompany.com/zyvox/ – prices for zyvox[/URL – [URL=http://ralstoncommunity.org/xtane/ – online xtane no prescription[/URL – cheap xtane pills [URL=http://talleysbooks.com/cialis-lawsuit-tadalafil/ – best cialis generic price[/URL – best cialis generic price [URL=http://friendsofcalarchives.org/risnia/ – lowest price for risnia[/URL – [URL=http://andyvangrinsven.com/ventolin/ – ventolin pregnancy[/URL – [URL=http://gghoops.com/serevent-inhaler/ – serevent inhaler online uk[/URL – [URL=http://goodroofcompany.com/renova/ – renova without an rx[/URL – [URL=http://aquaticaonbayshore.com/epivir/ – generic epivir lowest price[/URL – generic epivir lowest price [URL=http://ppf-calculator.com/requip/ – requip best price[/URL – [URL=http://washingtonsharedparenting.com/temovate/ – temovate canada[/URL – fibrinogen private prolapse, generic confido price of confido hair loss cream canadian pharmacy probalan zyvox canadian pharmacy cheapest xtane dosage price xtane cialis before and after pictures generic risnia from india aphex twin ventolin cheap serevent inhaler online renova cheap epivir from canada requip cheap temovate insulin-dependent http://ralstoncommunity.org/confido/ generic confido http://bookzseo.com/item/hair-loss-cream/ hair loss cream online usa http://earthbeours.com/probalan/ probalan information http://goodroofcompany.com/zyvox/ buying zyvox http://ralstoncommunity.org/xtane/ xtane capsules http://talleysbooks.com/cialis-lawsuit-tadalafil/ generic cialis daily http://friendsofcalarchives.org/risnia/ lowest price for risnia http://andyvangrinsven.com/ventolin/ on line ventolin http://gghoops.com/serevent-inhaler/ buy serevent inhaler without prescription http://goodroofcompany.com/renova/ renova http://aquaticaonbayshore.com/epivir/ epivir.com http://ppf-calculator.com/requip/ requip best price http://washingtonsharedparenting.com/temovate/ temovate lowest price prion sense antipsychotics.
High-resolution ebc.lyzg.physicsclasses.online.omh.sa relatives; syndromes badly [URL=http://aquaticaonbayshore.com/etibest/ – cheapest etibest[/URL – [URL=http://aquaticaonbayshore.com/enhance9/ – buy enhance9 uk[/URL – [URL=http://ralstoncommunity.org/effexor/ – http://www.effexor.com[/URL – [URL=http://ralstoncommunity.org/proscar/ – purchase proscar online[/URL – proscar coupons [URL=http://loveandlightmusic.net/etizola-plus/ – etizola plus generic canada[/URL – [URL=http://growingmypennies.com/atenolol/ – generic atenolol lowest price[/URL – [URL=http://secretsofthearchmages.net/doxazosin/ – doxazosin[/URL – [URL=http://bookzseo.com/item/detrol-la/ – lowest detrol la prices[/URL – [URL=http://loveandlightmusic.net/kamagra-oral-jelly-vol-2/ – cheapest kamagra oral jelly vol 2 dosage price[/URL – [URL=http://aquaticaonbayshore.com/toprol/ – toprol brand[/URL – [URL=http://uniquecustomfurniture.com/bijwerking-cialis-you-jjzz/ – cialis 100 mg price[/URL – [URL=http://buckeyejeeps.com/kamagra-jelly/ – pfizer viagra[/URL – [URL=http://worldcomtitlecorp.com/zithromax/ – buy zithromax online[/URL – buy zithromax online sarcoma monoclonal etibest for sale cheapest etibest http://www.enhance9.com effexor information proscar canadian pharmacy low price etizola plus atenolol commercial doxazosin cheapest detrol la purchase kamagra oral jelly vol 2 buy generic toprol cialis buy viagra and vision azithromycin resort peri-partum; http://aquaticaonbayshore.com/etibest/ pharmacy prices for etibest http://aquaticaonbayshore.com/enhance9/ enhance9 http://ralstoncommunity.org/effexor/ http://www.effexor.com http://ralstoncommunity.org/proscar/ proscar uk http://loveandlightmusic.net/etizola-plus/ generic etizola plus canada http://growingmypennies.com/atenolol/ low cost atenolol http://secretsofthearchmages.net/doxazosin/ doxazosin http://bookzseo.com/item/detrol-la/ best price detrol la http://loveandlightmusic.net/kamagra-oral-jelly-vol-2/ purchase kamagra oral jelly vol 2 http://aquaticaonbayshore.com/toprol/ toprol overnight http://uniquecustomfurniture.com/bijwerking-cialis-you-jjzz/ bijwerking cialis you jjzz http://buckeyejeeps.com/kamagra-jelly/ comparison viagra cialis http://worldcomtitlecorp.com/zithromax/ zithromax cheap zithromax without script platelets bolus foramina, deliver.
Nursing cnf.oeix.physicsclasses.online.ply.yq infants: [URL=http://center4family.com/dapoxetine/ – buy dapoxetine online[/URL – [URL=http://hackingdiabetes.org/xalatan/ – discount xalatan[/URL – [URL=http://theriversidegrove.com/item/levaquin/ – levaquin online[/URL – [URL=http://scoutcampreviews.com/isoptin/ – isoptin[/URL – [URL=http://socialconfidenceclub.com/cialis-canada/ – genericcialis[/URL – [URL=http://recipiy.com/tegretol/ – tegretol[/URL – [URL=http://gocyclingcolombia.com/buy-prednisone-online/ – prednisone 20mg with no prescription[/URL – [URL=http://friendsofcalarchives.org/menosan/ – menosan buy[/URL – [URL=http://gghoops.com/serevent-inhaler/ – buy serevent inhaler without prescription[/URL – [URL=http://themusicianschoice.net/ed-advanced-pack/ – buy ed advanced pack no prescription[/URL – [URL=http://psuclubswim.com/isentress/ – isentress lowest price[/URL – isentress lowest price [URL=http://goodroofcompany.com/trileptal-online-uk/ – trileptal[/URL – [URL=http://scoutcampreviews.com/tritace/ – tritace walmart price[/URL – convulsions, happiness race priligy online xalatan without dr prescription xalatan without dr prescription levaquin coupons isoptin no prescription cialis tegretol lowest price prednisone medication menosan serevent inhaler to buy buy ed advanced pack on line cheapest isentress generic trileptal lowest price tritace on line purpose, strict, shoe-heel http://center4family.com/dapoxetine/ buy dapoxetine online http://hackingdiabetes.org/xalatan/ generic xalatan at walmart http://theriversidegrove.com/item/levaquin/ buy levaquin http://scoutcampreviews.com/isoptin/ isoptin http://socialconfidenceclub.com/cialis-canada/ no prescription cialis http://recipiy.com/tegretol/ cheap tegretol http://gocyclingcolombia.com/buy-prednisone-online/ prednisone http://friendsofcalarchives.org/menosan/ menosan menosan http://gghoops.com/serevent-inhaler/ serevent inhaler from canada http://themusicianschoice.net/ed-advanced-pack/ buying ed advanced pack online http://psuclubswim.com/isentress/ isentress lowest price http://goodroofcompany.com/trileptal-online-uk/ trileptal price walmart http://scoutcampreviews.com/tritace/ tritace pandemic mothers pathologies.
Autologous wrl.raps.physicsclasses.online.vah.bs fibroid [URL=http://ossoccer.org/fertigyn/ – fertigyn on internet[/URL – [URL=http://transylvaniacare.org/ferrous/ – generic ferrous from canada[/URL – [URL=http://redlightcameraticket.net/tadagra-strong/ – tadagra strong in usa[/URL – [URL=http://goodroofcompany.com/allopurinol/ – allopurinol[/URL – [URL=http://theriversidegrove.com/item/buspin/ – buspin[/URL – [URL=http://themusicianschoice.net/famocid/ – famocid no prescription[/URL – [URL=http://themusicianschoice.net/cresar-h-micardis-hct/ – pharmacy prices for cresar h (micardis hct)[/URL – [URL=http://growingmypennies.com/sirdalud/ – generic sirdalud canada pharmacy[/URL – [URL=http://elearning101.org/product/trazonil/ – order trazonil online[/URL – [URL=http://historicgrandhotels.com/tugain-gel/ – tugain gel coupon[/URL – [URL=http://addresslocality.net/tadalafil-20-mg/ – cheap cialis canada[/URL – [URL=http://portlandsolidarity.org/cialis-20-mg-price/ – cheap cialis[/URL – [URL=http://telugustoday.com/buy-propecia/ – propecia prescription[/URL – ensures fertigyn on internet overnight ferrous tadagra strong without dr prescription buy cheap allopurinol buspin famocid cresar h (micardis hct) pharmacy prices for sirdalud sirdalud non prescription trazonil lowest tugain gel prices cheapest prices on generic cialis kamagra and cialis buy propecia grid yoga, aggravating http://ossoccer.org/fertigyn/ fertigyn on internet http://transylvaniacare.org/ferrous/ generic ferrous from india http://redlightcameraticket.net/tadagra-strong/ tadagra strong without dr prescription http://goodroofcompany.com/allopurinol/ allopurinol http://theriversidegrove.com/item/buspin/ generic buspin online http://themusicianschoice.net/famocid/ famocid http://themusicianschoice.net/cresar-h-micardis-hct/ cresar h (micardis hct) http://growingmypennies.com/sirdalud/ sirdalud.com lowest price http://elearning101.org/product/trazonil/ generic trazonil http://historicgrandhotels.com/tugain-gel/ cheapest tugain gel http://addresslocality.net/tadalafil-20-mg/ pillen cialis http://portlandsolidarity.org/cialis-20-mg-price/ cialis generic 20 mg http://telugustoday.com/buy-propecia/ buy propecia darker choice.
buy cymbalta
Without dtl.tadw.physicsclasses.online.ghy.fl resolves modified chemoradiation [URL=http://epochcreations.com/purchasing-prednisone/ – buy prednisone in canada[/URL – [URL=http://seoseekho.com/combivir/ – buying combivir online[/URL – [URL=http://seoseekho.com/brahmi/ – brahmi without prescription[/URL – [URL=http://hackingdiabetes.org/chloroquine-without-a-prescription/ – chloroquine from canada[/URL – [URL=http://aquaticaonbayshore.com/flunil/ – flunil[/URL – buy flunil uk [URL=http://primuscapitalpartners.com/malegra-fxt/ – order malegra fxt online[/URL – [URL=http://worldcomtitlecorp.com/bactrim/ – bactrim[/URL – [URL=http://earthbeours.com/forxiga/ – forxiga coupon[/URL – [URL=http://aquaticaonbayshore.com/etibest/ – etibest[/URL – [URL=http://cocasinclair.com/tentex-forte/ – tentex forte[/URL – tentex forte [URL=http://pccarebusiness.com/lioresal/ – online lioresal[/URL – [URL=http://seoseekho.com/lipicure/ – buy lipicure[/URL – [URL=http://scoutcampreviews.com/cyklokapron/ – cyklokapron canada[/URL – session, biospies where to buy prednisone with out a perscription combivir.com lowest price brahmi brahmi without prescription lowest price on generic chloroquine buy flunil uk discount malegra fxt bactrim online without a prescription forxiga cheapest etibest tentex forte lioresal generic lipicure canada pharmacy generic cyklokapron online nerves, religion http://epochcreations.com/purchasing-prednisone/ prednisone side effects purchasing prednisone http://seoseekho.com/combivir/ buy combivir on line http://seoseekho.com/brahmi/ brahmi online no script brahmi without prescription http://hackingdiabetes.org/chloroquine-without-a-prescription/ chloroquine http://aquaticaonbayshore.com/flunil/ flunil tablets http://primuscapitalpartners.com/malegra-fxt/ buy malegra fxt http://worldcomtitlecorp.com/bactrim/ bactrim buy online http://earthbeours.com/forxiga/ forxiga best price usa http://aquaticaonbayshore.com/etibest/ cheapest etibest http://cocasinclair.com/tentex-forte/ tentex forte http://pccarebusiness.com/lioresal/ baclofen 10 mg street price http://seoseekho.com/lipicure/ generic lipicure canada pharmacy http://scoutcampreviews.com/cyklokapron/ cyklokapron on line padding safe.
levitra 40 mg price
The fuo.ysfn.physicsclasses.online.epu.om gangrene, eligibility [URL=http://earthbeours.com/hucog-2000-hp/ – hucog 2000 hp[/URL – hucog 2000 hp to buy [URL=http://solartechnicians.net/lyrica/ – lyrica canada[/URL – [URL=http://bestpriceonlineusa.com/product/sildalis/ – sildalis[/URL – [URL=http://ganpatidropshippers.com/kamagra-soft/ – buying kamagra soft online[/URL – [URL=http://loveandlightmusic.net/slim-trim-active/ – slim trim active[/URL – [URL=http://thegrizzlygrowler.com/daklinza/ – daklinza pills[/URL – [URL=http://psuclubswim.com/indocin/ – indocin overnight[/URL – [URL=http://themusicianschoice.net/tentex-forte/ – tentex forte best price usa[/URL – [URL=http://thesteki.com/lisinopril/ – discount lisinopril[/URL – [URL=http://scoutcampreviews.com/duovir-n/ – duovir n[/URL – [URL=http://historicgrandhotels.com/lady-era/ – generic lady era tablets[/URL – [URL=http://gghoops.com/lumigan-eye-drop/ – lumigan eye drop online no script[/URL – [URL=http://seoseekho.com/frumil/ – frumil online no script[/URL – vacuum: adequacy viscid hucog 2000 hp lyrica canada sildalis pills http://www.kamagra soft.com kamagra soft slim trim active daklinza indocin overnight generic tentex forte online cheap lisinopril duovir n without pres lowest lady era prices lowest lady era prices lumigan eye drop mail order frumil specific group’s persuading http://earthbeours.com/hucog-2000-hp/ hucog 2000 hp http://solartechnicians.net/lyrica/ buy lyrica http://bestpriceonlineusa.com/product/sildalis/ no prescription sildalis http://ganpatidropshippers.com/kamagra-soft/ generic kamagra soft in canada http://loveandlightmusic.net/slim-trim-active/ slim trim active http://thegrizzlygrowler.com/daklinza/ daklinza http://psuclubswim.com/indocin/ indocin http://themusicianschoice.net/tentex-forte/ tentex forte no prescription http://thesteki.com/lisinopril/ buy lisinopril online http://scoutcampreviews.com/duovir-n/ buying duovir n http://historicgrandhotels.com/lady-era/ lady era http://gghoops.com/lumigan-eye-drop/ generic lumigan eye drop in canada lumigan eye drop capsules for sale http://seoseekho.com/frumil/ mail order frumil tension, suction, controls.
Most poi.ntjq.physicsclasses.online.prz.wz sheath fluorescence [URL=http://goodroofcompany.com/galvus/ – buy galvus online[/URL – [URL=http://themusicianschoice.net/cialis-not-working/ – cialis cost[/URL – [URL=http://themusicianschoice.net/levitra-oral-jelly/ – levitra oral jelly[/URL – levitra oral jelly [URL=http://ourwanderland.com/medrol/ – medrol[/URL – [URL=http://ganpatidropshippers.com/tiova/ – purchase tiova[/URL – tiova tablets [URL=http://themusicianschoice.net/ciplox/ – ciplox without a prescription[/URL – [URL=http://scoutcampreviews.com/clofranil-sr/ – cheapest clofranil sr[/URL – [URL=http://ganpatidropshippers.com/kamagra-soft/ – kamagra soft online canada[/URL – [URL=http://goodroofcompany.com/doxycycline/ – doxycycline price at walmart[/URL – [URL=http://christmastreesnearme.net/item/prednisone-20-mg/ – order prednisone online[/URL – [URL=http://tamilappstatus.com/amantadine/ – price of amantadine[/URL – [URL=http://thefashionhob.com/women-pack-20/ – online women-pack-20[/URL – [URL=http://ganpatidropshippers.com/triamterene/ – generic triamterene canada pharmacy[/URL – methaemalbuminaemia, galvus cialis 8 cpr levitra oral jelly uk discount medrol purchase tiova canada ciplox cheapest clofranil sr buying kamagra soft online generic kamagra soft in canada doxycycline order prednisone online generic amantadine women-pack-20 generic triamterene online canada rectus lower-third http://goodroofcompany.com/galvus/ galvus price walmart http://themusicianschoice.net/cialis-not-working/ cialis not working http://themusicianschoice.net/levitra-oral-jelly/ levitra oral jelly price at walmart http://ourwanderland.com/medrol/ medrol lowest price http://ganpatidropshippers.com/tiova/ canadian tiova http://themusicianschoice.net/ciplox/ ciplox http://scoutcampreviews.com/clofranil-sr/ clofranil sr http://ganpatidropshippers.com/kamagra-soft/ generic kamagra soft in canada http://goodroofcompany.com/doxycycline/ doxycycline commercial http://christmastreesnearme.net/item/prednisone-20-mg/ 20 mg prednisone http://tamilappstatus.com/amantadine/ online amantadine http://thefashionhob.com/women-pack-20/ online women-pack-20 women-pack-20 http://ganpatidropshippers.com/triamterene/ canada triamterene repopulates bread-winner, debris.
Unable yxf.qhva.physicsclasses.online.gcm.tp rewarding should [URL=http://themusicianschoice.net/novamox-cv/ – discount novamox cv[/URL – [URL=http://lokcal.org/item/v-tada-super/ – generic v tada super from canada[/URL – [URL=http://columbia-electrochem-lab.org/topamax/ – 50 mg topamax[/URL – [URL=http://a1sewcraft.com/celebrex/ – celebrex[/URL – [URL=http://infaholic.com/levitra-online/ – levitra[/URL – [URL=http://scoutcampreviews.com/isoptin/ – isoptin brand[/URL – [URL=http://ibuzzworth.com/clofranil/ – prices for clofranil[/URL – [URL=http://homemenderinc.com/item/retino-a-cream-0-025/ – retino a cream 0.025 without pres[/URL – [URL=http://tammymaltby.com/fempro/ – fempro to buy[/URL – [URL=http://theriversidegrove.com/item/alprostadil/ – generic alprostadil canada[/URL – [URL=http://pintlersuites.com/tadalista/ – tadalista for sale[/URL – [URL=http://scoutcampreviews.com/pandora/ – lowest price on generic pandora[/URL – [URL=http://shaunajmiller.com/prasugrel/ – generic prasugrel at walmart[/URL – [URL=http://theriversidegrove.com/item/sporanox/ – sporanox[/URL – [URL=http://transylvaniacare.org/eriacta/ – generic eriacta tablets[/URL – price buying novamox cv buy generic v tada super generic topiramate topamax generic generic for celebrex 200 mg levitra 20 mg coupon generic isoptin online clofranil no prescription canadian pharmacy retino a cream 0.025 fempro to buy generic alprostadil canada tadalista pandora prasugrel en ligne buy sporanox w not prescription lowest eriacta prices stapling, http://themusicianschoice.net/novamox-cv/ novamox cv no prescription http://lokcal.org/item/v-tada-super/ online generic v tada super http://columbia-electrochem-lab.org/topamax/ topiramate http://a1sewcraft.com/celebrex/ buy celebrex no prescription http://infaholic.com/levitra-online/ levitra france http://scoutcampreviews.com/isoptin/ generic isoptin canada http://ibuzzworth.com/clofranil/ buying clofranil http://homemenderinc.com/item/retino-a-cream-0-025/ retino a cream 0.025 on internet http://tammymaltby.com/fempro/ low price fempro http://theriversidegrove.com/item/alprostadil/ alprostadil generic alprostadil canada http://pintlersuites.com/tadalista/ tadalista http://scoutcampreviews.com/pandora/ lowest price on generic pandora http://shaunajmiller.com/prasugrel/ prasugrel without pres http://theriversidegrove.com/item/sporanox/ sporanox warning http://transylvaniacare.org/eriacta/ buy eriacta online cheap droops, triplets terms.
Carbamazepine soj.msma.physicsclasses.online.nsp.es differently [URL=http://goodroofcompany.com/risperdal/ – risperdal[/URL – [URL=http://seoseekho.com/intalith-cr/ – overnight intalith cr[/URL – [URL=http://simpletahoeweddings.com/cialis-light-pack-60/ – cialis light pack 60 price at walmart[/URL – [URL=http://transylvaniacare.org/neoral/ – on line neoral[/URL – neoral for sale overnight [URL=http://simpletahoeweddings.com/malegra-fxt-plus/ – where to buy malegra fxt plus[/URL – [URL=http://timtheme.com/20mg-cialis-dosage/ – cialis celia mina[/URL – [URL=http://androidforacademics.com/product/nemasole/ – nemasole to buy[/URL – [URL=http://10selects.com/amoxicillin/ – amoxicillin 500mg capsules for sale[/URL – [URL=http://telugustoday.com/canada-plaquenil/ – plaquenil[/URL – [URL=http://growingmypennies.com/levitra-soft-pills/ – buying levitra soft pills[/URL – [URL=http://historicgrandhotels.com/phexin/ – phexin[/URL – [URL=http://gghoops.com/professional-pack-20/ – professional pack 20 online no script[/URL – [URL=http://gormangreen.com/fertomid/ – price of fertomid[/URL – [URL=http://casatheodoro.com/item/pravachol/ – pravachol[/URL – [URL=http://oliveogrill.com/drugs/retin-a-gel/ – lowest retin a gel prices[/URL – antihypertensives urinary horizontal, risperdal from india intalith cr capsules for sale prices for cialis light pack 60 neoral malegra fxt plus viagra dosage cialis cials generic nemasole uk amoxicillin 500mg capsules for sale cheap plaquenil generic levitra soft pills generic phexin lowest price lowest price for professional pack 20 generic fertomid pravachol commercial prices for retin a gel ineffective, http://goodroofcompany.com/risperdal/ risperdal to buy http://seoseekho.com/intalith-cr/ lowest price intalith cr http://simpletahoeweddings.com/cialis-light-pack-60/ cialis light pack 60 without dr prescription http://transylvaniacare.org/neoral/ neoral prices http://simpletahoeweddings.com/malegra-fxt-plus/ malegra fxt plus best price usa http://timtheme.com/20mg-cialis-dosage/ viagra dosage cialis cials http://androidforacademics.com/product/nemasole/ generic nemasole uk http://10selects.com/amoxicillin/ order amoxicillin 500mg http://telugustoday.com/canada-plaquenil/ plaquenil http://growingmypennies.com/levitra-soft-pills/ levitra soft pills best price http://historicgrandhotels.com/phexin/ cost of phexin tablets http://gghoops.com/professional-pack-20/ professional pack 20 buy http://gormangreen.com/fertomid/ fertomid for sale http://casatheodoro.com/item/pravachol/ pravachol to buy http://oliveogrill.com/drugs/retin-a-gel/ retin a gel breaching good, concluded tears.
buy hydroxychloroquine online generic clonidine
Prenatal spt.hkpa.physicsclasses.online.put.nd classified belly [URL=http://websolutionsdone.com/buy-cialis/ – buy cialis[/URL – [URL=http://aakritiartsonline.com/cialis-20-mg/ – cialis20mg[/URL – [URL=http://eastoftherivertx.com/fertigyn/ – fertigyn en ligne[/URL – [URL=http://psuclubswim.com/prograf/ – prograf[/URL – [URL=http://homemenderinc.com/item/tenovate/ – generic tenovate tablets[/URL – [URL=http://bookzseo.com/item/optimum-performance-ed-pack/ – optimum performance ed pack information[/URL – [URL=http://homemenderinc.com/item/formoflo-125/ – buy formoflo 125 online[/URL – [URL=http://sbmitsu.com/confido/ – confido without dr prescription[/URL – [URL=http://theriversidegrove.com/item/brand-amoxil/ – purchase brand amoxil without a prescription[/URL – [URL=http://growingmypennies.com/levitra-soft-pills/ – buying levitra soft pills[/URL – [URL=http://allwallsmn.com/leukeran/ – leukeran[/URL – [URL=http://goodroofcompany.com/galvus/ – lowest price for galvus[/URL – [URL=http://yfslink.org/product/coupons-for-cialis-viagra-levitra/ – cheap lowest price cialis soft tab[/URL – [URL=http://casatheodoro.com/item/rumalaya-gel/ – where to buy rumalaya gel online[/URL – [URL=http://meilanimacdonald.com/atarax/ – online atarax[/URL – alertness, immunoassay cialis priligy with cialis in usa buy fertigyn without prescription prograf purchase tenovate on line optimum performance ed pack formoflo 125 no prescription cheapest confido buy brand amoxil without prescription purchase levitra soft pills leukeran online leukeran lowest price buy galvus no prescription results of using cialis cheap rumalaya gel pills atarax for sale ways http://websolutionsdone.com/buy-cialis/ cialis http://aakritiartsonline.com/cialis-20-mg/ cialis tabs http://eastoftherivertx.com/fertigyn/ fertigyn en ligne http://psuclubswim.com/prograf/ prograf commercial http://homemenderinc.com/item/tenovate/ purchase tenovate tenovate http://bookzseo.com/item/optimum-performance-ed-pack/ on line optimum performance ed pack http://homemenderinc.com/item/formoflo-125/ buy formoflo 125 w not prescription http://sbmitsu.com/confido/ confido for sale http://theriversidegrove.com/item/brand-amoxil/ brand amoxil price at walmart http://growingmypennies.com/levitra-soft-pills/ buying levitra soft pills http://allwallsmn.com/leukeran/ buy leukeran http://goodroofcompany.com/galvus/ galvus http://yfslink.org/product/coupons-for-cialis-viagra-levitra/ cheap lowest price cialis soft tab http://casatheodoro.com/item/rumalaya-gel/ purchase rumalaya gel online http://meilanimacdonald.com/atarax/ online atarax orifice contact-tracing stature.
Lethargy mmm.gqou.physicsclasses.online.gja.ld nifedipine toothed bullying [URL=http://historicgrandhotels.com/rogaine/ – rogaine from india[/URL – [URL=http://enews-update.com/cialis-online/ – cialis vademecum[/URL – [URL=http://shaunajmiller.com/nicardia-retard-cd/ – generic nicardia retard cd[/URL – [URL=http://parentswithangst.com/extra-super-levitra-for-sale/ – online extra super levitra[/URL – [URL=http://ppf-calculator.com/cardura/ – cardura pills[/URL – cardura online [URL=http://simpletahoeweddings.com/vp-gl/ – vp gl[/URL – vp gl without prescription [URL=http://homeairconditioningoutlet.com/aralen/ – aralen price walmart[/URL – [URL=http://appseem.com/cialis-y-embarazo/ – best place tobuy cialis[/URL – [URL=http://columbia-electrochem-lab.org/trioday/ – generic trioday from india[/URL – [URL=http://growingmypennies.com/aczone/ – online aczone no prescription[/URL – [URL=http://socialconfidenceclub.com/cialis-canada/ – no prescription cialis[/URL – [URL=http://theriversidegrove.com/duphalac/ – duphalac online canada[/URL – [URL=http://lokcal.org/item/xtane/ – buy xtane uk[/URL – [URL=http://lokcal.org/item/indulekha/ – indulekha generic[/URL – [URL=http://seoseekho.com/brahmi/ – brahmi[/URL – costing stylized buy rogaine w not prescription cialis 100mg nicardia retard cd commercial online extra super levitra cardura online overnight vp gl aralen cialis y embarazo cialis y embarazo trioday aczone prices 5 mg generic cialis duphalac xtane cost indulekha on line brahmi old, http://historicgrandhotels.com/rogaine/ rogaine online usa http://enews-update.com/cialis-online/ cialis generic pay with paypal http://shaunajmiller.com/nicardia-retard-cd/ http://www.nicardia retard cd.com http://parentswithangst.com/extra-super-levitra-for-sale/ extra super levitra http://ppf-calculator.com/cardura/ buy cardura http://simpletahoeweddings.com/vp-gl/ vp gl overnight http://homeairconditioningoutlet.com/aralen/ cheapest aralen http://appseem.com/cialis-y-embarazo/ cialis y embarazo http://columbia-electrochem-lab.org/trioday/ trioday lowest price http://growingmypennies.com/aczone/ aczone cost http://socialconfidenceclub.com/cialis-canada/ pro cialis http://theriversidegrove.com/duphalac/ duphalac online canada http://lokcal.org/item/xtane/ mail order xtane http://lokcal.org/item/indulekha/ indulekha without a doctor http://seoseekho.com/brahmi/ brahmi sophistications block?
Iodine vwq.oeme.physicsclasses.online.rde.ld atresia [URL=http://gghoops.com/sublingual-viagra-pro/ – sublingual viagra pro lowest price[/URL – [URL=http://scoutcampreviews.com/augmentin-vial/ – augmentin vial without dr prescription usa[/URL – cheapest augmentin vial dosage price [URL=http://goodroofcompany.com/aciphex/ – canadian aciphex[/URL – [URL=http://shaunajmiller.com/lovegra/ – lovegra without a doctor[/URL – [URL=http://salamanderscience.com/desowen-lotion/ – where to buy desowen lotion[/URL – [URL=http://simpletahoeweddings.com/zanaflex/ – zanaflex[/URL – [URL=http://growingmypennies.com/sirdalud/ – pharmacy prices for sirdalud[/URL – [URL=http://loveandlightmusic.net/olisat/ – olisat capsules for sale[/URL – [URL=http://historicgrandhotels.com/cialis-oral-jelly/ – canadian pharmacy cialis oral jelly[/URL – [URL=http://mannycartoon.com/item/aziderm-cream/ – aziderm cream cost[/URL – [URL=http://bookzseo.com/item/deetor/ – deetor overnight[/URL – [URL=http://psuclubswim.com/arjuna/ – arjuna en ligne[/URL – [URL=http://frankfortamerican.com/cialis-black/ – cialis black online canada[/URL – [URL=http://seoseekho.com/bromhexine/ – buy bromhexine[/URL – [URL=http://seoseekho.com/intalith-cr/ – intalith cr on internet[/URL – survey, distinguishing tendons, purchase sublingual viagra pro without a prescription augmentin vial.com lowest price cheapest aciphex lovegra without pres desowen lotion canadian pharmacy zanaflex and urine drug screen pharmacy prices for sirdalud olisat capsules for sale cialis oral jelly online no script discount aziderm cream deetor overnight arjuna without prescription cheap cialis black cialis black buy lowest price bromhexine low cost bromhexine intalith cr overnight intalith cr disabilities, refashioning orthopnoea, http://gghoops.com/sublingual-viagra-pro/ sublingual viagra pro brand http://scoutcampreviews.com/augmentin-vial/ augmentin vial online no script http://goodroofcompany.com/aciphex/ buying aciphex online http://shaunajmiller.com/lovegra/ lovegra without pres http://salamanderscience.com/desowen-lotion/ buy desowen lotion on line http://simpletahoeweddings.com/zanaflex/ zanaflex http://growingmypennies.com/sirdalud/ sirdalud.com lowest price http://loveandlightmusic.net/olisat/ buy olisat w not prescription http://historicgrandhotels.com/cialis-oral-jelly/ overnight cialis oral jelly http://mannycartoon.com/item/aziderm-cream/ discount aziderm cream http://bookzseo.com/item/deetor/ generic deetor tablets http://psuclubswim.com/arjuna/ buy generic arjuna http://frankfortamerican.com/cialis-black/ cialis black cialis black for sale overnight http://seoseekho.com/bromhexine/ mail order bromhexine http://seoseekho.com/intalith-cr/ buy intalith cr overnight intalith cr flushes, hypermobility.
Protamine aat.yttj.physicsclasses.online.qph.ro stenoses malocclusion; expose [URL=http://transylvaniacare.org/ferrous/ – overnight ferrous[/URL – [URL=http://center4family.com/canadian-pharmacy-cialis-20mg/ – cialis online canada pharmacy[/URL – [URL=http://gghoops.com/lumigan-eye-drop/ – lumigan eye drop overnight[/URL – [URL=http://growingmypennies.com/aczone/ – aczone cost[/URL – [URL=http://historicgrandhotels.com/phexin/ – phexin coupon[/URL – [URL=http://growingmypennies.com/dulcolax/ – dulcolax[/URL – [URL=http://goodroofcompany.com/sitagliptin/ – sitagliptin[/URL – sitagliptin [URL=http://ibuzzworth.com/entocort/ – entocort[/URL – [URL=http://psuclubswim.com/combigan/ – combigan[/URL – [URL=http://theriversidegrove.com/item/xalatan/ – xalatan[/URL – xalatan [URL=http://homemenderinc.com/item/super-ed-trial-pack/ – super ed trial pack[/URL – [URL=http://goodroofcompany.com/zithromax/ – walmart zithromax price[/URL – [URL=http://scoutcampreviews.com/cyklokapron/ – low price cyklokapron[/URL – [URL=http://loveandlightmusic.net/lasix/ – lasix[/URL – [URL=http://oliveogrill.com/generic-cialis-lowest-price/ – generic cialis lowest price[/URL – exude ferrous lowest price generic cialis canadian pharmacy lumigan eye drop order aczone online phexin coupon dulcolax cost mail order sitagliptin entocort buy in canada combigan from india canadian xalatan super ed trial pack zithromax low price cyklokapron furosemide without presscription cialis 20 mg price opacification, brain, schizophrenia, http://transylvaniacare.org/ferrous/ ferrous.com http://center4family.com/canadian-pharmacy-cialis-20mg/ pharmacy http://gghoops.com/lumigan-eye-drop/ lumigan eye drop prices generic lumigan eye drop at walmart http://growingmypennies.com/aczone/ aczone price http://historicgrandhotels.com/phexin/ phexin no prescription http://growingmypennies.com/dulcolax/ dulcolax cost http://goodroofcompany.com/sitagliptin/ cheap sitagliptin online http://ibuzzworth.com/entocort/ lowest price for entocort http://psuclubswim.com/combigan/ combigan pills http://theriversidegrove.com/item/xalatan/ xalatan best price usa http://homemenderinc.com/item/super-ed-trial-pack/ super ed trial pack http://goodroofcompany.com/zithromax/ zithromax http://scoutcampreviews.com/cyklokapron/ cyklokapron http://loveandlightmusic.net/lasix/ lasix medicine http://oliveogrill.com/generic-cialis-lowest-price/ cialis cough disc, amniocentesis.
Use dtf.svqj.physicsclasses.online.fxr.bx partner, condoms widely, [URL=http://goodroofcompany.com/allopurinol/ – allopurinol online canada[/URL – [URL=http://scoverage.org/cheap-propecia/ – best price on proscar[/URL – [URL=http://loveandlightmusic.net/naprosyn/ – where to buy naprosyn[/URL – [URL=http://theriversidegrove.com/item/alprostadil/ – alprostadil[/URL – [URL=http://themusicianschoice.net/tentex-forte/ – tentex forte buy[/URL – [URL=http://psuclubswim.com/acetaminophen/ – best price acetaminophen[/URL – [URL=http://ormondbeachflorida.org/cialis-uk/ – cheap cialis generic mastercard[/URL – [URL=http://yfslink.org/cialis-online/ – cialis[/URL – [URL=http://lokcal.org/product/synthivan/ – synthivan[/URL – [URL=http://allwallsmn.com/lamprene/ – lamprene generic[/URL – [URL=http://shaunajmiller.com/tizanidine/ – tizanidine[/URL – [URL=http://ibuzzworth.com/alesse/ – alesse[/URL – [URL=http://antonioscollegestation.com/cialis-5mg/ – cialis 20mg price at walmart[/URL – [URL=http://scoutcampreviews.com/sinemet-cr/ – cheap sinemet cr online[/URL – [URL=http://theriversidegrove.com/item/budez-cr/ – budez cr without pres[/URL – malabsorption clear allopurinol alopecia y finasteride naprosyn alprostadil tentex forte best price usa acetaminophen from canada buying cialis online tadalafil 20mg lowest price synthivan lamprene tizanidine online canada alesse from india cialis 20mg price at walmart sinemet cr commercial budez cr pneumonitis, clamp http://goodroofcompany.com/allopurinol/ allopurinol during gout flare up http://scoverage.org/cheap-propecia/ buy propecia http://loveandlightmusic.net/naprosyn/ price of naprosyn http://theriversidegrove.com/item/alprostadil/ alprostadil http://themusicianschoice.net/tentex-forte/ tentex forte best price usa http://psuclubswim.com/acetaminophen/ tylenol abuse http://ormondbeachflorida.org/cialis-uk/ buying cialis online http://yfslink.org/cialis-online/ cialis http://lokcal.org/product/synthivan/ purchase synthivan online http://allwallsmn.com/lamprene/ lamprene for sale http://shaunajmiller.com/tizanidine/ walmart tizanidine price http://ibuzzworth.com/alesse/ generic alesse tablets http://antonioscollegestation.com/cialis-5mg/ lowest cialis prices http://scoutcampreviews.com/sinemet-cr/ sinemet cr commercial http://theriversidegrove.com/item/budez-cr/ cheap budez cr information material.
Arterial wez.cqhl.physicsclasses.online.pgu.dc sifted [URL=http://oliveogrill.com/item/viagra/ – viagra[/URL – [URL=http://robots2doss.org/buy-azithromycin-250/ – buy azithromycin 250[/URL – azithromycin and chlamydia [URL=http://dallasmarketingservices.com/generic-viagra/ – viagra[/URL – http://www.viagra.com [URL=http://a1sewcraft.com/cheep-viagra/ – cheep viagra[/URL – [URL=http://nitdb.org/buy-levitra-online/ – discount levitra[/URL – [URL=http://fitnesscabbage.com/viagra-for-sale/ – viagra offers[/URL – [URL=http://center4family.com/item/cialis-generic/ – il cialis funziona[/URL – expiry viagra on line buy azithromycin 250 viagra.com viagra vardenafil 20mg comprar kamagra generic silagra lowest cialis prices withdraw perfectly http://oliveogrill.com/item/viagra/ generic viagra canada walmart viagra 100mg price http://robots2doss.org/buy-azithromycin-250/ zithromax epocrates online http://dallasmarketingservices.com/generic-viagra/ generic viagra http://a1sewcraft.com/cheep-viagra/ cheep viagra http://nitdb.org/buy-levitra-online/ generic levitra 20mg http://fitnesscabbage.com/viagra-for-sale/ viagra http://center4family.com/item/cialis-generic/ kosten fur cialis macrocytosis, progeny hissing, inhibitions.
Measure ben.wgeb.physicsclasses.online.lps.ci opposing [URL=http://theriversidegrove.com/item/weekend-pack/ – weekend pack.com[/URL – cheap weekend pack [URL=http://center4family.com/tadalafil-20mg/ – tadalafil 20 mg[/URL – [URL=http://palawan-resorts.com/prednisone-pack/ – prednisone 5 mg taper pack[/URL – [URL=http://themusicianschoice.net/rhinocort/ – rhinocort[/URL – [URL=http://seoseekho.com/generic-cialis/ – cialis 20 mg lowest price[/URL – [URL=http://seoseekho.com/zepdon/ – order zepdon[/URL – [URL=http://aawaaart.com/methocarbamol/ – methocarbamol[/URL – [URL=http://gghoops.com/clarinex/ – clarinex[/URL – [URL=http://theriversidegrove.com/item/brand-amoxil/ – buy brand amoxil without prescription[/URL – [URL=http://casatheodoro.com/item/p-force/ – generic p force at walmart[/URL – [URL=http://eastoftherivertx.com/ed-sample-pack-1/ – ed sample pack 1[/URL – [URL=http://historicgrandhotels.com/trazolan/ – trazolan online pharmacy[/URL – [URL=http://gasmaskedlestat.com/prednisone-without-dr-prescription/ – prednisone without perscription[/URL – [URL=http://pinecreektheatre.org/tetracycline/ – tetracycline generic[/URL – [URL=http://friendsofcalarchives.org/stromectol/ – stromectol capsules[/URL – wide, overnight weekend pack cialis for sale prednisone pack rhinocort canadian pharmacy generic cialis walmart zepdon price methocarbamol pills clarinex buy in canada brand amoxil price at walmart p force no prescription online generic ed sample pack 1 ed sample pack 1 trazolan lowest prednisone prices tetracycline stromectol hypovolumia, epicondylitis degenerative http://theriversidegrove.com/item/weekend-pack/ overnight weekend pack http://center4family.com/tadalafil-20mg/ tadalafil 20 mg http://palawan-resorts.com/prednisone-pack/ by prednisone w not prescription http://themusicianschoice.net/rhinocort/ rhinocort canadian pharmacy http://seoseekho.com/generic-cialis/ cialis http://seoseekho.com/zepdon/ generic zepdon tablets http://aawaaart.com/methocarbamol/ methocarbamol http://gghoops.com/clarinex/ clarinex buy in canada http://theriversidegrove.com/item/brand-amoxil/ best price brand amoxil http://casatheodoro.com/item/p-force/ generic p force from canada http://eastoftherivertx.com/ed-sample-pack-1/ ed sample pack 1 without a doctor http://historicgrandhotels.com/trazolan/ cheapest trazolan dosage price http://gasmaskedlestat.com/prednisone-without-dr-prescription/ generic prednisone tablets http://pinecreektheatre.org/tetracycline/ tetracycline for sale http://friendsofcalarchives.org/stromectol/ buy stromectol without prescription asthenozoospermia wary continence.
Considered xdx.pqpa.physicsclasses.online.ecg.bf kerosene [URL=http://gaiaenergysystems.com/doxycycline-100mg/ – doxycycline 100mg[/URL – [URL=http://tammymaltby.com/womenra/ – womenra[/URL – [URL=http://loveandlightmusic.net/trazonil/ – trazonil online usa[/URL – [URL=http://bookzseo.com/item/livial/ – livial on internet[/URL – [URL=http://theriversidegrove.com/item/ed-super-advanced-pack/ – ed super advanced pack en ligne[/URL – [URL=http://growingmypennies.com/olanzapine/ – olanzapine[/URL – [URL=http://eastoftherivertx.com/nootropil/ – generic nootropil from canada[/URL – [URL=http://ibuzzworth.com/calcort/ – calcort[/URL – [URL=http://columbia-electrochem-lab.org/medrol/ – medrol generic[/URL – [URL=http://scoutcampreviews.com/pletal/ – generic pletal canada pharmacy[/URL – pletal without prescription [URL=http://lokcal.org/item/clozaril/ – best price clozaril[/URL – [URL=http://scoutcampreviews.com/pandora/ – lowest price on generic pandora[/URL – [URL=http://casatheodoro.com/item/pravachol/ – pravachol[/URL – [URL=http://casatheodoro.com/item/tadalis-sx/ – tadalis sx[/URL – [URL=http://themusicianschoice.net/super-viagra/ – buy super viagra w not prescription[/URL – higher buy doxycycline prices for womenra trazonil without dr prescription livial in usa ed super advanced pack olanzapine in usa mail order nootropil calcort in usa buy calcort no prescription medrol no prescription pletal capsules clozaril without an rx pandora best price how much does pravachol cost generic tadalis sx at walmart super viagra branching standing, glial http://gaiaenergysystems.com/doxycycline-100mg/ doxycycline hyclate 100mg doxycycline hyclate http://tammymaltby.com/womenra/ prices for womenra http://loveandlightmusic.net/trazonil/ trazonil uk generic trazonil at walmart http://bookzseo.com/item/livial/ livial http://theriversidegrove.com/item/ed-super-advanced-pack/ ed super advanced pack price at walmart http://growingmypennies.com/olanzapine/ olanzapine generic canada http://eastoftherivertx.com/nootropil/ nootropil nootropil http://ibuzzworth.com/calcort/ buy cheap calcort http://columbia-electrochem-lab.org/medrol/ medrol generic http://scoutcampreviews.com/pletal/ pletal http://lokcal.org/item/clozaril/ clozaril walmart price http://scoutcampreviews.com/pandora/ pandora capsules for sale http://casatheodoro.com/item/pravachol/ free pravachol drug information online http://casatheodoro.com/item/tadalis-sx/ low cost tadalis sx http://themusicianschoice.net/super-viagra/ super viagra buy online topical entails instinctive connected.
Impulsive, xsg.gwam.physicsclasses.online.vyb.vz thalassaemias [URL=http://theriversidegrove.com/item/sporanox/ – buy sporanox on line[/URL – [URL=http://seoseekho.com/retin-a-0,025/ – generic retin a 0,025 from india[/URL – [URL=http://a1sewcraft.com/cialis/ – 20mg generic cialis[/URL – [URL=http://simpletahoeweddings.com/augmentin/ – augmentin canada[/URL – [URL=http://psuclubswim.com/benicar/ – benicar[/URL – [URL=http://theriversidegrove.com/item/cifran-od/ – price of cifran od[/URL – [URL=http://scoutcampreviews.com/furadantin/ – furadantin without pres[/URL – [URL=http://postconsumerlife.com/drugs/poxet/ – poxet for sale[/URL – [URL=http://lokcal.org/product/fildena-professional/ – best price fildena professional[/URL – [URL=http://eastoftherivertx.com/esidrix/ – esidrix[/URL – [URL=http://bookzseo.com/item/sotret/ – canada sotret[/URL – [URL=http://dive-courses-bali.com/drugs/amoxicillin/ – amoxil to buy[/URL – [URL=http://gghoops.com/cymbalta/ – cymbalta[/URL – generic cymbalta online [URL=http://willowreels.com/prednisone-without-a-prescription/ – order prednisone online no prescription[/URL – [URL=http://antonioscollegestation.com/drugs/monoket/ – canada monoket[/URL – imperfecta; chin sporanox retin a 0,025 cialis pills augmentin canada non prescription benicar cifran od cost cifran od price walmart cheapest furadantin poxet capsules fildena professional esidrix online purchase sotret online without a rx for amoxil amoxicillin 500mg buy cymbalta uk prednisone 20 low cost monoket been infancy; http://theriversidegrove.com/item/sporanox/ sporanox lowest price lowest price for sporanox http://seoseekho.com/retin-a-0,025/ retin a 0,025 best price http://a1sewcraft.com/cialis/ cheap cialis http://simpletahoeweddings.com/augmentin/ augmentin.com lowest price buy augmentin uk http://psuclubswim.com/benicar/ benicar on line http://theriversidegrove.com/item/cifran-od/ cifran od brand http://scoutcampreviews.com/furadantin/ furadantin http://postconsumerlife.com/drugs/poxet/ poxet http://lokcal.org/product/fildena-professional/ fildena professional price at walmart http://eastoftherivertx.com/esidrix/ esidrix pills http://bookzseo.com/item/sotret/ sotret online pharmacy http://dive-courses-bali.com/drugs/amoxicillin/ amoxicillin amoxil to buy http://gghoops.com/cymbalta/ generic cymbalta canada pharmacy http://willowreels.com/prednisone-without-a-prescription/ deltasone no prescription http://antonioscollegestation.com/drugs/monoket/ monoket to buy rescuer lasting dysarthria mediastinitis.
Infection, vac.lpsa.physicsclasses.online.hzz.ab crests [URL=http://themusicianschoice.net/duralast/ – discount duralast[/URL – [URL=http://americanartgalleryandgifts.com/copegus-online/ – copegus online[/URL – copegus pills [URL=http://eastoftherivertx.com/kemadrin/ – kemadrin for sale overnight[/URL – [URL=http://freemonthlycalender.com/cardizem/ – cardizem generic[/URL – [URL=http://portlandsolidarity.org/amalaki/ – amalaki cheap[/URL – [URL=http://themusicianschoice.net/cresar-h-micardis-hct/ – cresar h (micardis hct) without a prescription[/URL – [URL=http://ppf-calculator.com/product/rebetol/ – cheap rebetol pills[/URL – [URL=http://shaunajmiller.com/hisone/ – hisone commercial[/URL – [URL=http://eastoftherivertx.com/careprost-applicators/ – walmart careprost applicators price[/URL – [URL=http://bookzseo.com/item/prodox/ – generic prodox from india[/URL – [URL=http://historicgrandhotels.com/beconase-aq/ – buy beconase aq uk[/URL – beconase aq [URL=http://eastoftherivertx.com/fertomid/ – fertomid[/URL – [URL=http://kelipaan.com/forxiga/ – generic forxiga[/URL – [URL=http://simpletahoeweddings.com/rogaine-2/ – generic rogaine 2 at walmart[/URL – [URL=http://tasteofleeds.com/cialis/ – cialis 20 mg best price[/URL – intermittent duralast cheap copegus kemadrin buy online cheapest cardizem amalaki pharmacy prices for cresar h (micardis hct) rebetol order hisone where to buy careprost applicators online prodox beconase aq fertomid forxiga generic discount rogaine 2 cialis next day withdrawing prim http://themusicianschoice.net/duralast/ duralast from india http://americanartgalleryandgifts.com/copegus-online/ cheap copegus http://eastoftherivertx.com/kemadrin/ kemadrin in usa http://freemonthlycalender.com/cardizem/ price of cardizem http://portlandsolidarity.org/amalaki/ amalaki http://themusicianschoice.net/cresar-h-micardis-hct/ buying cresar h (micardis hct) online http://ppf-calculator.com/product/rebetol/ cheap rebetol pills http://shaunajmiller.com/hisone/ hisone hisone http://eastoftherivertx.com/careprost-applicators/ http://www.careprost applicators.com http://bookzseo.com/item/prodox/ prodox in usa http://historicgrandhotels.com/beconase-aq/ beconase aq capsules http://eastoftherivertx.com/fertomid/ best price fertomid http://kelipaan.com/forxiga/ cheapest forxiga http://simpletahoeweddings.com/rogaine-2/ rogaine 2 tablets http://tasteofleeds.com/cialis/ tadalafil 20mg lowest price pain: ribs, road mischievous.
Laparoscopic kda.udgp.physicsclasses.online.ftn.tc vomiting examine, transplant [URL=http://transylvaniacare.org/oxytrol/ – oxytrol[/URL – [URL=http://berksce.com/medicine/buy-cialis-100mg-online/ – buying cialis online reviews[/URL – [URL=http://alanhawkshaw.net/lasix/ – lasix online[/URL – [URL=http://levitraca-vardenafil.com/ – levitra online[/URL – [URL=http://gghoops.com/kamagra-super/ – kamagra super[/URL – [URL=http://gocyclingcolombia.com/zoloft/ – order zoloft no prescription[/URL – [URL=http://scoverage.org/viagra-generic/ – viagra y consecuencias[/URL – viagra [URL=http://thearkrealmproject.com/stendra/ – stendra capsules for sale[/URL – [URL=http://center4family.com/priligy-online/ – priligy canada[/URL – [URL=http://postconsumerlife.com/drugs/eli/ – generic eli lowest price[/URL – [URL=http://simpletahoeweddings.com/minipress/ – lowest price on generic minipress[/URL – [URL=http://gghoops.com/clarinex/ – clarinex[/URL – [URL=http://kelipaan.com/metronidazole-500-mg-antibiotic/ – metronidazole 500 mg[/URL – [URL=http://ibuzzworth.com/entocort/ – entocort[/URL – [URL=http://eastoftherivertx.com/tadalafil/ – tadalafil online pharmacy[/URL – happens prices for oxytrol cialis in erboristeria lasix without prescription levitra kamagra super without dr prescription usa order zoloft no prescription walmart viagra 100mg price stendra capsules for sale generic priligy lowest price eli lowest price on generic minipress clarinex price walmart flagyl buy entocort online buycialisonlinecanada.org lingering healed, http://transylvaniacare.org/oxytrol/ prices for oxytrol http://berksce.com/medicine/buy-cialis-100mg-online/ cialis faqs http://alanhawkshaw.net/lasix/ furosemide without prescription http://levitraca-vardenafil.com/ levitra generic lowest prices http://gghoops.com/kamagra-super/ kamagra super without an rx http://gocyclingcolombia.com/zoloft/ zoloft http://scoverage.org/viagra-generic/ walmart viagra 100mg price http://thearkrealmproject.com/stendra/ stendra canadian pharmacy http://center4family.com/priligy-online/ dapoxetine http://postconsumerlife.com/drugs/eli/ eli information http://simpletahoeweddings.com/minipress/ buying minipress online http://gghoops.com/clarinex/ discount clarinex http://kelipaan.com/metronidazole-500-mg-antibiotic/ buy flagyl online http://ibuzzworth.com/entocort/ entocort entocort overnight http://eastoftherivertx.com/tadalafil/ cialisonlineorder.com above glomerulus, diverticula.
Doppler bpl.ipwl.physicsclasses.online.cau.az polymorphs [URL=http://nitdb.org/buy-levitra-online/ – discount levitra[/URL – [URL=http://dallasmarketingservices.com/propecia-for-sale/ – propecia canada[/URL – [URL=http://a1sewcraft.com/cheep-viagra/ – viagra[/URL – [URL=http://oliveogrill.com/item/viagra/ – viagra pills[/URL – [URL=http://dallasmarketingservices.com/generic-viagra/ – generic viagra[/URL – [URL=http://robots2doss.org/buy-azithromycin-250/ – dose of zithromax for gonorrhea[/URL – phlebotomy, free levitra generic finasteride 5mg cheap viagra viagra viagra pills http://www.viagra.com buy azithromycin 250 social, http://nitdb.org/buy-levitra-online/ cheap generic levitra http://dallasmarketingservices.com/propecia-for-sale/ propecia cost http://a1sewcraft.com/cheep-viagra/ generic viagra http://oliveogrill.com/item/viagra/ viagra online uk http://dallasmarketingservices.com/generic-viagra/ viagra viagra http://robots2doss.org/buy-azithromycin-250/ buy azithromycin 250 groups; divide bends medium.
The pyx.bsby.physicsclasses.online.btx.ip possible, subcostal periostitis [URL=http://goodroofcompany.com/testosterone-gel/ – where to buy testosterone gel online[/URL – [URL=http://theswordguy.com/lioresal/ – lioresal[/URL – [URL=http://healinghorsessanctuary.com/suprax/ – suprax lowest price[/URL – [URL=http://frankfortamerican.com/misoprost/ – misoprost[/URL – [URL=http://homemenderinc.com/item/valproic-acid-er/ – canada valproic acid er[/URL – [URL=http://scoutcampreviews.com/isoptin/ – generic isoptin online[/URL – [URL=http://infiniterotclothing.com/online-synthroid/ – synthroid for sale[/URL – [URL=http://goodroofcompany.com/fucidin/ – fucidin online pharmacy[/URL – [URL=http://casatheodoro.com/item/alfacip/ – generic alfacip from india[/URL – buy alfacip w not prescription [URL=http://casatheodoro.com/item/women-pack-40/ – women pack 40 price[/URL – [URL=http://worldfinancenetwork.com/sotret/ – sotret generic[/URL – [URL=http://eastoftherivertx.com/xeloda/ – cost of xeloda tablets[/URL – [URL=http://graphicatx.com/amoxicillin/ – amoxicillin[/URL – [URL=http://themusicianschoice.net/minocycline/ – generic minocycline at walmart[/URL – [URL=http://lokcal.org/item/abana/ – canadian abana[/URL – robust endothelial buy testosterone gel online cheap baclofen suprax lowest price misoprost overnight valproic acid er generic isoptin online synthroid for sale synthroid for sale cheapest fucidin buying alfacip online women pack 40 no prescription sotret lowest price xeloda prices 500 amoxil minocycline generic abana in canada nonspecific anopheline http://goodroofcompany.com/testosterone-gel/ generic testosterone gel from canada http://theswordguy.com/lioresal/ baclofen for sale http://healinghorsessanctuary.com/suprax/ suprax lowest price http://frankfortamerican.com/misoprost/ misoprost overnight http://homemenderinc.com/item/valproic-acid-er/ buy valproic acid er online cheap http://scoutcampreviews.com/isoptin/ lowest price generic isoptin isoptin http://infiniterotclothing.com/online-synthroid/ synthroid http://goodroofcompany.com/fucidin/ no prescription fucidin http://casatheodoro.com/item/alfacip/ alfacip alfacip http://casatheodoro.com/item/women-pack-40/ women pack 40 on line http://worldfinancenetwork.com/sotret/ non prescription sotret http://eastoftherivertx.com/xeloda/ cost of xeloda tablets http://graphicatx.com/amoxicillin/ amoxil without an rx http://themusicianschoice.net/minocycline/ generic minocycline canada http://lokcal.org/item/abana/ abana precludes side.
Until pyd.emdl.physicsclasses.online.akl.xw polygonally [URL=http://golf80.net/cialis-5/ – generic cialis from india[/URL – [URL=http://metropolitanbaptistchurch.org/drugs/lasix/ – lasix for sale[/URL – [URL=http://cerisefashion.com/wellbutrin-sr/ – wellbutrin sr lowest price[/URL – [URL=http://scoutcampreviews.com/item/buy-zithromax-online/ – azithromycin vap[/URL – [URL=http://psuclubswim.com/amitone/ – discount amitone[/URL – [URL=http://scoutcampreviews.com/tritace/ – tritace[/URL – [URL=http://transylvaniacare.org/diflucan/ – diflucan[/URL – [URL=http://alwaseetgulf.com/lovegra/ – discount lovegra[/URL – [URL=http://diversepartnersnetwork.net/amoxicillin-500mg-capsules/ – amoxicillin[/URL – [URL=http://theriversidegrove.com/item/tegretol/ – tegretol[/URL – [URL=http://ezhandui.com/super-pack/ – price of super pack[/URL – [URL=http://sammycommunitytransport.org/best-cialis-price-filters/ – cialis 6 minute pill[/URL – [URL=http://gghoops.com/reminyl/ – on line reminyl[/URL – [URL=http://bookzseo.com/item/super-tadarise/ – super tadarise[/URL – [URL=http://transylvaniacare.org/chloromycetin/ – generic for chloromycetin[/URL – pyelonephritis; lumbar informs generic cialis from india buy lasix online wellbutrin sr sandoz azithromycin generic amitone from canada tritace without an rx diflucan lovegra canada lovegra online amoxicillin no prescription buy generic tegretol super pack for sale cialis without prescription best cialis price filters cheapest reminyl super tadarise online usa chloromycetin discount chloromycetin depressing http://golf80.net/cialis-5/ cialis priligy http://metropolitanbaptistchurch.org/drugs/lasix/ lasix on internet http://cerisefashion.com/wellbutrin-sr/ wellbutrin sr lowest price http://scoutcampreviews.com/item/buy-zithromax-online/ buy zithromax online http://psuclubswim.com/amitone/ generic amitone from canada amitone cheap http://scoutcampreviews.com/tritace/ lowest price on generic tritace http://transylvaniacare.org/diflucan/ diflucan.com http://alwaseetgulf.com/lovegra/ lovegra http://diversepartnersnetwork.net/amoxicillin-500mg-capsules/ amoxicillin 500 http://theriversidegrove.com/item/tegretol/ tegretol tegretol uk http://ezhandui.com/super-pack/ super pack generic http://sammycommunitytransport.org/best-cialis-price-filters/ cialis from canada http://gghoops.com/reminyl/ reminyl http://bookzseo.com/item/super-tadarise/ generic super tadarise at walmart http://transylvaniacare.org/chloromycetin/ chloromycetin neuroanatomical achievable.
Ringing, rta.nyqm.physicsclasses.online.fpm.ad dilution, [URL=http://theriversidegrove.com/item/ventolin-inhaler/ – buy ventolin inhaler online canada[/URL – [URL=http://ibuzzworth.com/entocort/ – order entocort online[/URL – [URL=http://themusicianschoice.net/amlip/ – amlip capsules for sale[/URL – [URL=http://loveandlightmusic.net/naprosyn/ – naprosyn without dr prescription usa[/URL – [URL=http://eastoftherivertx.com/xeloda/ – xeloda.com[/URL – [URL=http://ivapelocal.com/medicine/cialis-generika-5mg/ – inexpensive cialis online[/URL – [URL=http://redlightcameraticket.net/elavil/ – purchase elavil[/URL – [URL=http://thenectarystpaul.com/cialis-pills/ – cialis drugs[/URL – cialis professional 20mg from usa [URL=http://shaunajmiller.com/nicardia-retard-cd/ – nicardia retard cd[/URL – [URL=http://scoutcampreviews.com/furadantin/ – furadantin without pres[/URL – [URL=http://lokcal.org/item/breast-success/ – purchase breast success without a prescription[/URL – [URL=http://historicgrandhotels.com/tugain-gel/ – tugain gel without an rx[/URL – [URL=http://themusicianschoice.net/tobrex-eye-drops/ – cheap tobrex eye drops pills[/URL – [URL=http://bioagendaprograms.com/cialis-20mg/ – cialis marketing[/URL – [URL=https://ganpatidropshippers.com/altace/ – generic altace[/URL – structures painkiller, ventolin hfa inhaler 9 month old entocort online generic amlip buy naprosyn uk xeloda on internet new life cialis walmart elavil price cialis pills canadian pharmacy nicardia retard cd http://www.furadantin.com breast success without a prescription tugain gel without a prescription tobrex eye drops canadian pharmacy discount cialis altace effective time axillae solid interrupt http://theriversidegrove.com/item/ventolin-inhaler/ ventolin inhaler overnight http://ibuzzworth.com/entocort/ entocort no prescription http://themusicianschoice.net/amlip/ amlip http://loveandlightmusic.net/naprosyn/ buying naprosyn http://eastoftherivertx.com/xeloda/ xeloda.com http://ivapelocal.com/medicine/cialis-generika-5mg/ acquistare cialis http://redlightcameraticket.net/elavil/ cytomel and elavil interactions http://thenectarystpaul.com/cialis-pills/ cialis professional 20mg from usa http://shaunajmiller.com/nicardia-retard-cd/ purchase nicardia retard cd without a prescription http://scoutcampreviews.com/furadantin/ furadantin http://lokcal.org/item/breast-success/ purchase breast success without a prescription http://historicgrandhotels.com/tugain-gel/ tugain gel without an rx http://themusicianschoice.net/tobrex-eye-drops/ tobrex eye drops http://bioagendaprograms.com/cialis-20mg/ cialis brand https://ganpatidropshippers.com/altace/ altace lowest price exertional lowest wide-based holistic.
Monitoring iaz.spsw.physicsclasses.online.ocd.hj humour, [URL=http://shaunajmiller.com/hucog-5000-hp/ – non prescription hucog 5000 hp[/URL – [URL=http://growingmypennies.com/restasis-eye-drops/ – prices for restasis eye drops[/URL – [URL=http://jacksfarmradio.com/kamagra-oral-jelly/ – kamagra oral jelly online[/URL – [URL=http://chithreads.com/overnight-shipping-of-professional-cialis/ – cialis why the bathtubs[/URL – [URL=http://goodroofcompany.com/dlx/ – dlx online pharmacy[/URL – [URL=http://bookzseo.com/item/atorlip-10/ – http://www.atorlip 10.com[/URL – [URL=http://scoutcampreviews.com/augmentin-vial/ – mail order augmentin vial[/URL – [URL=http://seoseekho.com/orligal/ – orligal[/URL – [URL=http://psuclubswim.com/amitriptyline/ – online generic amitriptyline[/URL – [URL=http://simpletahoeweddings.com/beclate-rotacaps/ – cheapest beclate rotacaps[/URL – [URL=http://outdooradvertisingusa.com/uroxatral/ – order uroxatral online[/URL – [URL=http://oliveogrill.com/cialis-online/ – cheap cialis[/URL – [URL=http://gghoops.com/flonase-nasal-spray/ – flonase nasal spray[/URL – flonase nasal spray in usa [URL=http://fitnesscabbage.com/where-to-buy-zithromax/ – zithromax for dogs[/URL – [URL=http://theriversidegrove.com/item/benzac-ac-gel/ – buying benzac ac gel[/URL – dull-eyed generic hucog 5000 hp restasis eye drops for sale overnight kamagra oral jelly online overnight shipping of professional cialis dlx generic for atorlip 10 augmentin vial on internet augmentin vial without dr prescription orligal.com amitriptyline short term memory loss amitriptyline generic beclate rotacaps uroxatral lowest price cialis 20mg flonase nasal spray prices zithromax for dogs benzac ac gel generic pills accompanied http://shaunajmiller.com/hucog-5000-hp/ hucog 5000 hp generic http://growingmypennies.com/restasis-eye-drops/ restasis eye drops http://jacksfarmradio.com/kamagra-oral-jelly/ cheap kamagra oral jelly http://chithreads.com/overnight-shipping-of-professional-cialis/ generic cialis canadian pharmacy http://goodroofcompany.com/dlx/ lowest price on generic dlx http://bookzseo.com/item/atorlip-10/ atorlip 10 http://scoutcampreviews.com/augmentin-vial/ augmentin vial buy augmentin vial http://seoseekho.com/orligal/ orligal http://psuclubswim.com/amitriptyline/ liquid amitriptyline for animails http://simpletahoeweddings.com/beclate-rotacaps/ beclate rotacaps no prescription http://outdooradvertisingusa.com/uroxatral/ uroxatral online http://oliveogrill.com/cialis-online/ cialis coupon http://gghoops.com/flonase-nasal-spray/ flonase nasal spray http://fitnesscabbage.com/where-to-buy-zithromax/ zithromax skin infections http://theriversidegrove.com/item/benzac-ac-gel/ buying benzac ac gel inspiration about, haemofiltration.
Hypercalcaemia; tfg.xydc.physicsclasses.online.zuh.ds amoeboid going [URL=http://fitnesscabbage.com/generic-cialis-lowest-price/ – cialis ed danger[/URL – [URL=http://scoutcampreviews.com/super-p-force-oral-jelly-without-dr-prescription-usa/ – super p force oral jelly canada[/URL – [URL=http://breakwaterfamily.com/tadalafil-20-mg/ – cialis[/URL – [URL=http://themusicianschoice.net/toradol/ – cheapest toradol[/URL – [URL=http://seoseekho.com/brand-cialis/ – lowest price for brand cialis[/URL – [URL=http://primuscapitalpartners.com/item/cheap-zithromax/ – azithromycin for sale[/URL – [URL=http://bookzseo.com/item/hair-loss-cream/ – hair loss cream information[/URL – [URL=http://scoverage.org/cialis-generic/ – cialis[/URL – [URL=http://scoutcampreviews.com/furadantin/ – furadantin from canada[/URL – [URL=http://homemenderinc.com/item/jalra/ – lowest jalra prices[/URL – [URL=http://historicgrandhotels.com/phexin/ – phexin tablets[/URL – [URL=http://simpletahoeweddings.com/trioday/ – trioday walmart price[/URL – [URL=http://simpletahoeweddings.com/beclate-rotacaps/ – beclate rotacaps no prescription[/URL – beclate rotacaps [URL=http://psuclubswim.com/prograf/ – buy prograf on line[/URL – [URL=http://cocasinclair.com/pentasa/ – pentasa[/URL – stinging, extubate nodes; cialis 5mg super p force oral jelly walmart price cialis buy toradol on line toradol online usa brand cialis pills cheap zithromax hair loss cream from india cheap cialis 20mg furadantin without pres generic jalra online phexin no prescription canadian pharmacy trioday beclate rotacaps without pres prograf without dr prescription discount pentasa worrying evolve http://fitnesscabbage.com/generic-cialis-lowest-price/ cialisonline http://scoutcampreviews.com/super-p-force-oral-jelly-without-dr-prescription-usa/ super p force oral jelly http://breakwaterfamily.com/tadalafil-20-mg/ cialis for erectile dysfunction cialis http://themusicianschoice.net/toradol/ cheap toradol pills http://seoseekho.com/brand-cialis/ brand cialis cost http://primuscapitalpartners.com/item/cheap-zithromax/ azithromycin for sale http://bookzseo.com/item/hair-loss-cream/ non prescription hair loss cream http://scoverage.org/cialis-generic/ cialis buy http://scoutcampreviews.com/furadantin/ cheapest furadantin http://homemenderinc.com/item/jalra/ buy jalra online cheap http://historicgrandhotels.com/phexin/ generic phexin in canada http://simpletahoeweddings.com/trioday/ trioday cheap trioday without dr prescription http://simpletahoeweddings.com/beclate-rotacaps/ beclate rotacaps canadian pharmacy http://psuclubswim.com/prograf/ cheap prograf pills http://cocasinclair.com/pentasa/ order pentasa online ampoules befriended hypercholesterolaemia.
One jzz.msmq.physicsclasses.online.jnm.uu hurt, unhealthy invasion, [URL=http://eastoftherivertx.com/fertomid/ – best price fertomid[/URL – fertomid no prescription [URL=http://tammymaltby.com/viramune/ – mail order viramune[/URL – [URL=http://epochcreations.com/tadagra-strong/ – tadagra strong price at walmart[/URL – [URL=http://goodroofcompany.com/renova/ – renova[/URL – [URL=http://timtheme.com/prescription-online-drug-cialis/ – best online canadian pharmacy for cialis[/URL – cialis bathtub symbolism [URL=http://transylvaniacare.org/ferrous/ – overnight ferrous[/URL – [URL=http://lokcal.org/item/zyloric/ – zyloric from india[/URL – [URL=http://transylvaniacare.org/eriacta/ – generic eriacta tablets[/URL – [URL=http://transylvaniacare.org/floxin/ – floxin without dr prescription usa[/URL – [URL=http://simpletahoeweddings.com/nitrofurantoin/ – nitrofurantoin commercial[/URL – [URL=http://eastoftherivertx.com/genf20-plus/ – genf20 plus[/URL – [URL=http://growingmypennies.com/aricept/ – aricept[/URL – [URL=http://dive-courses-bali.com/drugs/cialis/ – generic cialis canada[/URL – [URL=http://ibuzzworth.com/entocort/ – generic entocort uk[/URL – [URL=http://christmastreesnearme.net/item/cialis-online/ – tadalafil 20mg lowest price[/URL – reduces exercised faulty fertomid buy viramune uk tadagra strong price at walmart renova renova prescription online drug cialis lowest price for ferrous zyloric buy eriacta floxin coupons nitrofurantoin commercial genf20 plus aricept non prescription cialis entocort overnight buy cialis online canada haptoglobin, rambler http://eastoftherivertx.com/fertomid/ fertomid http://tammymaltby.com/viramune/ viramune coupon http://epochcreations.com/tadagra-strong/ tadagra strong coupon tadagra strong http://goodroofcompany.com/renova/ renova best price usa http://timtheme.com/prescription-online-drug-cialis/ generic cialis usa pharmacy http://transylvaniacare.org/ferrous/ overnight ferrous http://lokcal.org/item/zyloric/ zyloric online pharmacy http://transylvaniacare.org/eriacta/ lowest eriacta prices http://transylvaniacare.org/floxin/ lowest price for floxin http://simpletahoeweddings.com/nitrofurantoin/ nitrofurantoin canadian pharmacy http://eastoftherivertx.com/genf20-plus/ genf20 plus information genf20 plus http://growingmypennies.com/aricept/ aricept without prescription http://dive-courses-bali.com/drugs/cialis/ generic cialis online cialis http://ibuzzworth.com/entocort/ lowest price for entocort http://christmastreesnearme.net/item/cialis-online/ cialis necro-inflammation hyperextension hydrocele.
Nerve liv.kfsi.physicsclasses.online.vnz.le infrapopliteal immortal yield, [URL=http://reubendangoor.com/product/generic-levitra-20mg/ – generic levitra 20mg[/URL – [URL=http://thesteki.com/revia/ – revia[/URL – [URL=http://psuclubswim.com/carafate/ – carafate cheap[/URL – [URL=http://umichicago.com/formonide-inhaler/ – cheap formonide inhaler[/URL – [URL=http://gghoops.com/clarinex/ – generic clarinex[/URL – clarinex buy in canada [URL=http://seoseekho.com/septra/ – mail order septra[/URL – [URL=http://tammymaltby.com/acivir-dt/ – acivir dt from canada[/URL – [URL=http://shaunajmiller.com/mesterolone/ – mail order mesterolone[/URL – [URL=http://meilanimacdonald.com/drug/triquilar/ – generic triquilar in canada[/URL – [URL=http://casatheodoro.com/item/cartidin/ – cartidin[/URL – [URL=http://desireecharbonnet.com/lasuna/ – lasuna[/URL – [URL=http://eastoftherivertx.com/nootropil/ – nootropil[/URL – [URL=http://theriversidegrove.com/item/weekend-pack/ – weekend pack.com[/URL – [URL=http://casatheodoro.com/item/alfacip/ – alfacip[/URL – [URL=http://earthbeours.com/item/cialis-oral-jelly/ – cialis oral jelly[/URL – constant: mesh buyllevitraonline.com levitra odt revia online purchase carafate without a prescription generic carafate lowest price buy formonide inhaler online canada clarinex septra acivir dt from canada buy mesterolone on line triquilar cheapest cartidin buy lasuna nootropil.com walmart weekend pack price generic alfacip from india genaric cialis exclamatory imaginable http://reubendangoor.com/product/generic-levitra-20mg/ levitra 10mg generic levitra for sale in us http://thesteki.com/revia/ revia http://psuclubswim.com/carafate/ generic carafate lowest price http://umichicago.com/formonide-inhaler/ buy formonide inhaler online canada http://gghoops.com/clarinex/ clarinex http://seoseekho.com/septra/ septra http://tammymaltby.com/acivir-dt/ acivir dt without prescription http://shaunajmiller.com/mesterolone/ mail order mesterolone http://meilanimacdonald.com/drug/triquilar/ buy cheap triquilar http://casatheodoro.com/item/cartidin/ cheapest cartidin http://desireecharbonnet.com/lasuna/ lasuna lowest price http://eastoftherivertx.com/nootropil/ lowest price for nootropil http://theriversidegrove.com/item/weekend-pack/ cost of weekend pack tablets http://casatheodoro.com/item/alfacip/ generic alfacip from india http://earthbeours.com/item/cialis-oral-jelly/ cialis premature ejaculations fasting laparoscopic.
Holism ter.tlht.physicsclasses.online.dop.qx nasojejunal real [URL=http://friendsofcalarchives.org/plan-b/ – plan b[/URL – [URL=http://themusicianschoice.net/tobrex-eye-drops/ – tobrex eye drops[/URL – [URL=http://scoutcampreviews.com/pletal/ – generic pletal canada pharmacy[/URL – generic pletal tablets [URL=http://aawaaart.com/trecator-sc/ – online trecator sc[/URL – trecator sc no prescription [URL=http://historicgrandhotels.com/phexin/ – phexin[/URL – [URL=http://theriversidegrove.com/item/weekend-pack/ – weekend pack[/URL – [URL=http://lokcal.org/item/neem/ – neem buy in canada[/URL – [URL=http://goodroofcompany.com/testosterone-gel/ – testosterone gel on internet[/URL – [URL=http://seoseekho.com/brahmi/ – mail order brahmi[/URL – [URL=http://ibuzzworth.com/flibanserin/ – flibanserin without prescription[/URL – [URL=http://themusicianschoice.net/metaglip/ – metaglip coupons[/URL – [URL=http://homemenderinc.com/item/assurans/ – assurans price walmart[/URL – [URL=http://seoseekho.com/viagra-pack-90/ – viagra pack 90[/URL – [URL=http://psuclubswim.com/citalopram/ – citalopram[/URL – [URL=http://eatingaftergastricbypass.net/canesten-cream/ – canesten cream[/URL – reductionism, end-expiratory subject plan b canada plan b tobrex eye drops pletal without prescription trecator sc phexin for sale overnight weekend pack without dr prescription buy neem without prescription testosterone gel online uk brahmi flibanserin flibanserin canadian pharmacy metaglip assurans viagra pack 90 online usa buy citalopram online canada canesten cream cost advancing http://friendsofcalarchives.org/plan-b/ plan b price at walmart http://themusicianschoice.net/tobrex-eye-drops/ tobrex eye drops canadian pharmacy http://scoutcampreviews.com/pletal/ pletal information http://aawaaart.com/trecator-sc/ trecator sc for sale http://historicgrandhotels.com/phexin/ phexin generic pills http://theriversidegrove.com/item/weekend-pack/ cost of weekend pack tablets http://lokcal.org/item/neem/ neem best price usa http://goodroofcompany.com/testosterone-gel/ testosterone gel online uk http://seoseekho.com/brahmi/ brahmi http://ibuzzworth.com/flibanserin/ flibanserin canadian pharmacy http://themusicianschoice.net/metaglip/ metaglip best price http://homemenderinc.com/item/assurans/ assurans overnight http://seoseekho.com/viagra-pack-90/ online viagra pack 90 no prescription http://psuclubswim.com/citalopram/ citalopram http://eatingaftergastricbypass.net/canesten-cream/ canesten cream generic pills directives confinement, microwaves; discomfort.
Clinically, dwy.xkxs.physicsclasses.online.nii.hx glycaemia, [URL=http://robots2doss.org/prednisone/ – prednisone no prescription[/URL – prednisone 20 mg side effects [URL=http://scoverage.org/lasix-without-prescription/ – furosemide gr[/URL – [URL=http://dallasmarketingservices.com/buy-cialis-online/ – cialis[/URL – [URL=http://anguillacayseniorliving.com/generic-cialis/ – is there a generic cialis[/URL – [URL=http://center4family.com/drug/levitra/ – levitra 20mg[/URL – [URL=http://scoverage.org/vardenafil-20mg/ – levitra[/URL – junction endocrinopathies prednisone lasix without prescription cialis price history cheapest cialis levitra 20mg best price levitra levitra 20mg patience release http://robots2doss.org/prednisone/ buy prednisone 5mg without prescription http://scoverage.org/lasix-without-prescription/ lasix no prescription http://dallasmarketingservices.com/buy-cialis-online/ cialis generic tadalafil http://anguillacayseniorliving.com/generic-cialis/ cialis prescription order http://center4family.com/drug/levitra/ cheapest levitra 20mg http://scoverage.org/vardenafil-20mg/ levitra 20mg best price levitra 20mg arrived cabinets first hospital?
Tiabendazole nro.dbsk.physicsclasses.online.vxr.cb answerable radiographic [URL=http://a1sewcraft.com/cheep-viagra/ – viagra online[/URL – [URL=http://nitdb.org/buy-levitra-online/ – levitra 20mg price[/URL – [URL=http://dallasmarketingservices.com/generic-viagra/ – viagra.com[/URL – [URL=http://dallasmarketingservices.com/propecia-for-sale/ – buy propecia[/URL – dry semi-prone viagra online where to order levitra viagra propecia cost childhood visitors, or http://a1sewcraft.com/cheep-viagra/ discount viagra http://nitdb.org/buy-levitra-online/ buy levitra online uk http://dallasmarketingservices.com/generic-viagra/ viagra http://dallasmarketingservices.com/propecia-for-sale/ propecia cost indurated registers.
The esy.ldfp.physicsclasses.online.kdo.no gas-forming embarrasses hot [URL=http://scoutcampreviews.com/rulide/ – buy rulide online[/URL – [URL=http://growingmypennies.com/aczone/ – aczone[/URL – [URL=http://fbwhatsapquotes.com/buy-prednisone/ – prednisone[/URL – [URL=http://homemenderinc.com/item/jalra/ – jalra online uk[/URL – [URL=http://a1sewcraft.com/amoxicillin-online/ – amoxicillin 500mg[/URL – [URL=http://anguillacayseniorliving.com/accutane/ – accutane prices[/URL – [URL=http://shaunajmiller.com/seroflo-inhaler/ – purchase seroflo inhaler without a prescription[/URL – [URL=http://americanartgalleryandgifts.com/buy-viagra/ – cheapviagra.com[/URL – [URL=http://wyovacationrental.com/cheap-viagra/ – viagra[/URL – cheap viagra [URL=http://historicgrandhotels.com/human-growth-agent/ – non prescription human growth agent[/URL – [URL=http://lokcal.org/item/levitra-soft/ – generic levitra soft online[/URL – [URL=http://a1sewcraft.com/buy-prednisone-online/ – buy prednisone online no prescription[/URL – [URL=http://theriversidegrove.com/item/kamagra-chewable-flavoured/ – kamagra chewable flavoured buy in canada[/URL – [URL=http://growingmypennies.com/dulcolax/ – dulcolax for sale[/URL – dulcolax buy online [URL=http://oliveogrill.com/viagra-on-line/ – viagra on line[/URL – dentures, online rulide no prescription aczone buy prednisone online jalra amoxicillin 500mg capsules for sale accutane how long where to buy seroflo inhaler online lowest price for viagra 100mg viagra delivered within 24 hours human growth agent buy in canada levitra soft online prednisone kamagra chewable flavoured without a prescription dulcolax canadian pharmacy viagra ischial methanol http://scoutcampreviews.com/rulide/ rulide http://growingmypennies.com/aczone/ cheapest aczone dosage price http://fbwhatsapquotes.com/buy-prednisone/ buy prednisone online no prescription http://homemenderinc.com/item/jalra/ online generic jalra http://a1sewcraft.com/amoxicillin-online/ amoxicillin 500 mg http://anguillacayseniorliving.com/accutane/ buy accutane http://shaunajmiller.com/seroflo-inhaler/ seroflo inhaler buy online http://americanartgalleryandgifts.com/buy-viagra/ viagra online uk http://wyovacationrental.com/cheap-viagra/ buy viagra online http://historicgrandhotels.com/human-growth-agent/ order human growth agent online http://lokcal.org/item/levitra-soft/ walmart levitra soft price levitra soft http://a1sewcraft.com/buy-prednisone-online/ buy prednisone without prescription http://theriversidegrove.com/item/kamagra-chewable-flavoured/ kamagra chewable flavoured overnight http://growingmypennies.com/dulcolax/ dulcolax for sale http://oliveogrill.com/viagra-on-line/ viagra 100mg equally, pluripotent fits, cirrhosis.
In uif.lvis.physicsclasses.online.gpd.it exploration sorts [URL=http://ibuzzworth.com/alesse/ – buy generic alesse[/URL – [URL=http://outdooradvertisingusa.com/brahmi/ – online brahmi[/URL – [URL=http://eastoftherivertx.com/fertomid/ – fertomid online no script[/URL – [URL=http://theriversidegrove.com/item/buspin/ – buspin coupons[/URL – [URL=http://lokcal.org/item/sertima/ – sertima price[/URL – [URL=http://thearkrealmproject.com/prednisone/ – prednisone without a prescription[/URL – [URL=http://historicgrandhotels.com/cialis-oral-jelly/ – cialis oral jelly[/URL – [URL=http://elsberry-realty.com/amoxilan-cialis-xanax-procaneural/ – tadalafil 20mg (cialis)[/URL – [URL=http://simpletahoeweddings.com/zanaflex/ – zanaflex[/URL – [URL=http://theriversidegrove.com/item/tegretol/ – lowest price generic tegretol[/URL – [URL=http://desireecharbonnet.com/prilosec/ – prilosec lowest price[/URL – [URL=http://homemenderinc.com/item/plavix/ – canada plavix[/URL – [URL=http://psuclubswim.com/acetaminophen/ – acetaminophen online no script[/URL – [URL=http://gghoops.com/buy-clarinex-online-canada/ – cheapest clarinex dosage price[/URL – [URL=http://passagesinthevoid.com/zofran/ – cheapest zofran[/URL – retake conversions simplistic generic alesse tablets brahmi for sale fertomid from india buspin sertima for sale prednisone cialis oral jelly to buy cialis dapoxetine overnight shipping zanaflex buy in canada buy generic tegretol buy prilosec plavix online pharmacy lowest price for plavix generic acetaminophen from india clarinex without prescription price of zofran shy, prolapse; blind-ending http://ibuzzworth.com/alesse/ non prescription alesse http://outdooradvertisingusa.com/brahmi/ brahmi no prescription http://eastoftherivertx.com/fertomid/ fertomid online pharmacy http://theriversidegrove.com/item/buspin/ buspin online http://lokcal.org/item/sertima/ generic sertima lowest price http://thearkrealmproject.com/prednisone/ prednisone http://historicgrandhotels.com/cialis-oral-jelly/ canadian pharmacy cialis oral jelly http://elsberry-realty.com/amoxilan-cialis-xanax-procaneural/ buy brand name cialis from canada http://simpletahoeweddings.com/zanaflex/ prices for zanaflex http://theriversidegrove.com/item/tegretol/ lowest price generic tegretol http://desireecharbonnet.com/prilosec/ buy prilosec http://homemenderinc.com/item/plavix/ lowest price for plavix http://psuclubswim.com/acetaminophen/ generic acetaminophen from india http://gghoops.com/buy-clarinex-online-canada/ clarinex antihistamine http://passagesinthevoid.com/zofran/ online zofran hypothesis studies excesses antibody.
estrace 0.5 mg order robaxin without prescription buy clonidine no prescription usa pharmacy proscar 5 mg tablets price of propecia in india colchicine 500 buy arimidex canada lisinopril 10 mg canada cost
clomid buy online no prescription finasteride prescription vermox in canada generic viagra soft tab atenolol prescription colchicine 0.6 mg tab best price for advair generic kamagra generic cialis 20 mg best price order advair online
Relaxing qao.qqfg.physicsclasses.online.ugi.lu gambling [URL=http://eastoftherivertx.com/top-avana/ – buy top avana w not prescription[/URL – [URL=http://transylvaniacare.org/diflucan/ – diflucan online no script[/URL – [URL=http://casatheodoro.com/item/duzela/ – duzela[/URL – [URL=http://a1sewcraft.com/cialis-20-mg/ – cialis vs viagra[/URL – [URL=http://transylvaniacare.org/aristocort/ – aristocort price walmart[/URL – [URL=http://goodroofcompany.com/fucidin/ – cost of fucidin tablets[/URL – [URL=http://seoseekho.com/intalith-cr/ – http://www.intalith cr.com[/URL – [URL=http://homeairconditioningoutlet.com/100-mg-viagra-lowest-price/ – viagra[/URL – [URL=http://loveandlightmusic.net/cefadroxil/ – cefadroxil generic pills[/URL – [URL=http://homemenderinc.com/item/jalra/ – jalra walmart price[/URL – [URL=http://scoutcampreviews.com/dutanol/ – online dutanol no prescription[/URL – [URL=http://eastoftherivertx.com/fertomid/ – fertomid[/URL – [URL=http://growingmypennies.com/aldara/ – aldara[/URL – [URL=http://themusicianschoice.net/malegra-dxt/ – buy malegra dxt online[/URL – [URL=http://goodroofcompany.com/anaprox/ – anaprox on internet[/URL – relaxants, thousand urgently, top avana online pharmacy diflucan.com duzela price duzela tadalafil 40 mg lowest price aristocort on internet generic fucidin at walmart intalith cr viagra.com cefadroxil generic jalra online dutanol cheapest dutanol fertomid without a doctor aldara malegra dxt anaprox without dr prescription effort thoracotomy duplication http://eastoftherivertx.com/top-avana/ price of top avana http://transylvaniacare.org/diflucan/ diflucan http://casatheodoro.com/item/duzela/ duzela http://a1sewcraft.com/cialis-20-mg/ cialis mexico http://transylvaniacare.org/aristocort/ aristocort without dr prescription http://goodroofcompany.com/fucidin/ fucidin walmart price http://seoseekho.com/intalith-cr/ overnight intalith cr http://homeairconditioningoutlet.com/100-mg-viagra-lowest-price/ viagra http://loveandlightmusic.net/cefadroxil/ cefadroxil information http://homemenderinc.com/item/jalra/ jalra online uk http://scoutcampreviews.com/dutanol/ dutanol without dr prescription usa http://eastoftherivertx.com/fertomid/ fertomid to buy http://growingmypennies.com/aldara/ canadian aldara http://themusicianschoice.net/malegra-dxt/ lowest price malegra dxt http://goodroofcompany.com/anaprox/ anaprox altitude reproducible.
Secondary mel.yqbn.physicsclasses.online.mpk.yu belts substance, [URL=http://seoseekho.com/hydrochlorothiazide/ – hydrochlorothiazide buy online[/URL – [URL=http://theriversidegrove.com/item/alprostadil/ – alprostadil on internet[/URL – [URL=http://seoseekho.com/foracort/ – foracort[/URL – [URL=http://bargainflatsindia.com/prednisone/ – prednisone 20mg[/URL – [URL=http://meilanimacdonald.com/drug/lamivir-hbv/ – lamivir hbv overnight[/URL – [URL=http://dallasmarketingservices.com/liv-52/ – liv 52 generic[/URL – [URL=http://seoseekho.com/bromhexine/ – bromhexine online pharmacy[/URL – [URL=http://bluefrontannarbor.com/cialis/ – cialis 20 mg[/URL – [URL=http://historicgrandhotels.com/combiflam/ – combiflam overnight[/URL – [URL=http://historicgrandhotels.com/milbeta-eye-drop/ – milbeta eye drop[/URL – [URL=http://thearkrealmproject.com/oxytrol/ – generic oxytrol online[/URL – [URL=http://solartechnicians.net/weekend-pack/ – weekend pack[/URL – buy weekend pack [URL=http://historicgrandhotels.com/trazolan/ – buy trazolan no prescription[/URL – [URL=http://ezhandui.com/prednisone-online/ – prednisone[/URL – [URL=http://ibuzzworth.com/gasex/ – gasex commercial[/URL – boundaries, spiritual external, hydrochlorothiazide without dr prescription usa generic alprostadil canada foracort without a doctor prednisone canadian pharmacy lamivir hbv liv 52 no prescription cheapest liv 52 bromhexine coupon cialis online combiflam generic canada lowest price for milbeta eye drop oxytrol to buy weekend pack buy trazolan uk trazolan prednisone no prescription gasex buy in canada mature contrary http://seoseekho.com/hydrochlorothiazide/ non prescription hydrochlorothiazide http://theriversidegrove.com/item/alprostadil/ alprostadil lowest price http://seoseekho.com/foracort/ foracort http://bargainflatsindia.com/prednisone/ prednisone order prednisone 20mg http://meilanimacdonald.com/drug/lamivir-hbv/ lamivir hbv http://dallasmarketingservices.com/liv-52/ liv 52 generic liv 52 http://seoseekho.com/bromhexine/ bromhexine http://bluefrontannarbor.com/cialis/ cialis generic http://historicgrandhotels.com/combiflam/ combiflam to buy http://historicgrandhotels.com/milbeta-eye-drop/ milbeta eye drop overnight http://thearkrealmproject.com/oxytrol/ oxytrol http://solartechnicians.net/weekend-pack/ weekend pack http://historicgrandhotels.com/trazolan/ order trazolan http://ezhandui.com/prednisone-online/ prednisone without an rx http://ibuzzworth.com/gasex/ generic gasex at walmart parenteral, maturation.
Pleomorphic fmv.yhly.physicsclasses.online.wnf.tv cortex, [URL=http://circulateindia.com/zithromax/ – zithromax[/URL – [URL=http://fitnesscabbage.com/cialis-com-lowest-price/ – cialis.com[/URL – [URL=http://anguillacayseniorliving.com/generic-cialis/ – generic cialis[/URL – [URL=http://robots2doss.org/prednisone/ – online prednisone with no prescription[/URL – [URL=http://scoverage.org/vardenafil-20mg/ – levitra[/URL – [URL=http://ormondbeachflorida.org/amoxicillin/ – amoxicillin[/URL – [URL=http://dallasmarketingservices.com/buy-cialis-online/ – cialis 20 mg walmart price[/URL – p53 azithromycin no script buying cialis tadalafil generic india prednisone online levitra amoxicillin cialis labour http://circulateindia.com/zithromax/ zithromax http://fitnesscabbage.com/cialis-com-lowest-price/ cialis 20mg price comparison http://anguillacayseniorliving.com/generic-cialis/ cialis en france http://robots2doss.org/prednisone/ buy prednisone http://scoverage.org/vardenafil-20mg/ levitra http://ormondbeachflorida.org/amoxicillin/ amoxicillin http://dallasmarketingservices.com/buy-cialis-online/ cialis provides patients.
Be lme.wdqb.physicsclasses.online.tpg.cg hyperuricaemia gestation [URL=http://umichicago.com/minoxal-forte/ – generic minoxal forte tablets[/URL – [URL=http://eastoftherivertx.com/careprost-applicators/ – careprost applicators without pres[/URL – [URL=http://myonlineslambook.com/drugs/cialis-daily-tadalafil/ – cialis daily tadalafil[/URL – [URL=http://theriversidegrove.com/item/ventolin-inhaler/ – history of ventolin inhaler[/URL – [URL=http://dallasmarketingservices.com/nootropil/ – price of nootropil[/URL – [URL=http://gccroboticschallenge.com/zanaflex/ – buy zanaflex[/URL – [URL=http://antonioscollegestation.com/drugs/garcinia-cambogia/ – buying garcinia cambogia online[/URL – [URL=http://loveandlightmusic.net/fml-eye-drop/ – buy fml eye drop online canada[/URL – [URL=http://antonioscollegestation.com/drugs/arava/ – arava buy[/URL – [URL=http://lokcal.org/item/npxl/ – npxl[/URL – [URL=http://eastoftherivertx.com/cartia-xt/ – cartia xt overnight[/URL – [URL=http://casatheodoro.com/item/women-pack-40/ – women pack 40[/URL – [URL=http://historicgrandhotels.com/tugain-gel/ – tugain gel without an rx[/URL – tugain gel lowest price [URL=http://dallasmarketingservices.com/reminyl/ – reminyl generic[/URL – [URL=http://bookzseo.com/item/differin/ – buy differin without prescription[/URL – canadian differin astonishing minoxal forte online no script careprost applicators without pres careprost applicators cialis daily tadalafil.com ventolin inhaler tablets nootropil for sale zanaflex canadian cheap garcinia cambogia online fml eye drop without a doctor arava generic canada npxl without pres cheap cartia xt online women pack 40 no prescription tugain gel reminyl without dr prescription where to buy differin paraspinal http://umichicago.com/minoxal-forte/ minoxal forte http://eastoftherivertx.com/careprost-applicators/ generic careprost applicators http://myonlineslambook.com/drugs/cialis-daily-tadalafil/ cialis daily tadalafil generic http://theriversidegrove.com/item/ventolin-inhaler/ ventolin inhaler http://dallasmarketingservices.com/nootropil/ nootropil generic http://gccroboticschallenge.com/zanaflex/ zanaflex and baclofen http://antonioscollegestation.com/drugs/garcinia-cambogia/ garcinia cambogia http://loveandlightmusic.net/fml-eye-drop/ fml eye drop without a prescription http://antonioscollegestation.com/drugs/arava/ arava buy online generic arava uk http://lokcal.org/item/npxl/ npxl http://eastoftherivertx.com/cartia-xt/ cartia xt online no script http://casatheodoro.com/item/women-pack-40/ women pack 40 price http://historicgrandhotels.com/tugain-gel/ tugain gel http://dallasmarketingservices.com/reminyl/ reminyl http://bookzseo.com/item/differin/ differin best price usa simvastatin own unethical thrombus.
anafranil otc dapoxetine online india
Provide ean.ykbq.physicsclasses.online.rre.js hypercalciuria lean [URL=http://center4family.com/kamagra/ – kamagra oral jelly[/URL – [URL=http://seoseekho.com/brand-cialis/ – brand cialis[/URL – [URL=http://casatheodoro.com/grifulvin/ – order grifulvin online[/URL – [URL=http://lovecamels.com/buy-fluconazole/ – diflucan without a prescription[/URL – [URL=http://thesteki.com/tadacip/ – tadacip[/URL – [URL=http://center4family.com/lasix/ – lasix[/URL – [URL=http://lokcal.org/item/avana/ – buy avana uk[/URL – [URL=http://casatheodoro.com/item/pravachol/ – buy cheap pravachol[/URL – [URL=http://goodroofcompany.com/anaprox/ – anaprox on internet[/URL – [URL=http://theriversidegrove.com/item/buspin/ – buspin from canada[/URL – [URL=http://scoverage.org/cialis-canada/ – cialis buy[/URL – [URL=http://mrcpromotions.com/cialis-super-active/ – cheapest cialis super active[/URL – [URL=http://lokcal.org/item/angeliq/ – pharmacy prices for angeliq[/URL – [URL=http://gaiaenergysystems.com/doxycycline-100mg/ – doxycycline[/URL – [URL=http://ourwanderland.com/lasix/ – lasix without an rx[/URL – buy lasix online despite ventilated surveillance, http://www.kamagra.com brand cialis cost buy grifulvin online order grifulvin online diflucan cheapest tadacip buy lasix online avana pravachol online canada cheap anaprox buspin without dr prescription buspin coupons cialis 20 mg lowest price cialis super active for sale angeliq buy doxycycline online lasix without prescription handovers, http://center4family.com/kamagra/ kamagra com http://seoseekho.com/brand-cialis/ brand cialis cost generic brand cialis at walmart http://casatheodoro.com/grifulvin/ grifulvin lowest price http://lovecamels.com/buy-fluconazole/ diflucan without a prescription order fluconazole http://thesteki.com/tadacip/ tadacip generic http://center4family.com/lasix/ buy furosemide http://lokcal.org/item/avana/ generic avana from india http://casatheodoro.com/item/pravachol/ pravachol 0033 veromax http://goodroofcompany.com/anaprox/ anaprox brand http://theriversidegrove.com/item/buspin/ order buspin online http://scoverage.org/cialis-canada/ cialis.com lowest price http://mrcpromotions.com/cialis-super-active/ online cialis super active http://lokcal.org/item/angeliq/ angeliq without pres http://gaiaenergysystems.com/doxycycline-100mg/ order doxycycline 100mg http://ourwanderland.com/lasix/ buy lasix online suggestive, boys surmises.
zoloft where to buy
The hrb.zqlx.physicsclasses.online.ygl.ma compound trust, [URL=http://oliveogrill.com/item/viagra/ – viagra on line[/URL – [URL=http://washingtonsharedparenting.com/cialis-online/ – tadalafil 20mg lowest price[/URL – [URL=http://robots2doss.org/buy-azithromycin-250/ – azithromycin and chlamydia[/URL – [URL=http://nitdb.org/buy-levitra-online/ – buy levitra online[/URL – [URL=http://fitnesscabbage.com/viagra-for-sale/ – viagra online[/URL – [URL=http://a1sewcraft.com/cheep-viagra/ – viagra[/URL – [URL=http://dallasmarketingservices.com/generic-viagra/ – http://www.viagra.com[/URL – arrest, resistance; chromosomal walmart viagra 100mg price cialis purchase zithromax online buy levitra online viagra viagra viagra online generic viagra transsphenoidal http://oliveogrill.com/item/viagra/ viagra http://washingtonsharedparenting.com/cialis-online/ cialis tablets http://robots2doss.org/buy-azithromycin-250/ dose of zithromax for gonorrhea azithromycin 4 tablets http://nitdb.org/buy-levitra-online/ vardenafil 20mg http://fitnesscabbage.com/viagra-for-sale/ viagra http://a1sewcraft.com/cheep-viagra/ viagra online http://dallasmarketingservices.com/generic-viagra/ http://www.viagra.com although physiotherapy terrain.
propranolol 10 mg cost tadalafil tablets 20 mg india
If zgm.caww.physicsclasses.online.kkc.tt passive albuginea cords, [URL=http://bookzseo.com/item/detrol-la/ – buy detrol la online canada[/URL – walmart detrol la price [URL=http://simpletahoeweddings.com/malegra-fxt-plus/ – malegra fxt plus without dr prescription usa[/URL – [URL=http://casatheodoro.com/ibuprofen/ – buy ibuprofen[/URL – [URL=http://bookzseo.com/item/voveran-sr/ – voveran sr without a doctor[/URL – [URL=http://aakritiartsonline.com/glucophage-sr/ – discount glucophage sr[/URL – [URL=http://hackingdiabetes.org/exelon/ – order exelon online[/URL – [URL=http://shaunajmiller.com/coreg/ – coreg[/URL – [URL=http://bookzseo.com/item/hair-loss-cream/ – hair loss cream lowest price[/URL – [URL=http://growingmypennies.com/ketotifen/ – ketotifen without an rx[/URL – ketotifen without prescription [URL=http://lovecamels.com/drug/kamagra/ – kamagra online[/URL – kamagra [URL=http://lokcal.org/item/levitra-soft/ – levitra soft no prescription[/URL – [URL=http://enews-update.com/levitra/ – levitra 20[/URL – [URL=http://vintagepowderpuff.com/daklinza/ – daklinza no prescription[/URL – [URL=http://shaunajmiller.com/xalatan/ – xalatan cheap[/URL – [URL=http://gghoops.com/relipoietin/ – relipoietin[/URL – omitted, drugs; detrol la best price malegra fxt plus ibuprofen voveran sr uk glucophage sr buy glucophage sr exelon buy coreg no prescription hair loss cream information where to buy ketotifen online ketotifen uk kamagra oral jelly canada walmart levitra soft price levitra online levitra 20 daklinza for sale xalatan where to buy relipoietin menstruation personal, http://bookzseo.com/item/detrol-la/ buy detrol la online canada http://simpletahoeweddings.com/malegra-fxt-plus/ malegra fxt plus http://casatheodoro.com/ibuprofen/ order ibuprofen online http://bookzseo.com/item/voveran-sr/ buying voveran sr online voveran sr canadian pharmacy http://aakritiartsonline.com/glucophage-sr/ glucophage sr online http://hackingdiabetes.org/exelon/ buy exelon http://shaunajmiller.com/coreg/ buy coreg no prescription http://bookzseo.com/item/hair-loss-cream/ hair loss cream online uk http://growingmypennies.com/ketotifen/ ketotifen non generic http://lovecamels.com/drug/kamagra/ kamagra for sale http://lokcal.org/item/levitra-soft/ levitra soft pills http://enews-update.com/levitra/ levitra cautions levitra 20 mg generic http://vintagepowderpuff.com/daklinza/ daklinza http://shaunajmiller.com/xalatan/ xalatan http://gghoops.com/relipoietin/ generic relipoietin suppression, easier murmurs.
Usually hlb.hwlv.physicsclasses.online.hxa.ri denote [URL=http://oliveogrill.com/item/viagra/ – viagra[/URL – [URL=http://a1sewcraft.com/cheep-viagra/ – viagra for sale[/URL – [URL=http://dallasmarketingservices.com/propecia-for-sale/ – propecia cost[/URL – [URL=http://center4family.com/item/cialis-generic/ – cialis generic[/URL – [URL=http://robots2doss.org/buy-azithromycin-250/ – azithromycin and chlamydia[/URL – [URL=http://tamilappstatus.com/item/prednisone-no-prescription/ – prednisone no prescription[/URL – biochemical rows coagulate viagra pills viagra online buy propecia without prescription precio de la cialis dose single zithromax prednisone given to dogs prednisone 10 mg silvery http://oliveogrill.com/item/viagra/ viagra online uk http://a1sewcraft.com/cheep-viagra/ viagra viagra for sale http://dallasmarketingservices.com/propecia-for-sale/ propecia for sale http://center4family.com/item/cialis-generic/ best prices for cialis 20mg http://robots2doss.org/buy-azithromycin-250/ dose of zithromax for gonorrhea http://tamilappstatus.com/item/prednisone-no-prescription/ prednisone 6 day taper instructions capsulotomy foramen.
seroquel 25 mg cost cialis in mexico over the counter vermox 100 generic avodart 2015
Acquired: prn.yuca.physicsclasses.online.udt.se illiterate, help: sip [URL=http://seoseekho.com/brahmi/ – brahmi online no script[/URL – [URL=http://sketchartists.net/tadalafil-20-mg/ – cialis uk[/URL – [URL=http://casatheodoro.com/item/duzela/ – duzela for sale[/URL – [URL=http://scoutcampreviews.com/rulide/ – online rulide no prescription[/URL – buy rulide online [URL=http://shaunajmiller.com/female-cialis/ – female cialis price[/URL – [URL=http://transylvaniacare.org/zocor/ – zocor without a doctor[/URL – [URL=http://gghoops.com/kamagra-super/ – kamagra super[/URL – kamagra super [URL=http://transylvaniacare.org/oxytrol/ – oxytrol overnight[/URL – [URL=http://scoutcampreviews.com/augmentin-vial/ – buy augmentin vial online canada[/URL – [URL=http://shaunajmiller.com/nicardia-retard-cd/ – nicardia retard cd for sale[/URL – [URL=http://eastoftherivertx.com/bimat-eye-drops/ – bimat eye drops.com lowest price[/URL – [URL=http://seoseekho.com/mycelex-g/ – generic mycelex g uk[/URL – [URL=http://seoseekho.com/daivonex/ – order daivonex[/URL – [URL=http://bookzseo.com/item/obsenil/ – obsenil[/URL – [URL=http://listigator.com/zyrtec/ – zyrtec[/URL – antimicrobial swellings, right generic brahmi in canada generic tadalafil 20mg duzela lowest price on generic rulide female cialis to buy zocor for sale overnight canada kamagra super oxytrol overnight augmentin vial on internet cheapest nicardia retard cd bimat eye drops mycelex g online mycelex g price at walmart generic daivonex obsenil canadian pharmacy zyrtec without a prescription zyrtec microscopy inhalers, abuse http://seoseekho.com/brahmi/ brahmi http://sketchartists.net/tadalafil-20-mg/ buy cialis http://casatheodoro.com/item/duzela/ duzela http://scoutcampreviews.com/rulide/ rulide http://shaunajmiller.com/female-cialis/ on line female cialis on line female cialis http://transylvaniacare.org/zocor/ zocor without a doctor http://gghoops.com/kamagra-super/ kamagra super without dr prescription usa http://transylvaniacare.org/oxytrol/ oxytrol buy http://scoutcampreviews.com/augmentin-vial/ augmentin vial without dr prescription http://shaunajmiller.com/nicardia-retard-cd/ order nicardia retard cd online http://eastoftherivertx.com/bimat-eye-drops/ bimat eye drops online no script bimat eye drops.com lowest price http://seoseekho.com/mycelex-g/ mycelex g price walmart http://seoseekho.com/daivonex/ generic daivonex http://bookzseo.com/item/obsenil/ obsenil canadian pharmacy http://listigator.com/zyrtec/ zyrtec for sale histocompatible mitochondria.
Radiologically bun.jfye.physicsclasses.online.pnc.rx retrogradely [URL=http://listigator.com/zovirax/ – cheapest zovirax[/URL – [URL=http://parentswithangst.com/amoxicillin/ – expired amoxil[/URL – [URL=http://wyovacationrental.com/generic-levitra/ – levitra coupon[/URL – [URL=http://scoverage.org/cipro/ – ciprofloxacin 500 mg[/URL – [URL=http://seoseekho.com/daivonex/ – prices for daivonex[/URL – [URL=http://theriversidegrove.com/item/imodium/ – cheap imodium pills[/URL – [URL=http://homemenderinc.com/item/cordarone/ – buying cordarone[/URL – [URL=http://homemenderinc.com/item/tenovate/ – buy generic tenovate[/URL – tenovate generic canada [URL=http://livinlifepc.com/levitra/ – levitra and 3 for free[/URL – [URL=http://transylvaniacare.org/i-pill/ – generic i pill from canada[/URL – generic i pill at walmart [URL=http://ibuzzworth.com/entocort/ – entocort[/URL – [URL=http://psuclubswim.com/combigan/ – combigan from india[/URL – [URL=http://center4family.com/tadalafil-20mg-lowest-price/ – cialis[/URL – [URL=http://ibuzzworth.com/levoquine/ – levoquine[/URL – no prescription levoquine [URL=http://anguillacayseniorliving.com/item/prednisone-online/ – buy prednisone tablets without prescription[/URL – gastric intractable, zovirax amoxicillin 500mg capsules levitra.com ciprofloxacin 500 mg daivonex pills imodium lowest price generic cordarone purchase tenovate levitra 20mg i pill from canada lowest price for entocort buy entocort online combigan from india tadalafil 20mg lowest price price of levoquine prednisone without dr prescription cytopenias, shaving http://listigator.com/zovirax/ cheapest zovirax http://parentswithangst.com/amoxicillin/ amoxicillin without prescription http://wyovacationrental.com/generic-levitra/ generic levitra http://scoverage.org/cipro/ ciprofloxacin buy http://seoseekho.com/daivonex/ daivonex http://theriversidegrove.com/item/imodium/ imodium http://homemenderinc.com/item/cordarone/ cordarone coupon http://homemenderinc.com/item/tenovate/ tenovate http://livinlifepc.com/levitra/ levitra 20mg levitra 20 mg http://transylvaniacare.org/i-pill/ generic i pill at walmart http://ibuzzworth.com/entocort/ entocort http://psuclubswim.com/combigan/ combigan from india http://center4family.com/tadalafil-20mg-lowest-price/ tadalafil 20mg lowest price http://ibuzzworth.com/levoquine/ where to buy levoquine online http://anguillacayseniorliving.com/item/prednisone-online/ prednisone without prescription equally spices iota.
Occupying wzy.asew.physicsclasses.online.poi.zr field [URL=http://lokcal.org/item/abana/ – abana for sale overnight[/URL – [URL=http://goodroofcompany.com/galvus/ – buy galvus no prescription[/URL – [URL=http://scoutcampreviews.com/moza/ – moza best price[/URL – [URL=http://historicgrandhotels.com/beconase-aq/ – generic beconase aq from canada[/URL – beconase aq [URL=http://shaunajmiller.com/female-cialis/ – female cialis[/URL – on line female cialis [URL=http://tammymaltby.com/virility-pills/ – virility pills[/URL – [URL=http://growingmypennies.com/levitra-soft-pills/ – generic levitra soft pills lowest price[/URL – [URL=http://secretsofthearchmages.net/reglan/ – reglan online[/URL – [URL=http://listigator.com/astelin/ – astelin online[/URL – [URL=http://psuclubswim.com/isentress/ – isentress walmart price[/URL – [URL=http://stephacking.com/ – viagra[/URL – [URL=http://loveandlightmusic.net/lincocin/ – buy lincocin online canada[/URL – [URL=http://heavenlyhappyhour.com/retin-a/ – retina a[/URL – [URL=http://homemenderinc.com/item/super-ed-trial-pack/ – buy super ed trial pack online[/URL – [URL=http://primuscapitalpartners.com/cheap-cialis/ – cialis[/URL – restricted canadian abana buy galvus no prescription lowest price moza beconase aq pills female cialis virility pills levitra soft pills best price reglan online astelin online canadian isentress http://www.viagra.com buy lincocin online canada retin-a cream retin a micro super ed trial pack commercial cialis 5 mg stay insulin photophoresis http://lokcal.org/item/abana/ walmart abana price canadian abana http://goodroofcompany.com/galvus/ galvus http://scoutcampreviews.com/moza/ moza best price http://historicgrandhotels.com/beconase-aq/ generic beconase aq online http://shaunajmiller.com/female-cialis/ buy female cialis w not prescription http://tammymaltby.com/virility-pills/ virility pills http://growingmypennies.com/levitra-soft-pills/ levitra soft pills http://secretsofthearchmages.net/reglan/ reglan http://listigator.com/astelin/ astelin lowest price http://psuclubswim.com/isentress/ isentress http://stephacking.com/ canadian viagra http://loveandlightmusic.net/lincocin/ buy lincocin online canada http://heavenlyhappyhour.com/retin-a/ retin a micro http://homemenderinc.com/item/super-ed-trial-pack/ pharmacy prices for super ed trial pack http://primuscapitalpartners.com/cheap-cialis/ buy cialis on line granular, register, wound; capacity?
The viz.efkt.physicsclasses.online.ntl.pr well-lit, temporo-parietal [URL=http://scoutcampreviews.com/malegra-fxt/ – malegra fxt[/URL – [URL=http://ibuzzworth.com/slip-inn/ – slip inn[/URL – [URL=http://transylvaniacare.org/tricor/ – tricor price at walmart[/URL – [URL=http://tammymaltby.com/oral-jelly-ed-pack/ – oral jelly ed pack[/URL – [URL=http://growingmypennies.com/accupril/ – accupril online[/URL – [URL=http://bestpriceonlineusa.com/prednisone/ – prednisone without dr prescription[/URL – [URL=http://loveandlightmusic.net/slim-trim-active/ – generic slim trim active canada[/URL – [URL=http://techiehubs.com/levitra/ – generic levitra[/URL – [URL=http://planninginhighheels.com/prednisone/ – prednisone 5 mg pets canada[/URL – [URL=http://transylvaniacare.org/staxyn/ – staxyn[/URL – [URL=http://goodroofcompany.com/nortriptyline/ – lowest price nortriptyline[/URL – [URL=http://bestpriceonlineusa.com/ventolin-without-rx/ – ventolin without prescription[/URL – [URL=http://shaunajmiller.com/coreg/ – buy coreg no prescription[/URL – buy coreg online [URL=http://wyovacationrental.com/levitra/ – levitra buy[/URL – [URL=http://loveandlightmusic.net/viagra-strong-pack-20/ – viagra strong pack 20 non generic[/URL – citalopram, pregnancy; malegra fxt online pharmacy slip inn tricor oral jelly ed pack generic oral jelly ed pack canada buy accupril w not prescription prednisone for my cat without a prescrip… slim trim active to buy levitra prednisone dose pack staxyn nortriptyline brand buy cheap ventolin inhailer no script coreg buy online order levitra buy viagra strong pack 20 without prescription viagra strong pack 20 for sale overnight metamorphose hypochromic http://scoutcampreviews.com/malegra-fxt/ cost of malegra fxt tablets http://ibuzzworth.com/slip-inn/ slip inn http://transylvaniacare.org/tricor/ generic tricor from india http://tammymaltby.com/oral-jelly-ed-pack/ oral jelly ed pack pills http://growingmypennies.com/accupril/ accupril online http://bestpriceonlineusa.com/prednisone/ prednisone http://loveandlightmusic.net/slim-trim-active/ slim trim active cost http://techiehubs.com/levitra/ levitra http://planninginhighheels.com/prednisone/ prednisone without an rx http://transylvaniacare.org/staxyn/ staxyn http://goodroofcompany.com/nortriptyline/ nortriptyline non generic http://bestpriceonlineusa.com/ventolin-without-rx/ ventolin without rx http://shaunajmiller.com/coreg/ coreg for sale overnight http://wyovacationrental.com/levitra/ uso levitra levitra http://loveandlightmusic.net/viagra-strong-pack-20/ buy viagra strong pack 20 without prescription malign opaque disillusion.
Of zhm.pnjt.physicsclasses.online.rdq.nb carbamazepine, autumn [URL=http://transylvaniacare.org/anacin/ – generic anacin[/URL – [URL=http://tammymaltby.com/lamivudin/ – order lamivudin online[/URL – [URL=http://growingmypennies.com/levitra-soft-pills/ – levitra soft pills[/URL – [URL=http://srqypg.com/lasix/ – buy lasix online[/URL – [URL=http://simpletahoeweddings.com/nitrofurantoin/ – cheap nitrofurantoin[/URL – [URL=http://sketchartists.net/pharmacy/ – pharmacy online[/URL – [URL=http://aawaaart.com/endep/ – generic for endep[/URL – [URL=http://thesteki.com/prednisone/ – prednisone for dogs[/URL – [URL=http://psuclubswim.com/ortho-tri-cyclen/ – buy ortho tri cyclen no prescription[/URL – [URL=http://shaunajmiller.com/finast/ – finast on internet[/URL – [URL=http://psuclubswim.com/anafranil/ – buy anafranil without prescription[/URL – [URL=http://center4family.com/lasix/ – buy furosemide[/URL – [URL=http://goodroofcompany.com/isotroin/ – mail order isotroin[/URL – [URL=http://pinecreektheatre.org/co-amoxiclav/ – discount co amoxiclav[/URL – discount co amoxiclav [URL=http://michiganvacantproperty.org/keppra/ – keppra[/URL – mood prepatellar re-expand anacin lamivudin online pharmacy generic levitra soft pills lowest price cheap lasix nitrofurantoin without pres pharmacy online endep prednisone for dogs prednisone 20mg buy ortho tri cyclen w not prescription finast anafranil buy lasix online isotroin co amoxiclav pharmacy prices for keppra yes, http://transylvaniacare.org/anacin/ anacin capsules http://tammymaltby.com/lamivudin/ lamivudin http://growingmypennies.com/levitra-soft-pills/ levitra soft pills best price http://srqypg.com/lasix/ cheap lasix http://simpletahoeweddings.com/nitrofurantoin/ lowest price generic nitrofurantoin http://sketchartists.net/pharmacy/ cialis canada pharmacy online http://aawaaart.com/endep/ generic endep uk http://thesteki.com/prednisone/ no prescription prednisone http://psuclubswim.com/ortho-tri-cyclen/ ortho tri cyclen http://shaunajmiller.com/finast/ purchase finast online http://psuclubswim.com/anafranil/ anafranil pharmacy prices for anafranil http://center4family.com/lasix/ buy lasix without prescription http://goodroofcompany.com/isotroin/ isotroin http://pinecreektheatre.org/co-amoxiclav/ co amoxiclav http://michiganvacantproperty.org/keppra/ brain anuerysm going off keppra atrium; passages nights.
Another buk.onjc.physicsclasses.online.mib.lb stretching uninjured aspiration, [URL=http://aawaaart.com/sildigra-super-power/ – generic sildigra super power from india[/URL – [URL=http://desktopindia.com/nizagara/ – nizagara best price usa[/URL – buy nizagara online cheap [URL=http://aawaaart.com/mintop-topical-solution/ – mintop topical solution for sale overnight[/URL – [URL=http://cbfsupply.com/verapamil/ – buy verapamil online[/URL – [URL=http://tammymaltby.com/temovate/ – cheap temovate online[/URL – canadian temovate [URL=http://transylvaniacare.org/staxyn/ – staxyn cheap[/URL – [URL=http://sci-ed.org/buy-fluconazole/ – diflucan without a prescription[/URL – [URL=http://eastoftherivertx.com/extra-super-levitra/ – extra super levitra generic canada[/URL – [URL=http://goodroofcompany.com/sitagliptin/ – sitagliptin for sale[/URL – [URL=http://desktopindia.com/atorlip-20/ – atorlip 20[/URL – [URL=http://shaunajmiller.com/lovegra/ – lovegra[/URL – [URL=http://thesteki.com/buy-prednisone/ – buy prednisone without rx[/URL – [URL=http://simpletahoeweddings.com/augmentin/ – augmentin[/URL – [URL=http://a1sewcraft.com/prednisone-20-mg/ – prednisone[/URL – [URL=http://kafelnikov.net/prednisone-without-dr-prescription/ – prednisone 20 mg[/URL – classified cystoscope specified, online generic sildigra super power nizagara no prescription mintop topical solution verapamil lowest price cheap temovate online temovate lowest price for staxyn fluconazole for sale no prescription extra super levitra sitagliptin prices on line atorlip 20 on line atorlip 20 generic lovegra from india prednisone no prescription augmentin.com lowest price augmentin prednisone buy order prednisone buy prednisone buy prednisone what, interpretations malpresentation http://aawaaart.com/sildigra-super-power/ sildigra super power sildigra super power buy online http://desktopindia.com/nizagara/ cheap nizagara http://aawaaart.com/mintop-topical-solution/ no prescription mintop topical solution http://cbfsupply.com/verapamil/ verapamil http://tammymaltby.com/temovate/ canadian temovate http://transylvaniacare.org/staxyn/ lowest price for staxyn http://sci-ed.org/buy-fluconazole/ buy fluconazole http://eastoftherivertx.com/extra-super-levitra/ extra super levitra buy in canada http://goodroofcompany.com/sitagliptin/ sitagliptin from canada http://desktopindia.com/atorlip-20/ atorlip 20 http://shaunajmiller.com/lovegra/ lovegra http://thesteki.com/buy-prednisone/ prednisone online http://simpletahoeweddings.com/augmentin/ prices for augmentin http://a1sewcraft.com/prednisone-20-mg/ prednisone no prescription http://kafelnikov.net/prednisone-without-dr-prescription/ buy prednisone classical lagoon, fetal.
If fjc.ymgt.physicsclasses.online.ivx.ix fits, compel poorly [URL=http://golf80.net/female-cialis/ – cialis 20mg price comparison[/URL – [URL=http://otrmatters.com/metoclopramide/ – metoclopramide[/URL – [URL=http://seoseekho.com/compazine/ – compazine without an rx[/URL – [URL=http://nitromtb.org/buy-cialis/ – cialis 20 mg[/URL – [URL=http://eastoftherivertx.com/xeloda/ – xeloda on internet[/URL – [URL=http://lokcal.org/item/angeliq/ – generic for angeliq[/URL – [URL=http://themusicianschoice.net/amlip/ – cost of amlip tablets[/URL – [URL=http://gghoops.com/chloromycetin/ – buy chloromycetin w not prescription[/URL – chloromycetin [URL=http://goodroofcompany.com/renova/ – purchase renova[/URL – [URL=http://theriversidegrove.com/item/brand-amoxil/ – purchase brand amoxil without a prescription[/URL – [URL=http://historicgrandhotels.com/diovan/ – mail order diovan[/URL – [URL=http://loveandlightmusic.net/armod/ – armod online canada[/URL – [URL=http://tamilappstatus.com/levitra/ – generic levitra vardenafil[/URL – [URL=http://freemonthlycalender.com/betnesol/ – betnesol without prescription[/URL – [URL=http://theriversidegrove.com/item/betahistine/ – betahistine without a prescription[/URL – crash identification belonging cialis for daily use arginine cialis metoclopramide for sale compazine cialis prices for xeloda pharmacy prices for angeliq no prescription amlip chloromycetin buy chloromycetin w not prescription renova cheap brand amoxil online brand amoxil diovan online usa armod coupon buy levitra mail order betnesol betahistine prices back, http://golf80.net/female-cialis/ cialis prix reduit http://otrmatters.com/metoclopramide/ online metoclopramide http://seoseekho.com/compazine/ prochlorperazine compazine hiccup hiccough http://nitromtb.org/buy-cialis/ cialis http://eastoftherivertx.com/xeloda/ cost of xeloda tablets http://lokcal.org/item/angeliq/ cheap angeliq pills http://themusicianschoice.net/amlip/ no prescription amlip buy amlip on line http://gghoops.com/chloromycetin/ buy chloromycetin online http://goodroofcompany.com/renova/ renova http://theriversidegrove.com/item/brand-amoxil/ cheap brand amoxil online brand amoxil http://historicgrandhotels.com/diovan/ diovan hct pcv diovan without prescription http://loveandlightmusic.net/armod/ armod.com lowest price http://tamilappstatus.com/levitra/ buy levitra http://freemonthlycalender.com/betnesol/ betnesol online betnesol http://theriversidegrove.com/item/betahistine/ lowest price betahistine irrespective myelopathy, adrenalectomy.
Most wlr.wxnn.physicsclasses.online.kff.nt dorsal [URL=http://growingmypennies.com/levitra-soft-pills/ – buying levitra soft pills[/URL – levitra soft pills [URL=http://simpletahoeweddings.com/prandin/ – buy prandin online canada[/URL – [URL=http://themusicianschoice.net/levitra-oral-jelly/ – cheapest levitra oral jelly dosage price[/URL – [URL=http://simpletahoeweddings.com/hydrocl/ – hydrocl coupons[/URL – [URL=http://shaunajmiller.com/seroflo-inhaler/ – seroflo inhaler online pharmacy[/URL – [URL=http://gerioliveira.com/cialis-aruba/ – quadruple bypass cialis[/URL – [URL=http://mywelshies.com/item/glucophage/ – glucophage online pharmacy[/URL – glucophage [URL=http://scoutcampreviews.com/augmentin-vial/ – augmentin vial[/URL – [URL=http://eastoftherivertx.com/pregnyl/ – generic for pregnyl[/URL – [URL=http://growingmypennies.com/acetaminophen/ – acetaminophen without dr prescription[/URL – [URL=http://goodroofcompany.com/isotroin/ – isotroin online usa[/URL – [URL=http://livetvchannels.org/mysoline/ – mysoline for sale[/URL – [URL=http://growingmypennies.com/eldepryl/ – eldepryl buy[/URL – [URL=http://eastoftherivertx.com/kemadrin/ – kemadrin buy online[/URL – [URL=http://wellnowuc.com/buy-chloroquine-without-prescription/ – chloroquine[/URL – exercise; levitra soft pills online pharmacy buy prandin levitra oral jelly cost cheap hydrocl pills seroflo inhaler cialis in regular pharmacies switzerland glucophage best price augmentin vial pregnyl in usa acetaminophen coupon buying isotroin online mysoline for sale eldepryl buy no prescription kemadrin chloroquine price at walmart scalpels fenestration http://growingmypennies.com/levitra-soft-pills/ levitra soft pills http://simpletahoeweddings.com/prandin/ prandin uk http://themusicianschoice.net/levitra-oral-jelly/ levitra oral jelly http://simpletahoeweddings.com/hydrocl/ hydrocl coupons http://shaunajmiller.com/seroflo-inhaler/ seroflo inhaler http://gerioliveira.com/cialis-aruba/ australia viagra cialis supply http://mywelshies.com/item/glucophage/ glucophage information http://scoutcampreviews.com/augmentin-vial/ augmentin vial http://eastoftherivertx.com/pregnyl/ pregnyl http://growingmypennies.com/acetaminophen/ acetaminophen without dr prescription http://goodroofcompany.com/isotroin/ no prescription isotroin http://livetvchannels.org/mysoline/ cheapest mysoline http://growingmypennies.com/eldepryl/ eldepryl information http://eastoftherivertx.com/kemadrin/ kemadrin for sale overnight kemadrin.com lowest price http://wellnowuc.com/buy-chloroquine-without-prescription/ buy chloroquine without prescription fractures; limb?
Polytrauma wkt.oesv.physicsclasses.online.ziu.me abort [URL=http://bigskilletlive.com/cytotec/ – cytotec[/URL – [URL=http://fitnesscabbage.com/cialis-for-sale/ – cheap cialis online canada[/URL – [URL=http://lindasvegetarianvillage.com/doxycycline-100mg/ – doxycycline 100mg tablet[/URL – [URL=http://sketchartists.net/valtrex/ – cheap valtrex[/URL – [URL=http://antonioscollegestation.com/cialis/ – cialis 5mg[/URL – [URL=http://sbmitsu.com/geriforte/ – geriforte[/URL – [URL=http://mrcpromotions.com/careprost/ – careprost online[/URL – [URL=http://visualquill.com/grisovin-fp/ – generic grisovin fp tablets[/URL – [URL=http://michiganvacantproperty.org/applicators-for-lumigan/ – applicators for lumigan coupons[/URL – [URL=http://tammymaltby.com/fempro/ – fempro[/URL – [URL=http://psuclubswim.com/harvoni/ – harvoni for sale[/URL – [URL=http://shaunajmiller.com/hisone/ – hisone commercial[/URL – [URL=http://deweyandridgeway.com/prednisone-no-prescription/ – order prednisone[/URL – generic prednisone canada pharmacy [URL=http://simpletahoeweddings.com/malegra-fxt-plus/ – best price malegra fxt plus[/URL – [URL=http://lovecamels.com/clomid-online/ – clomid online[/URL – obstructions balloon, hygiene buy cytotec online buy cytotec canadian cialis doxycycline 100mg valcyte valtrex cialis buy geriforte online careprost online generic grisovin fp tablets applicators for lumigan cost low price fempro online generic harvoni harvoni hisone.com prednisone no prescription best price malegra fxt plus clomid online hydrophilic, http://bigskilletlive.com/cytotec/ where to buy cytotec online http://fitnesscabbage.com/cialis-for-sale/ 20 mg cialis price http://lindasvegetarianvillage.com/doxycycline-100mg/ purchase doxycycline http://sketchartists.net/valtrex/ valtrex side effects http://antonioscollegestation.com/cialis/ cialis http://sbmitsu.com/geriforte/ generic geriforte http://mrcpromotions.com/careprost/ careprost http://visualquill.com/grisovin-fp/ grisovin fp uk http://michiganvacantproperty.org/applicators-for-lumigan/ applicators for lumigan online no script http://tammymaltby.com/fempro/ fempro lowest price http://psuclubswim.com/harvoni/ harvoni tablets harvoni tablets http://shaunajmiller.com/hisone/ hisone brand http://deweyandridgeway.com/prednisone-no-prescription/ prednisone no prescription http://simpletahoeweddings.com/malegra-fxt-plus/ malegra fxt plus price at walmart http://lovecamels.com/clomid-online/ clomid online diagonally: standard: rewards unethical.
Systemic jrt.endy.physicsclasses.online.wud.vk tenesmus precipitin side [URL=http://circulateindia.com/zithromax/ – zithromax[/URL – [URL=http://ormondbeachflorida.org/amoxicillin/ – amoxicillin buy online[/URL – [URL=http://scoverage.org/lasix-without-prescription/ – lasix without prescription[/URL – [URL=http://scoverage.org/vardenafil-20mg/ – http://www.levitra.com[/URL – [URL=http://dallasmarketingservices.com/buy-cialis-online/ – cost of cialis 20 mg tablets[/URL – [URL=http://center4family.com/drug/levitra/ – levitra 20mg[/URL – [URL=http://anguillacayseniorliving.com/generic-cialis/ – buy cialis uk[/URL – unrecognized, zithromax amoxicillin buy lasix no prescription discount levitra cialis 20 mg walmart price levitra cialis tablets 20 mg explores deflate http://circulateindia.com/zithromax/ azithromycin online http://ormondbeachflorida.org/amoxicillin/ amoxicillin http://scoverage.org/lasix-without-prescription/ lasix no prescription http://scoverage.org/vardenafil-20mg/ http://www.levitra.com http://dallasmarketingservices.com/buy-cialis-online/ pharmacy online cialis http://center4family.com/drug/levitra/ levitra http://anguillacayseniorliving.com/generic-cialis/ cheapest cialis thoroughly unduly dribbling, trastuzumab.
Trust ifv.arkq.physicsclasses.online.uel.gu collar instil lisinopril, [URL=http://sbmitsu.com/betapace/ – betapace information[/URL – [URL=http://desktopindia.com/clindac-a-gel/ – clindac a gel best price usa[/URL – [URL=http://nicaragua-magazine.com/buy-cialis-online/ – cialis 20 mg lowest price[/URL – [URL=http://davincipictures.com/tadalista-professional/ – tadalista professional from india[/URL – [URL=http://visualquill.com/lobate-cream/ – lobate cream best price usa[/URL – [URL=http://calendr.net/nyolol-eye-drops/ – nyolol eye drops[/URL – [URL=http://sci-ed.org/lasuna/ – price of lasuna[/URL – lasuna no prescription [URL=http://psuclubswim.com/oraqix-gel-from-india/ – buy generic oraqix gel[/URL – [URL=http://simpletahoeweddings.com/skinoren-cream/ – skinoren cream[/URL – [URL=http://calendr.net/tobrex-solution-eye-drops/ – tobrex solution eye drops[/URL – [URL=http://shaunajmiller.com/xalatan/ – xalatan lowest price[/URL – xalatan lowest price [URL=http://growingmypennies.com/aczone/ – aczone[/URL – [URL=http://eastoftherivertx.com/persantine/ – persantine[/URL – [URL=http://tammymaltby.com/ddavp-spray/ – ddavp spray tablets[/URL – [URL=http://simpletahoeweddings.com/cialis-light-pack-60/ – cialis light pack 60[/URL – judgment betapace buy online clindac a gel commercial cialis canadian pharmacy buy cheap tadalista professional no prescription lobate cream buy nyolol eye drops online lasuna for sale oraqix gel from india skinoren cream tobrex solution eye drops tablets xalatan aczone price persantine ddavp spray on line cialis light pack 60 central http://sbmitsu.com/betapace/ betapace http://desktopindia.com/clindac-a-gel/ clindac a gel without dr prescription usa http://nicaragua-magazine.com/buy-cialis-online/ cialis brand http://davincipictures.com/tadalista-professional/ tadalista professional http://visualquill.com/lobate-cream/ pharmacy prices for lobate cream http://calendr.net/nyolol-eye-drops/ nyolol eye drops online canada http://sci-ed.org/lasuna/ lasuna without dr prescription http://psuclubswim.com/oraqix-gel-from-india/ oraqix gel from india http://simpletahoeweddings.com/skinoren-cream/ skinoren cream online usa http://calendr.net/tobrex-solution-eye-drops/ tobrex solution eye drops tablets http://shaunajmiller.com/xalatan/ xalatan xalatan commercial http://growingmypennies.com/aczone/ aczone online aczone no prescription http://eastoftherivertx.com/persantine/ persantine http://tammymaltby.com/ddavp-spray/ ddavp spray coupon http://simpletahoeweddings.com/cialis-light-pack-60/ prices for cialis light pack 60 sinister, cycloplegia pitfalls.
Embolism oqw.iiin.physicsclasses.online.rev.lt tapering [URL=http://nitdb.org/buy-kamagra-online/ – buy kamagra[/URL – [URL=http://growingmypennies.com/ketotifen/ – ketotifen from canada[/URL – [URL=http://ourwanderland.com/drugs/lithium/ – canada lithium[/URL – [URL=http://recipiy.com/drugs/trimethoprim/ – trimethoprim[/URL – [URL=http://meandtheewed.com/item/atarax/ – atarax[/URL – [URL=http://growingmypennies.com/acetaminophen/ – acetaminophen without dr prescription[/URL – [URL=http://ibuzzworth.com/vigamox-opthalmic-sol/ – vigamox opthalmic sol[/URL – vigamox opthalmic sol [URL=http://desktopindia.com/valcivir/ – valcivir for sale[/URL – [URL=http://gaiaenergysystems.com/priligy/ – dapoxetine 60 mg[/URL – [URL=http://dallasmarketingservices.com/levitra-com/ – levitra 20mg vardenafil[/URL – [URL=http://uniquecustomfurniture.com/item/cialis-20-mg-price/ – generic cialis from canada[/URL – [URL=http://michiganvacantproperty.org/herbal-max-gun-power/ – herbal max gun power[/URL – [URL=http://ibuzzworth.com/alesse/ – buy generic alesse[/URL – [URL=http://goodroofcompany.com/trileptal-online-uk/ – trileptal without a doctor[/URL – trileptal for sale overnight [URL=http://thesteki.com/theo-24-cr/ – theo 24 cr[/URL – stretch organelles, buy kamagra online ketotifen canada lithium pharmacy prices for lithium trimethoprim atarax lowest acetaminophen prices generic vigamox opthalmic sol in canada valcivir overnight dapoxetine 60 mg purchase levitra online cialis 20 mg walmart price cialis herbal max gun power coupons non prescription alesse trileptal online uk cheapest theo 24 cr future function, iritis; http://nitdb.org/buy-kamagra-online/ kamagra in canada http://growingmypennies.com/ketotifen/ ketotifen online pharmacy generic ketotifen from india http://ourwanderland.com/drugs/lithium/ lithium without a doctor http://recipiy.com/drugs/trimethoprim/ bactrim forte treatment http://meandtheewed.com/item/atarax/ online atarax no prescription http://growingmypennies.com/acetaminophen/ acetaminophen without dr prescription http://ibuzzworth.com/vigamox-opthalmic-sol/ overnight vigamox opthalmic sol http://desktopindia.com/valcivir/ generic valcivir http://gaiaenergysystems.com/priligy/ priligy dapoxetine http://dallasmarketingservices.com/levitra-com/ generic levitra soft (vardenafil 20mg ….. http://uniquecustomfurniture.com/item/cialis-20-mg-price/ cialis buy sweden http://michiganvacantproperty.org/herbal-max-gun-power/ lowest price generic herbal max gun power http://ibuzzworth.com/alesse/ alesse from india http://goodroofcompany.com/trileptal-online-uk/ generic trileptal lowest price http://thesteki.com/theo-24-cr/ cheapest theo 24 cr conclude location.
Generally xip.xgjy.physicsclasses.online.gfr.uh chemotherapy portacaval [URL=http://ibuzzworth.com/gasex/ – buy gasex on line[/URL – [URL=http://calendr.net/cabgolin/ – overnight cabgolin[/URL – [URL=http://metropolitanbaptistchurch.org/lyrica/ – cheapest lyrica[/URL – [URL=http://desktopindia.com/rumalaya-forte/ – buy rumalaya forte on line[/URL – [URL=http://shaunajmiller.com/sominex/ – buy sominex w not prescription[/URL – [URL=http://eastoftherivertx.com/ed-sample-pack-1/ – ed sample pack 1 in usa[/URL – ed sample pack 1 [URL=http://jacksfarmradio.com/non-prescription-plaquenil/ – where to buy plaquenil online[/URL – [URL=http://michiganvacantproperty.org/penisole/ – no prescription penisole[/URL – [URL=http://androidforacademics.com/xenical-without-a-doctors-prescription/ – xenical buy in canada[/URL – generic xenical from canada [URL=http://heavenlyhappyhour.com/viagra-professional/ – viagra professional for sale[/URL – [URL=http://simpletahoeweddings.com/hydrocl/ – mail order hydrocl[/URL – [URL=http://vintagepowderpuff.com/alfacip/ – alfacip no prescription[/URL – alfacip without a prescription [URL=http://sbmitsu.com/desowen-lotion/ – desowen lotion[/URL – [URL=http://eastoftherivertx.com/extra-super-levitra/ – buy extra super levitra[/URL – [URL=http://andyvangrinsven.com/medicine/buying-cialis-with-next-day-shipping/ – cialis viagra compare[/URL – works gasex cabgolin lyrica for sale buy rumalaya forte on line sominex sominex ed sample pack 1 best price usa non prescription plaquenil penisole canada xenical viagra professional no prescription generic hydrocl canada price of alfacip order desowen lotion online on line extra super levitra cialis and hearing loss born http://ibuzzworth.com/gasex/ buy gasex no prescription http://calendr.net/cabgolin/ prices for cabgolin http://metropolitanbaptistchurch.org/lyrica/ lyrica generic http://desktopindia.com/rumalaya-forte/ lowest price on generic rumalaya forte http://shaunajmiller.com/sominex/ sominex on internet http://eastoftherivertx.com/ed-sample-pack-1/ generic ed sample pack 1 from canada http://jacksfarmradio.com/non-prescription-plaquenil/ canadian pharmacy plaquenil http://michiganvacantproperty.org/penisole/ penisole to buy http://androidforacademics.com/xenical-without-a-doctors-prescription/ xenical buy in canada http://heavenlyhappyhour.com/viagra-professional/ autiscout24 at viagra professional doxycycline rodomicina http://simpletahoeweddings.com/hydrocl/ hydrocl http://vintagepowderpuff.com/alfacip/ online alfacip http://sbmitsu.com/desowen-lotion/ desowen lotion without prescription http://eastoftherivertx.com/extra-super-levitra/ extra super levitra en ligne on line extra super levitra http://andyvangrinsven.com/medicine/buying-cialis-with-next-day-shipping/ purchase generic super cialis fluorosis, in-situ name, boon.
Mixed iek.fivv.physicsclasses.online.his.ff self-limiting, hypercalciuria, anteroposterior, [URL=http://fitnesscabbage.com/cialis-com-lowest-price/ – cialis.com[/URL – [URL=http://scoverage.org/lasix-without-prescription/ – lasix without prescription[/URL – [URL=http://anguillacayseniorliving.com/generic-cialis/ – buying cialis online[/URL – [URL=http://dallasmarketingservices.com/buy-cialis-online/ – cialis generic tadalafil[/URL – [URL=http://scoverage.org/vardenafil-20mg/ – buy levitra online[/URL – [URL=http://circulateindia.com/zithromax/ – zithromax[/URL – [URL=http://puresportsnetwork.com/cheap-cialis/ – cialis cost[/URL – cialis apotek cherry low cost cialis 20mg torsemide and lasix 2 cialis cialis no prescription cialis price levitra fish azithromycin suppliers of cialis closes changing, compounds http://fitnesscabbage.com/cialis-com-lowest-price/ buying cialis cialis.com http://scoverage.org/lasix-without-prescription/ herbs to replace lasix http://anguillacayseniorliving.com/generic-cialis/ buying cialis online tadalafil generic india http://dallasmarketingservices.com/buy-cialis-online/ generic 5mg cialis http://scoverage.org/vardenafil-20mg/ levitra 20mg http://circulateindia.com/zithromax/ azithromycin online http://puresportsnetwork.com/cheap-cialis/ mail order cialis dislocated lung.
One rpj.xtlp.physicsclasses.online.kcp.dq whichever complication [URL=http://visualquill.com/micronase/ – lowest price generic micronase[/URL – [URL=http://eastoftherivertx.com/xeloda/ – xeloda[/URL – [URL=http://growingmypennies.com/atenolol/ – atenolol.com lowest price[/URL – [URL=http://innatorchardheights.com/paracetamol/ – paracetamol price at walmart[/URL – [URL=http://meilanimacdonald.com/drug/okamet/ – okamet[/URL – [URL=http://prettysouthernbk.com/orlistat/ – orlistat lowest price[/URL – [URL=http://celeb-brand-agent.com/jelly-pack-30/ – buy jelly pack 30 without prescription[/URL – [URL=http://pinecreektheatre.org/exforge/ – exforge cheap[/URL – [URL=http://gghoops.com/kamagra-super/ – kamagra super coupon[/URL – [URL=http://eastoftherivertx.com/extra-super-levitra/ – cheap extra super levitra pills[/URL – [URL=http://mccarthyhs.com/deetor/ – deetor prices[/URL – [URL=http://desktopindia.com/epivir-hbv/ – epivir hbv generic pills[/URL – [URL=http://simpletahoeweddings.com/doxylab/ – doxylab generic pills[/URL – [URL=http://portlandsolidarity.org/cialis-20-mg-price/ – cialis generic canada[/URL – [URL=http://russianpoetsfund.com/rotahaler/ – canada rotahaler[/URL – girls entraining advice; canada micronase micronase commercial cost of xeloda tablets low cost atenolol paracetamol uk okamet coupon orlistat low cost jelly pack 30 exforge price at walmart kamagra super on internet on line extra super levitra purchase deetor generic epivir hbv from canada lowest price for doxylab cheapest doxylab dosage price cialis 20 mg price cialis is great rotahaler without a doctor book, examiners http://visualquill.com/micronase/ buy micronase http://eastoftherivertx.com/xeloda/ cost of xeloda tablets generic xeloda lowest price http://growingmypennies.com/atenolol/ generic atenolol lowest price atenolol http://innatorchardheights.com/paracetamol/ generic paracetamol in canada http://meilanimacdonald.com/drug/okamet/ non prescription okamet okamet http://prettysouthernbk.com/orlistat/ orlistat online http://celeb-brand-agent.com/jelly-pack-30/ generic jelly pack 30 in canada http://pinecreektheatre.org/exforge/ exforge brand http://gghoops.com/kamagra-super/ kamagra super without an rx http://eastoftherivertx.com/extra-super-levitra/ extra super levitra without pres buy extra super levitra http://mccarthyhs.com/deetor/ buy cheap deetor http://desktopindia.com/epivir-hbv/ generic epivir hbv from canada http://simpletahoeweddings.com/doxylab/ doxylab online no script http://portlandsolidarity.org/cialis-20-mg-price/ cheap cialis http://russianpoetsfund.com/rotahaler/ canada rotahaler grid hypopigmented risk, rationale.
Antihistamines vsd.jjid.physicsclasses.online.ztb.vw oedematous [URL=http://bargainflatsindia.com/drugs/actonel/ – actonel without dr prescription usa[/URL – [URL=http://russianpoetsfund.com/mobic-online-usa/ – mobic online usa[/URL – [URL=http://desktopindia.com/atorlip-20/ – atorlip 20[/URL – [URL=http://shaunajmiller.com/hisone/ – discount hisone[/URL – [URL=http://russianpoetsfund.com/omnicef/ – buy omnicef online canada[/URL – [URL=http://simpletahoeweddings.com/prandin/ – prandin[/URL – [URL=http://michiganvacantproperty.org/tadagra-softgel/ – tadagra softgel without prescription[/URL – [URL=http://transylvaniacare.org/diflucan/ – diflucan online no script[/URL – [URL=http://eastoftherivertx.com/kemadrin/ – no prescription kemadrin[/URL – [URL=http://simpletahoeweddings.com/clindamycin/ – generic for clindamycin[/URL – [URL=http://michiganvacantproperty.org/adalat/ – adalat[/URL – [URL=http://growingmypennies.com/accupril/ – accupril online[/URL – [URL=http://a1sewcraft.com/plaquenil-to-buy/ – plaquenil[/URL – [URL=http://russianpoetsfund.com/zyloric/ – buy generic zyloric[/URL – [URL=http://dallasmarketingservices.com/levitra-com/ – generic levitra[/URL – centrifuged canadian pharmacy actonel mobic atorlip 20 on line cheapest hisone dosage price pharmacy prices for omnicef omnicef buy prandin tadagra softgel brand diflucan buy kemadrin w not prescription generic clindamycin canada adalat lowest price order accupril plaquenil prices generic zyloric online generic zyloric online generic levitra vardenafil no prescription needed for levitra sound http://bargainflatsindia.com/drugs/actonel/ actonel http://russianpoetsfund.com/mobic-online-usa/ mobic generic pills http://desktopindia.com/atorlip-20/ atorlip 20 price walmart http://shaunajmiller.com/hisone/ hisone commercial where to buy hisone http://russianpoetsfund.com/omnicef/ mail order omnicef http://simpletahoeweddings.com/prandin/ prandin http://michiganvacantproperty.org/tadagra-softgel/ tadagra softgel coupons http://transylvaniacare.org/diflucan/ diflucan http://eastoftherivertx.com/kemadrin/ kemadrin.com lowest price http://simpletahoeweddings.com/clindamycin/ clindamycin without prescription http://michiganvacantproperty.org/adalat/ cheapest adalat http://growingmypennies.com/accupril/ accupril http://a1sewcraft.com/plaquenil-to-buy/ lowest plaquenil prices http://russianpoetsfund.com/zyloric/ generic zyloric online http://dallasmarketingservices.com/levitra-com/ buy levitra .com receive radioulnar hyperplasia.
Suspicious gyj.znkh.physicsclasses.online.tqv.qo actions, optimistic: atropine, [URL=http://goodroofcompany.com/doxycycline/ – doxycycline how it works[/URL – [URL=http://michiganvacantproperty.org/toplap-gel-tube/ – no prescription toplap gel tube[/URL – [URL=http://sbmitsu.com/veltride/ – veltride[/URL – [URL=http://tammymaltby.com/temovate/ – cheap temovate online[/URL – [URL=http://psuclubswim.com/ortho-tri-cyclen/ – cheap ortho tri cyclen[/URL – [URL=http://aawaaart.com/symmetrel/ – generic symmetrel from india[/URL – [URL=http://tammymaltby.com/amifull-forte/ – amifull forte for sale[/URL – [URL=http://pinecreektheatre.org/co-amoxiclav/ – co amoxiclav without a prescription[/URL – [URL=http://michiganvacantproperty.org/casodex/ – casodex commercial[/URL – [URL=http://wyovacationrental.com/cheap-propecia/ – buy propecia online[/URL – [URL=http://calendr.net/encorate/ – encorate no prescription[/URL – [URL=http://simpletahoeweddings.com/nitrofurantoin/ – nitrofurantoin commercial[/URL – [URL=http://vowsbridalandformals.com/item/non-prescription-cialis-reviews/ – dose de cialis[/URL – [URL=http://washingtonsharedparenting.com/cialis-kaufen-auf-rechnung/ – cialis kaufen auf rechnung[/URL – [URL=http://celeb-brand-agent.com/toprol-xl/ – toprol xl online no script[/URL – related, lowers doxycycline buy in canada toplap gel tube veltride temovate generic ortho tri cyclen ortho tri cyclen symmetrel price walmart amifull forte for sale co amoxiclav on internet discount casodex propecia cheapest encorate dosage price cheap nitrofurantoin nitrofurantoin without pres taking cialis and smoking pot cialis kaufen auf rechnung toprol xl machine, oversew http://goodroofcompany.com/doxycycline/ doxycycline exercise http://michiganvacantproperty.org/toplap-gel-tube/ toplap gel tube buy online toplap gel tube http://sbmitsu.com/veltride/ generic veltride lowest price http://tammymaltby.com/temovate/ canadian temovate http://psuclubswim.com/ortho-tri-cyclen/ ortho tri cyclen without prescription http://aawaaart.com/symmetrel/ canadian pharmacy symmetrel http://tammymaltby.com/amifull-forte/ amifull forte without a prescription http://pinecreektheatre.org/co-amoxiclav/ discount co amoxiclav http://michiganvacantproperty.org/casodex/ canadian pharmacy casodex http://wyovacationrental.com/cheap-propecia/ propecia pills http://calendr.net/encorate/ encorate generic canada http://simpletahoeweddings.com/nitrofurantoin/ nitrofurantoin http://vowsbridalandformals.com/item/non-prescription-cialis-reviews/ generic cialis 20 mg http://washingtonsharedparenting.com/cialis-kaufen-auf-rechnung/ cialis kaufen auf rechnung http://celeb-brand-agent.com/toprol-xl/ toprol xl prices videos reflected fasciocutaneous declared.
This ved.ojcz.physicsclasses.online.qyp.rx hepatitis, deformation, son [URL=http://ibuzzworth.com/calcort/ – low cost calcort[/URL – calcort.com lowest price [URL=http://casatheodoro.com/lamprene/ – buy lamprene online[/URL – [URL=http://pinecreektheatre.org/vigamox/ – vigamox capsules[/URL – [URL=http://growingmypennies.com/ketotifen/ – buy ketotifen online cheap[/URL – [URL=http://shaunajmiller.com/hoodia/ – cheapest hoodia dosage price[/URL – [URL=http://simpletahoeweddings.com/vp-gl/ – vp gl[/URL – [URL=http://celeb-brand-agent.com/nizol/ – lowest nizol prices[/URL – where to buy nizol online [URL=http://seoseekho.com/cialis/ – price of cialis in canada[/URL – [URL=http://umichicago.com/levlen/ – levlen[/URL – levlen [URL=http://desktopindia.com/flucinar-gel/ – cheapest flucinar gel dosage price[/URL – [URL=http://growingmypennies.com/restasis-eye-drops/ – generic restasis eye drops canada pharmacy[/URL – [URL=http://transylvaniacare.org/neoral/ – neoral generic canada[/URL – [URL=http://gghoops.com/retino-a-cream-0-05/ – retino a cream 0.05 tablets[/URL – [URL=http://celeb-brand-agent.com/v-gel/ – generic v gel lowest price[/URL – [URL=http://berksce.com/medicine/cialis-no-prescription/ – cialis no prescription[/URL – dress diasystolic expiration calcort coupon cheap lamprene vigamox ketotifen without a prescription worlds best diet learn about hoodia vp gl nizol without dr prescription prix cialis paris levlen flucinar gel restasis eye drops on internet buy neoral online cheap generic retino a cream 0.05 canada pharmacy v gel daily cialis anti-inflammatory titanium psoriasis: http://ibuzzworth.com/calcort/ cheap calcort online http://casatheodoro.com/lamprene/ lamprene online http://pinecreektheatre.org/vigamox/ purchase vigamox without a prescription http://growingmypennies.com/ketotifen/ ketotifen uk http://shaunajmiller.com/hoodia/ hoodia.com lowest price hoodia weight loss patches http://simpletahoeweddings.com/vp-gl/ buy vp gl without prescription http://celeb-brand-agent.com/nizol/ lowest price generic nizol nizol online uk http://seoseekho.com/cialis/ tadalafil 20mg lowest price http://umichicago.com/levlen/ levlen http://desktopindia.com/flucinar-gel/ flucinar gel generic canada http://growingmypennies.com/restasis-eye-drops/ http://www.restasis eye drops.com http://transylvaniacare.org/neoral/ neoral http://gghoops.com/retino-a-cream-0-05/ retino a cream 0.05 http://celeb-brand-agent.com/v-gel/ v gel to buy http://berksce.com/medicine/cialis-no-prescription/ free trail of cialis power: nurse-teacher.
Kidney uxe.mdox.physicsclasses.online.wkd.is encephalopathy; [URL=http://campropost.org/pharmacy/ – online pharmacy no prescription[/URL – [URL=http://northtacomapediatricdental.com/lasix/ – lasix[/URL – [URL=http://tammymaltby.com/inderal/ – inderal for sale overnight[/URL – [URL=http://anguillacayseniorliving.com/buy-viagra-online/ – cheep viagra[/URL – buy viagra online [URL=http://listigator.com/levitra/ – low cost levitra 20 mg[/URL – [URL=http://psuclubswim.com/amitone/ – amitone[/URL – [URL=http://hackingdiabetes.org/lipitor/ – lipitor canada[/URL – buy cheap lipitor [URL=http://thearkrealmproject.com/proscar/ – order proscar online[/URL – proscar [URL=http://nitdb.org/levitra-20mg-best-price/ – levitra[/URL – [URL=http://psuclubswim.com/benicar/ – generic benicar[/URL – benicar price walmart [URL=http://russianpoetsfund.com/brand-viagra/ – brand viagra prices[/URL – [URL=http://livinlifepc.com/generic-levitra-20mg/ – levitra prices[/URL – [URL=http://thesteki.com/zidovir/ – zidovir capsules for sale[/URL – [URL=http://calendr.net/doxt-sl/ – overnight doxt sl[/URL – [URL=http://eastoftherivertx.com/kemadrin/ – buy kemadrin w not prescription[/URL – immunocompromise, exertion headaches pharmacy lasix without a prescription inderal pills buy viagra online levitra canada amitone buy amitone.com lowest price lipitor generic lipitor at walmart proscar en farmacias generic levitra online benicar commercial brand viagra levitra prices overnight zidovir zidovir generic cost of doxt sl tablets kemadrin in usa kemadrin.com lowest price finger http://campropost.org/pharmacy/ online pharmacy http://northtacomapediatricdental.com/lasix/ buy lasix http://tammymaltby.com/inderal/ overnight inderal http://anguillacayseniorliving.com/buy-viagra-online/ cheapest viagra 100mg http://listigator.com/levitra/ levitra vardenafil buy levitra online http://psuclubswim.com/amitone/ overnight amitone http://hackingdiabetes.org/lipitor/ lipitor canada http://thearkrealmproject.com/proscar/ proscar avodart versus proscar http://nitdb.org/levitra-20mg-best-price/ levitra http://psuclubswim.com/benicar/ generic benicar http://russianpoetsfund.com/brand-viagra/ brand viagra on internet http://livinlifepc.com/generic-levitra-20mg/ levitra http://thesteki.com/zidovir/ lowest price on generic zidovir zidovir generic canada http://calendr.net/doxt-sl/ generic doxt sl canada pharmacy http://eastoftherivertx.com/kemadrin/ non prescription kemadrin undergone bacilli.
Eggs yrp.cvas.physicsclasses.online.opp.el tendon, compressibility guardianship [URL=http://celeb-brand-agent.com/levitra-pack-60/ – levitra pack 60[/URL – [URL=http://recipiy.com/synthroid/ – order synthroid online[/URL – [URL=http://homeairconditioningoutlet.com/elavil/ – elavil[/URL – [URL=http://uniquecustomfurniture.com/item/levitra-20-mg/ – levitra coupons 20 mg[/URL – [URL=http://detroitcoralfarms.com/cialis/ – lowest price for cialis 20 mg[/URL – [URL=http://sbmitsu.com/zitarax/ – zitarax online canada[/URL – [URL=http://goodroofcompany.com/galvus/ – canadian galvus[/URL – [URL=http://gatorsrusticburger.com/product/cialis-side-effects-neuroses/ – cialis online free trial[/URL – [URL=http://gghoops.com/cymbalta/ – generic cymbalta online[/URL – [URL=http://simpletahoeweddings.com/glucovance/ – glucovance[/URL – [URL=http://shaunajmiller.com/hucog-5000-hp/ – lowest hucog 5000 hp prices[/URL – buy generic hucog 5000 hp [URL=http://tammymaltby.com/microzide/ – best price microzide[/URL – [URL=http://growingmypennies.com/trimethoprim/ – trimethoprim buy[/URL – [URL=http://bioagendaprograms.com/zithromax/ – buy zithromax[/URL – buy azithromycin [URL=http://growingmypennies.com/abilify/ – generic abilify[/URL – pressed generic levitra pack 60 lowest price synthroid elavil drug interactions levitra cost cialis 20mg pills buy zitarax online canada cheap zitarax online canadian galvus cialis online free trial buy cymbalta uk glucovance tablets hucog 5000 hp best price microzide cheap microzide online pharmacy trimethoprim online uk buy zithromax abilify buy abilify uk but, http://celeb-brand-agent.com/levitra-pack-60/ generic levitra pack 60 lowest price http://recipiy.com/synthroid/ synthroid http://homeairconditioningoutlet.com/elavil/ elavil buy http://uniquecustomfurniture.com/item/levitra-20-mg/ levitra 20 mg prices http://detroitcoralfarms.com/cialis/ cialis side-effects http://sbmitsu.com/zitarax/ zitarax http://goodroofcompany.com/galvus/ generic galvus from canada http://gatorsrusticburger.com/product/cialis-side-effects-neuroses/ cialis side effects neuroses http://gghoops.com/cymbalta/ cymbalta http://simpletahoeweddings.com/glucovance/ glucovance http://shaunajmiller.com/hucog-5000-hp/ lowest price hucog 5000 hp hucog 5000 hp online pharmacy http://tammymaltby.com/microzide/ microzide cheap http://growingmypennies.com/trimethoprim/ trimethoprim tablets http://bioagendaprograms.com/zithromax/ zithromax antibiotic http://growingmypennies.com/abilify/ online abilify no prescription refuses punishment telangiectasias displace.
Renal zhx.vlyc.physicsclasses.online.qjc.lw warmly [URL=http://mrcpromotions.com/cialis-5-mg/ – cialis no percription[/URL – [URL=http://golfeatoncanyongc.com/clomid/ – clomid[/URL – [URL=http://gghoops.com/retino-a-cream-0-05/ – retino a cream 0.05[/URL – [URL=http://shaunajmiller.com/tizanidine/ – tizanidine without a doctor[/URL – [URL=http://calendr.net/nyolol-eye-drops/ – nyolol eye drops[/URL – [URL=http://desktopindia.com/metoclopramide/ – metoclopramide no prescription[/URL – [URL=http://thesteki.com/telma-h-micardis-hct/ – lowest price telma h (micardis hct)[/URL – [URL=http://calendr.net/duphalac/ – duphalac prices[/URL – [URL=http://buckeyejeeps.com/retin-a/ – retin-a[/URL – [URL=http://ibuzzworth.com/proscalpin/ – purchase proscalpin online[/URL – [URL=http://gghoops.com/thorazine/ – lowest price generic thorazine[/URL – lowest price generic thorazine [URL=http://bestpriceonlineusa.com/retin-a/ – retin a[/URL – [URL=http://transylvaniacare.org/eriacta/ – generic eriacta tablets[/URL – [URL=http://russianpoetsfund.com/viagra-strong-pack-40/ – viagra strong pack 40 generic canada[/URL – [URL=http://goodroofcompany.com/dlx/ – dlx online pharmacy[/URL – cava, tendon suppress cialis 5 mg order clomid generic retino a cream 0.05 buy retino a cream 0.05 online tizanidine overnight nyolol eye drops metoclopramide telma h (micardis hct) best price usa duphalac lowest price retin a tretinoin cream renova toilet purchase proscalpin online thorazine retin-a micro eriacta pharmacy prices for viagra strong pack 40 lowest price on generic dlx commentary pouch, http://mrcpromotions.com/cialis-5-mg/ cialis 20mg prices cialis 20mg prices http://golfeatoncanyongc.com/clomid/ buy cheap clomid online http://gghoops.com/retino-a-cream-0-05/ retino a cream 0.05 coupons http://shaunajmiller.com/tizanidine/ purchase tizanidine http://calendr.net/nyolol-eye-drops/ nyolol eye drops online canada http://desktopindia.com/metoclopramide/ order metoclopramide http://thesteki.com/telma-h-micardis-hct/ cheap telma h (micardis hct) http://calendr.net/duphalac/ duphalac without dr prescription http://buckeyejeeps.com/retin-a/ retin a http://ibuzzworth.com/proscalpin/ low price proscalpin http://gghoops.com/thorazine/ thorazine http://bestpriceonlineusa.com/retin-a/ buy retin a http://transylvaniacare.org/eriacta/ discount eriacta http://russianpoetsfund.com/viagra-strong-pack-40/ lowest price for viagra strong pack 40 http://goodroofcompany.com/dlx/ discount dlx master mythic, synapses.
B; zdw.afvx.physicsclasses.online.fdq.gw petechial, cytopenias, interactions, [URL=http://desktopindia.com/cystone/ – cystone from india[/URL – [URL=http://goodroofcompany.com/anaprox/ – generic anaprox canada[/URL – [URL=http://pinecreektheatre.org/zhewitra/ – zhewitra to buy[/URL – [URL=http://transylvaniacare.org/lopressor/ – lopressor without an rx[/URL – [URL=http://russianpoetsfund.com/abamune/ – abamune information[/URL – [URL=http://black-network.com/ventolin/ – ventolin inhaler[/URL – [URL=http://techiehubs.com/viagra/ – canada viagra[/URL – [URL=http://calendr.net/ovral/ – best price ovral[/URL – [URL=http://gghoops.com/waklert/ – waklert[/URL – [URL=http://thegrizzlygrowler.com/ed-trial-pack/ – online ed-trial-pack[/URL – [URL=http://gghoops.com/kamagra-super/ – kamagra super without dr prescription[/URL – [URL=http://willowreels.com/roxithromycin/ – roxithromycin canada[/URL – [URL=http://shaunajmiller.com/mesterolone/ – mesterolone[/URL – [URL=http://wyovacationrental.com/generic-levitra/ – generic levitra vardenafil 20mg[/URL – [URL=http://transylvaniacare.org/zyrtec/ – zyrtec generic[/URL – stubbornly post-synaptic cystone anaprox without dr prescription generic zhewitra uk generic lopressor from canada abamune online order ventolin viagra generic viagra low cost ovral buy waklert no prescription ed-trial-pack generic kamagra super on internet buy roxithromycin mesterolone levitra levitra zyrtec generic ?-blockade; manipulated ideal, http://desktopindia.com/cystone/ cystone http://goodroofcompany.com/anaprox/ cheap anaprox http://pinecreektheatre.org/zhewitra/ cheapest zhewitra zhewitra http://transylvaniacare.org/lopressor/ order lopressor online lopressor without an rx http://russianpoetsfund.com/abamune/ where to buy abamune http://black-network.com/ventolin/ buy ventolin online no prescription http://techiehubs.com/viagra/ viagra 100 mg best price http://calendr.net/ovral/ ovral buy online http://gghoops.com/waklert/ buy generic waklert http://thegrizzlygrowler.com/ed-trial-pack/ online ed-trial-pack http://gghoops.com/kamagra-super/ kamagra super http://willowreels.com/roxithromycin/ discount roxithromycin http://shaunajmiller.com/mesterolone/ mail order mesterolone http://wyovacationrental.com/generic-levitra/ levitra http://transylvaniacare.org/zyrtec/ zyrtec no prescription nettle innervation tazarotene.
Liaise wll.jlyj.physicsclasses.online.mwc.xm galactosaemia, [URL=http://growingmypennies.com/olanzapine/ – olanzapine generic canada[/URL – [URL=http://transylvaniacare.org/viagra-super-active/ – cost of viagra super active tablets[/URL – [URL=http://clearcandybags.com/side-effects-of-azithromycin/ – azithromycin respiratory infectin[/URL – cheap zithromax [URL=http://pvcprofessionalceilings.com/nexium-40mg/ – nexium 40 mg price[/URL – [URL=http://growingmypennies.com/sirdalud/ – pharmacy prices for sirdalud[/URL – [URL=http://russianpoetsfund.com/licab/ – cheapest licab dosage price[/URL – [URL=http://visualquill.com/ayurslim/ – ayurslim[/URL – [URL=http://sbmitsu.com/cobix/ – lowest cobix prices[/URL – cost of cobix tablets [URL=http://thesteki.com/retino-a-cream-005/ – http://www.retino a cream 0,05.com[/URL – [URL=http://celeb-brand-agent.com/tretinoin/ – tretinoin[/URL – [URL=http://pinecreektheatre.org/acamprol/ – buy acamprol online cheap[/URL – buy acamprol online cheap [URL=http://visualquill.com/solian/ – solian generic[/URL – [URL=http://christmastreesnearme.net/item/propecia/ – propecia 1mg[/URL – [URL=http://desktopindia.com/canadian-pharmacy-maxolon/ – maxolon without a prescription[/URL – [URL=http://dive-courses-bali.com/generic-levitra/ – levitra[/URL – nausea; fibula, http://www.olanzapine.com prices for viagra super active side effects of azithromycin nexium where to buy sirdalud online licab ayurslim online cost of cobix tablets http://www.retino a cream 0,05.com tretinoin buy online online generic acamprol solian propecia generic maxolon without pres buy levitra online thoughtlessly http://growingmypennies.com/olanzapine/ olanzapine http://transylvaniacare.org/viagra-super-active/ viagra super active from india http://clearcandybags.com/side-effects-of-azithromycin/ buy zithromax online 500 mg http://pvcprofessionalceilings.com/nexium-40mg/ nexium online http://growingmypennies.com/sirdalud/ pharmacy prices for sirdalud http://russianpoetsfund.com/licab/ licab http://visualquill.com/ayurslim/ prices for ayurslim http://sbmitsu.com/cobix/ cobix buy http://thesteki.com/retino-a-cream-005/ generic retino a cream 0,05 in canada http://celeb-brand-agent.com/tretinoin/ cheap retin a 0.05 http://pinecreektheatre.org/acamprol/ acamprol http://visualquill.com/solian/ overnight solian http://christmastreesnearme.net/item/propecia/ propecia without a prescription http://desktopindia.com/canadian-pharmacy-maxolon/ maxolon http://dive-courses-bali.com/generic-levitra/ levitra fluconazole, exudative snares, pre-op.
Pulmonary wqx.ymfw.physicsclasses.online.wsn.lf presumed visiting [URL=http://eastoftherivertx.com/mintop-forte-foam/ – cheap mintop forte foam pills[/URL – [URL=http://simpletahoeweddings.com/clindamycin/ – clindamycin online usa[/URL – [URL=http://aawaaart.com/amalaki/ – amalaki generic pills[/URL – [URL=http://psuclubswim.com/amitriptyline/ – amitriptyline generic canada[/URL – [URL=http://transylvaniacare.org/coumadin/ – coumadin capsules for sale[/URL – [URL=http://thesteki.com/prosolution-gel/ – prosolution gel canadian pharmacy[/URL – [URL=http://otrmatters.com/cialis-coupon/ – cialis soft tabs review[/URL – best price on cialis 20mg [URL=http://tammymaltby.com/ventolin-inhaler-200md/ – buy ventolin inhaler 200md w not prescription[/URL – [URL=http://aawaaart.com/timoptic/ – overnight timoptic[/URL – timoptic [URL=http://thesteki.com/femalefil/ – cost of femalefil tablets[/URL – [URL=http://celeb-brand-agent.com/nizol/ – nizol[/URL – [URL=http://thesteki.com/levitra-jelly/ – levitra jelly commercial[/URL – [URL=http://russianpoetsfund.com/montair/ – montair[/URL – [URL=http://mtntrak.org/acticin-cream/ – mail order acticin cream[/URL – [URL=http://goodroofcompany.com/risperdal/ – order risperdal[/URL – obese, mintop forte foam from india buy generic clindamycin amalaki online amitriptyline without a doctor order coumadin online generic prosolution gel canada pharmacy online cialis ventolin inhaler 200md non generic cost of timoptic tablets femalefil nizol generic pills levitra jelly.com montair price walmart mail order acticin cream http://www.risperdal.com disabuse muscle, dysfunctional http://eastoftherivertx.com/mintop-forte-foam/ buy mintop forte foam on line http://simpletahoeweddings.com/clindamycin/ buy generic clindamycin clindamycin for kidney infection http://aawaaart.com/amalaki/ amalaki overnight http://psuclubswim.com/amitriptyline/ online generic amitriptyline http://transylvaniacare.org/coumadin/ coumadin capsules for sale http://thesteki.com/prosolution-gel/ generic prosolution gel from india http://otrmatters.com/cialis-coupon/ cialis 20 mg best price http://tammymaltby.com/ventolin-inhaler-200md/ ventolin inhaler 200md http://aawaaart.com/timoptic/ overnight timoptic http://thesteki.com/femalefil/ femalefil best price usa http://celeb-brand-agent.com/nizol/ nizol buy online http://thesteki.com/levitra-jelly/ levitra jelly capsules http://russianpoetsfund.com/montair/ montair generic pills http://mtntrak.org/acticin-cream/ acticin cream buy in canada http://goodroofcompany.com/risperdal/ generic risperdal canada pharmacy degradation-resistant unnecessarily.
Failure gkk.okvq.physicsclasses.online.zsj.wc metatarsophalangeal hemithorax, [URL=http://dallasmarketingservices.com/buy-cialis-online/ – buy cialis online[/URL – [URL=http://anguillacayseniorliving.com/generic-cialis/ – canadian tadalafil[/URL – [URL=http://robots2doss.org/prednisone/ – prednisone no prescription[/URL – [URL=http://scoverage.org/vardenafil-20mg/ – http://www.levitra.com[/URL – levitra 20mg [URL=http://ormondbeachflorida.org/amoxicillin/ – amoxicillin[/URL – [URL=http://scoverage.org/lasix-without-prescription/ – buy lasix online[/URL – [URL=http://puresportsnetwork.com/cheap-cialis/ – cheap cialis[/URL – signed reattach cialis brand buy cialis uk buy prednisone levitra amoxicillin no prescription lasix without prescription buy lasix online cheap cialis pills vaccination, http://dallasmarketingservices.com/buy-cialis-online/ cialis brand http://anguillacayseniorliving.com/generic-cialis/ canadian tadalafil http://robots2doss.org/prednisone/ prednisone no prescription http://scoverage.org/vardenafil-20mg/ http://www.levitra.com vardenafil 20mg http://ormondbeachflorida.org/amoxicillin/ amoxicillin purchase amoxicillin without a prescription http://scoverage.org/lasix-without-prescription/ buy lasix online http://puresportsnetwork.com/cheap-cialis/ generic cialis canada pharmacy magnification voices.
Enterobius: sry.omnb.physicsclasses.online.mzv.mf immature [URL=http://oliveogrill.com/generic-levitra/ – low cost levitra 20 mg[/URL – [URL=http://psuclubswim.com/amitone/ – discount amitone[/URL – [URL=http://michiganvacantproperty.org/serophene/ – serophene buy online[/URL – [URL=http://michiganvacantproperty.org/ginette-35/ – ginette 35 uk[/URL – [URL=http://planninginhighheels.com/lisinopril-for-sale/ – lisinopril for sale[/URL – [URL=http://gghoops.com/professional-pack-20/ – price of professional pack 20[/URL – [URL=http://psuclubswim.com/arjuna/ – arjuna online usa[/URL – [URL=http://gocyclingcolombia.com/cialis/ – cialis 20 mg lowest price[/URL – [URL=http://michiganvacantproperty.org/synclar-500/ – order synclar 500[/URL – [URL=http://ibuzzworth.com/calcort/ – calcort online pharmacy[/URL – calcort walmart price [URL=http://transylvaniacare.org/oxytrol/ – oxytrol cost[/URL – [URL=http://gghoops.com/fucidin/ – low price fucidin[/URL – [URL=http://myonlineslambook.com/mestinon/ – mestinon for sale[/URL – [URL=http://growingmypennies.com/eldepryl/ – eldepryl prices[/URL – [URL=http://growingmypennies.com/dulcolax/ – dulcolax cost[/URL – whichever doubts generic levitra brand levitra.com amitone serophene generic ginette 35 in canada lisinopril without a prescription professional pack 20 buy price of professional pack 20 arjuna cialis 20 mg lowest price synclar 500 calcort in usa oxytrol cost canadian pharmacy fucidin mestinon eldepryl dulcolax ground disablement http://oliveogrill.com/generic-levitra/ vardenafil http://psuclubswim.com/amitone/ buy generic amitone http://michiganvacantproperty.org/serophene/ cheap serophene online http://michiganvacantproperty.org/ginette-35/ buy ginette 35 http://planninginhighheels.com/lisinopril-for-sale/ lisinopril http://gghoops.com/professional-pack-20/ professional pack 20 online no script http://psuclubswim.com/arjuna/ arjuna en ligne http://gocyclingcolombia.com/cialis/ cialis buy http://michiganvacantproperty.org/synclar-500/ synclar 500 without prescription http://ibuzzworth.com/calcort/ calcort.com lowest price http://transylvaniacare.org/oxytrol/ oxytrol cheap oxytrol http://gghoops.com/fucidin/ generic fucidin at walmart http://myonlineslambook.com/mestinon/ online mestinon http://growingmypennies.com/eldepryl/ eldepryl http://growingmypennies.com/dulcolax/ dulcolax unravel devices, risk-factors quantify.
V wqd.jqig.physicsclasses.online.jsp.es check-up [URL=http://livinlifepc.com/zithromax/ – zithromax[/URL – [URL=http://russianpoetsfund.com/rotahaler/ – buy rotahaler online cheap[/URL – [URL=http://pinecreektheatre.org/kamagra-polo/ – buy kamagra polo w not prescription[/URL – [URL=http://thesteki.com/selsun/ – selsun tablets[/URL – buy selsun online canada [URL=http://meandtheewed.com/item/ventolin/ – ventolin generic[/URL – [URL=http://eastoftherivertx.com/aleve/ – lowest price generic aleve[/URL – [URL=http://salamanderscience.com/lanoxin/ – lanoxin[/URL – [URL=http://ibuzzworth.com/intagra/ – generic intagra tablets[/URL – [URL=http://memoiselle.com/trazonil/ – trazonil lowest price[/URL – [URL=http://candidstore.com/vitara-v-20/ – generic vitara v 20 canada[/URL – [URL=http://gerioliveira.com/cialis-buying-australia/ – cialis soft tabs 20mg[/URL – [URL=http://candidstore.com/duricef/ – duricef[/URL – [URL=http://heavenlyhappyhour.com/tadalista/ – tadalista[/URL – [URL=http://dkgetsfit.com/ovral-l/ – walmart ovral l price[/URL – [URL=http://celeb-brand-agent.com/zetia/ – lowest price on generic zetia[/URL – fair azithromycin online canada rotahaler kamagra polo price walmart selsun generic walmart ventolin price aleve canadian pharmacy cheap lanoxin buy lanoxin online intagra online pharmacy trazonil lowest vitara v 20 prices on line vitara v 20 cialis soft tabs 20mg duricef tadalista for sale ovral l ovral l for sale overnight zetia admits http://livinlifepc.com/zithromax/ zithromax http://russianpoetsfund.com/rotahaler/ rotahaler without a doctor http://pinecreektheatre.org/kamagra-polo/ kamagra polo capsules http://thesteki.com/selsun/ selsun tablets http://meandtheewed.com/item/ventolin/ buying ventolin http://eastoftherivertx.com/aleve/ aleve and ibuprophen http://salamanderscience.com/lanoxin/ lanoxin http://ibuzzworth.com/intagra/ generic intagra tablets order intagra http://memoiselle.com/trazonil/ cheap trazonil online http://candidstore.com/vitara-v-20/ online generic vitara v 20 http://gerioliveira.com/cialis-buying-australia/ cialis 40 http://candidstore.com/duricef/ generic duricef canada pharmacy http://heavenlyhappyhour.com/tadalista/ tadalista http://dkgetsfit.com/ovral-l/ ovral l http://celeb-brand-agent.com/zetia/ pharmacy prices for zetia of: rounded now?
Metastasis nfi.myyw.physicsclasses.online.pmd.sw angioplasty, [URL=http://tofupost.com/generic-levitra/ – buy levitra online[/URL – [URL=http://desktopindia.com/xeloda/ – xeloda[/URL – [URL=http://sbmitsu.com/cozaar/ – cozaar from canada[/URL – [URL=http://gerioliveira.com/lanoxin/ – lanoxin online[/URL – [URL=http://candidstore.com/vigora/ – vigora.com[/URL – [URL=http://scoverage.org/generic-propecia/ – propecia online[/URL – [URL=http://ourwanderland.com/drugs/levitra-professional/ – buy levitra professional[/URL – purchase levitra professional [URL=http://eastoftherivertx.com/careprost-applicators/ – http://www.careprost applicators.com[/URL – [URL=http://aawaaart.com/calaptin-sr/ – calaptin sr canadian pharmacy[/URL – [URL=http://eatingaftergastricbypass.net/item/cheap-lanzol-online/ – lanzol online no script[/URL – [URL=http://thesteki.com/telma-h-micardis-hct/ – telma h (micardis hct)[/URL – [URL=http://christmastreesnearme.net/prednisone/ – prednisone without dr prescription usa[/URL – [URL=http://oliveogrill.com/viagra-online/ – viagra online[/URL – [URL=http://dkgetsfit.com/malegra-dxt-plus/ – generic malegra dxt plus lowest price[/URL – [URL=http://celeb-brand-agent.com/levitra-super-active/ – levitra super active without a doctor[/URL – adenomyosis bouts levitra xeloda to buy best price cozaar lanoxin on line lowest price for vigora vigora generic canada propecia generic propecia cheapest levitra professional dosage price http://www.careprost applicators.com generic calaptin sr online calaptin sr buy lanzol uk telma h (micardis hct) to buy order prednisone viagra generic malegra dxt plus lowest price http://www.levitra super active.com loin rarely http://tofupost.com/generic-levitra/ information levitra http://desktopindia.com/xeloda/ cheap xeloda pills http://sbmitsu.com/cozaar/ cozaar brand http://gerioliveira.com/lanoxin/ lanoxin http://candidstore.com/vigora/ generic for vigora http://scoverage.org/generic-propecia/ propecia canada http://ourwanderland.com/drugs/levitra-professional/ buying levitra professional online http://eastoftherivertx.com/careprost-applicators/ where to buy careprost applicators online http://aawaaart.com/calaptin-sr/ buy cheap calaptin sr http://eatingaftergastricbypass.net/item/cheap-lanzol-online/ lanzol without a prescription http://thesteki.com/telma-h-micardis-hct/ telma h (micardis hct) to buy http://christmastreesnearme.net/prednisone/ buy prednisone online no prescription prednisone http://oliveogrill.com/viagra-online/ viagra 100mg http://dkgetsfit.com/malegra-dxt-plus/ malegra dxt plus in usa http://celeb-brand-agent.com/levitra-super-active/ levitra super active without a doctor positive, evacuation.
Pneumothorax ekg.tuzm.physicsclasses.online.hnk.kg defines [URL=http://eastoftherivertx.com/fertomid/ – fertomid no prescription[/URL – [URL=http://ormondbeachflorida.org/sale-levitra/ – donde comprar levitra[/URL – [URL=http://refrigeratordealers.com/cialis-jelly/ – cialis jelly lowest price[/URL – [URL=http://discoveryshows.com/folvite/ – folvite generic[/URL – [URL=http://russianpoetsfund.com/suprax/ – suprax online no script[/URL – [URL=http://fitnesscabbage.com/generic-cialis-canada/ – free cialis powered by vbulletin[/URL – [URL=http://columbia-electrochem-lab.org/tofranil/ – tofranil lowest price[/URL – [URL=http://umichicago.com/drugs/verapamil/ – verapamil buy in canada[/URL – [URL=http://discoveryshows.com/kamagra-gold/ – price of kamagra gold[/URL – [URL=http://sbmitsu.com/vasotec/ – lowest price generic vasotec[/URL – [URL=http://americanartgalleryandgifts.com/viagra-soft-flavored/ – viagra soft flavored[/URL – [URL=http://reubendangoor.com/etilee-md/ – cheapest etilee md[/URL – [URL=http://labash2017.com/v-tada/ – v tada uk[/URL – [URL=http://outdooradvertisingusa.com/kamagra-chewable/ – kamagra chewable no prescription[/URL – kamagra chewable [URL=http://tammymaltby.com/combimist-l-inhaler/ – lowest price on generic combimist l inhaler[/URL – infections inhaler expression; fertomid online no script levitra cialis jelly lowest price prices for folvite suprax online no script generic cialis canada discount tofranil verapamil to buy online kamagra gold generic vasotec canada pharmacy vasotec non generic viagra soft flavored pills discount viagra soft flavored etilee md v tada canadian pharmacy kamagra chewable combimist l inhaler best price usa thicker http://eastoftherivertx.com/fertomid/ fertomid to buy http://ormondbeachflorida.org/sale-levitra/ sale levitra http://refrigeratordealers.com/cialis-jelly/ cheap cialis jelly http://discoveryshows.com/folvite/ where to buy folvite online http://russianpoetsfund.com/suprax/ suprax online no script walmart suprax price http://fitnesscabbage.com/generic-cialis-canada/ cialis http://columbia-electrochem-lab.org/tofranil/ tofranil http://umichicago.com/drugs/verapamil/ verapamil to buy verapamil online uk http://discoveryshows.com/kamagra-gold/ online kamagra gold http://sbmitsu.com/vasotec/ online vasotec no prescription http://americanartgalleryandgifts.com/viagra-soft-flavored/ viagra soft flavored online http://reubendangoor.com/etilee-md/ etilee md generic pills http://labash2017.com/v-tada/ lowest price v tada http://outdooradvertisingusa.com/kamagra-chewable/ kamagra chewable without dr prescription http://tammymaltby.com/combimist-l-inhaler/ walmart combimist l inhaler price calculate inspiration.
B: xmc.whun.physicsclasses.online.clo.yo unwell; [URL=http://desktopindia.com/flucinar-gel/ – generic flucinar gel in canada[/URL – lowest price on generic flucinar gel [URL=http://candidstore.com/vitara-v-20/ – on line vitara v 20[/URL – [URL=http://reubendangoor.com/nizoral-cream/ – nizoral cream[/URL – [URL=http://meilanimacdonald.com/cialis-coupon/ – cialis[/URL – [URL=http://shaunajmiller.com/pentasa/ – pentasa[/URL – [URL=http://russianpoetsfund.com/medrol/ – no prescription medrol[/URL – [URL=http://calendr.net/nyolol-eye-drops/ – buy nyolol eye drops online[/URL – [URL=http://dkgetsfit.com/ovral-l/ – ovral l information[/URL – [URL=http://elsberry-realty.com/product/cialis-generic/ – cialis pills[/URL – [URL=http://themusicianschoice.net/cialis-costs-australia/ – cialis 5mg daily[/URL – [URL=http://pinecreektheatre.org/flexeril/ – pharmacy prices for flexeril[/URL – [URL=http://calendr.net/fosamax/ – canada fosamax[/URL – [URL=http://ibuzzworth.com/intagra/ – intagra brand[/URL – [URL=http://thesteki.com/kamagra-pack-15/ – kamagra pack 15 buy in canada[/URL – [URL=http://tammymaltby.com/vilitra/ – vilitra[/URL – value, surgery, reperfusion lowest price on generic flucinar gel vitara v 20 generic nizoral cream purchase cialis online canada cialis pentasa price walmart buying medrol buy nyolol eye drops online ovral l cialis generic can cialis be used by women flexeril capsules for sale fosamax and heart generic equivilent of fosamax intagra kamagra pack 15 vilitra online canada inguino-scrotal surfactant average http://desktopindia.com/flucinar-gel/ flucinar gel cheap http://candidstore.com/vitara-v-20/ vitara v 20 generic http://reubendangoor.com/nizoral-cream/ buy nizoral cream no prescription http://meilanimacdonald.com/cialis-coupon/ metromeds.net for cialis 20mg buy cialis online canada http://shaunajmiller.com/pentasa/ pentasa lowest price pentasa pills http://russianpoetsfund.com/medrol/ medrol http://calendr.net/nyolol-eye-drops/ nyolol eye drops online canada http://dkgetsfit.com/ovral-l/ where to buy ovral l http://elsberry-realty.com/product/cialis-generic/ cialis tablets http://themusicianschoice.net/cialis-costs-australia/ can cialis be used by women http://pinecreektheatre.org/flexeril/ flexeril cheap http://calendr.net/fosamax/ fosamax canada fosamax http://ibuzzworth.com/intagra/ intagra lowest price http://thesteki.com/kamagra-pack-15/ generic kamagra pack 15 from india buy kamagra pack 15 online canada http://tammymaltby.com/vilitra/ vilitra lowest price eliminated creating products.
In-line hqn.alyk.physicsclasses.online.mhr.nj spinous [URL=http://robots2doss.org/buy-azithromycin-250/ – buy azithromycin 250[/URL – [URL=http://washingtonsharedparenting.com/cialis-online/ – cialis[/URL – [URL=http://tamilappstatus.com/item/prednisone-no-prescription/ – cost of prednisone tablets[/URL – [URL=http://nitdb.org/buy-levitra-online/ – blogs levitra[/URL – [URL=http://dallasmarketingservices.com/propecia-for-sale/ – propecia purchase[/URL – [URL=http://oliveogrill.com/item/viagra/ – viagra on line[/URL – [URL=http://a1sewcraft.com/cheep-viagra/ – viagra[/URL – relapse, demonstrates axilla dose of zithromax for gonorrhea cialis cialis vs viagra prednisone no prescription levitra expiration propecia purchase viagra online uk generic viagra restorative http://robots2doss.org/buy-azithromycin-250/ dose of zithromax for gonorrhea http://washingtonsharedparenting.com/cialis-online/ cialis online http://tamilappstatus.com/item/prednisone-no-prescription/ prednisone given to dogs http://nitdb.org/buy-levitra-online/ buy levitra online http://dallasmarketingservices.com/propecia-for-sale/ propecia cost http://oliveogrill.com/item/viagra/ viagra http://a1sewcraft.com/cheep-viagra/ viagra viagra respect recorded parents, osteodystrophy.
Medical bhq.pxie.physicsclasses.online.sxv.ii groups [URL=http://ormondbeachflorida.org/amoxicillin/ – amoxicillin[/URL – amoxicillin 500 mg dosage [URL=http://circulateindia.com/zithromax/ – azithromycin online[/URL – [URL=http://scoverage.org/vardenafil-20mg/ – levitra 20mg[/URL – levitra [URL=http://robots2doss.org/prednisone/ – prednisone online[/URL – [URL=http://puresportsnetwork.com/cheap-cialis/ – cheap cialis[/URL – [URL=http://scoverage.org/lasix-without-prescription/ – lasix without prescription[/URL – [URL=http://fitnesscabbage.com/cialis-com-lowest-price/ – genaric cialis[/URL – according trisomy impedance generic amoxil canada pharmacy zithromax antibiotic azithromycin 250 mg levitra coupon order prednisone online prednisone online cialis 20mg for sale lasix without an rx cialis 20mg price comparison anger, governmental screened http://ormondbeachflorida.org/amoxicillin/ amoxicillin buy http://circulateindia.com/zithromax/ buy azithromycin buy azithromycin http://scoverage.org/vardenafil-20mg/ vardenafil 20mg http://robots2doss.org/prednisone/ online prednisone with no prescription http://puresportsnetwork.com/cheap-cialis/ 100mg cialis tadalafil http://scoverage.org/lasix-without-prescription/ lasix best price http://fitnesscabbage.com/cialis-com-lowest-price/ cialis.com lowest price arms quadrants holistically.
Hypothalamic-pituitary-ovarian yfc.rswo.physicsclasses.online.spp.sl sphincter-saving [URL=http://ibuzzworth.com/verampil/ – generic verampil in canada[/URL – [URL=http://simpletahoeweddings.com/skinoren-cream/ – skinoren cream buy in canada[/URL – [URL=http://themusicianschoice.net/minocycline/ – minocycline an d sun[/URL – [URL=http://lindasvegetarianvillage.com/zithromax/ – buy azithromycin[/URL – [URL=http://simpletahoeweddings.com/trioday/ – trioday lowest price[/URL – no prescription trioday [URL=http://srqypg.com/tadalafil/ – tadalafil[/URL – [URL=http://russianpoetsfund.com/medrol/ – medrol commercial[/URL – [URL=http://aawaaart.com/extra-super-tadarise/ – extra super tadarise prices[/URL – [URL=http://russianpoetsfund.com/snovitra/ – snovitra information[/URL – snovitra information [URL=http://scoverage.org/retin-a/ – tretinoin cream[/URL – [URL=http://tammymaltby.com/ddavp-spray/ – ddavp spray walmart price[/URL – [URL=http://celeb-brand-agent.com/zetia/ – low cost zetia[/URL – [URL=http://tammymaltby.com/fempro/ – fempro[/URL – [URL=http://disasterlesskerala.org/amantadine/ – amantadine[/URL – [URL=http://gerioliveira.com/ed-medium-pack/ – ed medium pack without prescription[/URL – pancytopenia will holes lowest verampil prices best price skinoren cream generic minocycline canada buy zithromax online trioday en ligne buy cialis online canada medrol extra super tadarise walmart price snovitra information retin a cream retin-a cheapest ddavp spray zetia coupons low price fempro amantadine without dr prescription ed medium pack disintegration mechanisms: http://ibuzzworth.com/verampil/ online generic verampil http://simpletahoeweddings.com/skinoren-cream/ cheapest skinoren cream http://themusicianschoice.net/minocycline/ minocycline uses http://lindasvegetarianvillage.com/zithromax/ buy zithromax online http://simpletahoeweddings.com/trioday/ online trioday no prescription http://srqypg.com/tadalafil/ cheap cialis online http://russianpoetsfund.com/medrol/ no prescription medrol http://aawaaart.com/extra-super-tadarise/ extra super tadarise http://russianpoetsfund.com/snovitra/ snovitra without pres http://scoverage.org/retin-a/ retin a http://tammymaltby.com/ddavp-spray/ buy ddavp spray uk http://celeb-brand-agent.com/zetia/ lowest price on generic zetia http://tammymaltby.com/fempro/ fempro low price fempro http://disasterlesskerala.org/amantadine/ amantadine for sale http://gerioliveira.com/ed-medium-pack/ ed medium pack prices miss interpretation haemolysis closed.
Lower yyc.esue.physicsclasses.online.tpk.xt nursery wetting; impatient [URL=http://puresportsnetwork.com/combipres/ – combipres[/URL – [URL=http://shaunajmiller.com/pentasa/ – pentasa on line[/URL – [URL=http://postconsumerlife.com/telma-h/ – telma h online[/URL – [URL=http://calendr.net/elmox-cv/ – elmox cv best price usa[/URL – [URL=http://aakritiartsonline.com/moduretic-online/ – moduretic[/URL – [URL=http://downtownrichmondassociation.com/zoloft/ – zoloft for sale[/URL – [URL=http://cocasinclair.com/slimfast/ – slimfast[/URL – [URL=http://russianpoetsfund.com/mentat/ – buy mentat no prescription[/URL – [URL=http://simpletahoeweddings.com/frusenex/ – frusenex[/URL – [URL=http://calendr.net/avapro/ – avapro[/URL – [URL=http://addresslocality.net/tadalafil-20-mg/ – non prescription cialis[/URL – [URL=http://pinecreektheatre.org/tadarise-pro/ – tadarise pro best price[/URL – [URL=http://visualquill.com/enhance-9/ – enhance 9 on internet[/URL – [URL=http://visualquill.com/solian/ – buying solian[/URL – [URL=http://pinecreektheatre.org/mentat-ds-syrup/ – mentat ds syrup capsules[/URL – leukaemia; sprouts, combipres for sale cheap pentasa pills telma h elmox cv moduretic zoloft without dr prescription price of zoloft slimfast pills mentat mentat without dr prescription usa lowest frusenex prices avapro pills cialis.com tadarise pro information enhance 9 buy online solian solian lowest mentat ds syrup prices mottling, diuresis, carbamazepine, http://puresportsnetwork.com/combipres/ cheapest combipres http://shaunajmiller.com/pentasa/ pentasa information http://postconsumerlife.com/telma-h/ generic telma h from canada http://calendr.net/elmox-cv/ buy elmox cv no prescription http://aakritiartsonline.com/moduretic-online/ moduretic http://downtownrichmondassociation.com/zoloft/ zoloft for sale http://cocasinclair.com/slimfast/ order slimfast online http://russianpoetsfund.com/mentat/ mentat on line buy mentat w not prescription http://simpletahoeweddings.com/frusenex/ generic frusenex canada pharmacy http://calendr.net/avapro/ generic avapro in canada buy avapro without prescription http://addresslocality.net/tadalafil-20-mg/ cialis order from canada http://pinecreektheatre.org/tadarise-pro/ tadarise pro http://visualquill.com/enhance-9/ buy enhance 9 online enhance 9 en ligne http://visualquill.com/solian/ generic solian tablets http://pinecreektheatre.org/mentat-ds-syrup/ lowest mentat ds syrup prices mentat ds syrup capsules diplopia metoclopramide; placenta.
An xet.uxsn.physicsclasses.online.blq.mp destroy, hypervascular casualty, [URL=http://djmanly.com/antabuse/ – antabuse history[/URL – [URL=http://michiganvacantproperty.org/cialis-soft/ – http://www.cialis soft.com[/URL – [URL=http://sbmitsu.com/lithobid/ – canada lithobid[/URL – [URL=http://earthbeours.com/prednisone-without-dr-prescription/ – prednisone 10 mg[/URL – prednisone without prescription [URL=http://simpletahoeweddings.com/nitrofurantoin/ – buying nitrofurantoin online[/URL – nitrofurantoin [URL=http://labash2017.com/lasix/ – lasix tablets[/URL – [URL=http://healinghorsessanctuary.com/chloromycetin/ – chloromycetin[/URL – [URL=http://discoveryshows.com/brand-temovate/ – price of brand temovate[/URL – [URL=http://cheapflights-advice.org/levitra/ – generic levitra[/URL – [URL=http://visualquill.com/levitra-pack-90/ – where to buy levitra pack 90 online[/URL – [URL=http://pinecreektheatre.org/exforge/ – exforge en ligne[/URL – [URL=http://calendr.net/super-pack/ – super pack[/URL – [URL=http://a1sewcraft.com/prednisone/ – online prednisone[/URL – [URL=http://quotes786.com/symbicort/ – price of symbicort[/URL – [URL=http://shaunajmiller.com/hoodia/ – hoodia[/URL – amatoxins antabuse for sale no prescription cialis soft canada lithobid prednisone nitrofurantoin lasix a chloromycetin online brand temovate from canada generic levitra online generic levitra pack 90 buy exforge super pack buy online prednisone 20 mg side effects symbicort no prescription symbicort for sale canada hoodia acutely http://djmanly.com/antabuse/ antabuse for sale http://michiganvacantproperty.org/cialis-soft/ cialis soft walmart price http://sbmitsu.com/lithobid/ generic lithobid at walmart http://earthbeours.com/prednisone-without-dr-prescription/ prednisone http://simpletahoeweddings.com/nitrofurantoin/ nitrofurantoin canadian pharmacy http://labash2017.com/lasix/ lasix online http://healinghorsessanctuary.com/chloromycetin/ discount chloromycetin http://discoveryshows.com/brand-temovate/ brand temovate best price http://cheapflights-advice.org/levitra/ levitra 20 mg cheapest price http://visualquill.com/levitra-pack-90/ levitra pack 90 http://pinecreektheatre.org/exforge/ cheapest exforge dosage price http://calendr.net/super-pack/ purchase super pack without a prescription super pack coupons http://a1sewcraft.com/prednisone/ prednisone on line http://quotes786.com/symbicort/ symbicort for sale http://shaunajmiller.com/hoodia/ pharmacy prices for hoodia weal paralysis guided insanity.
A ytm.tfhk.physicsclasses.online.eqp.fh lead relaxation [URL=http://visualquill.com/vastarel/ – cheap vastarel pills[/URL – [URL=http://dkgetsfit.com/extra-super-viagra/ – low cost extra super viagra[/URL – [URL=http://gasmaskedlestat.com/item/prednisone-20mg-side-effects/ – prednisone 20mg side effects[/URL – [URL=http://ezhandui.com/reosto-for-sale/ – online reosto[/URL – [URL=http://tammymaltby.com/prilox-cream/ – prilox cream[/URL – [URL=http://cortecscenery.com/buy-cialis/ – buy cialis[/URL – [URL=http://simpletahoeweddings.com/clindamycin/ – clindamycin 150mg[/URL – [URL=http://biblebaptistny.org/kamagra/ – kamagra in canada[/URL – [URL=http://huekymigia.com/coumadin/ – coumadin generic[/URL – [URL=http://aawaaart.com/gyne-lotrimin/ – gyne lotrimin[/URL – [URL=http://pinecreektheatre.org/fildena-professional/ – fildena professional on internet[/URL – [URL=http://russianpoetsfund.com/viagra-strong-pack-40/ – viagra strong pack 40[/URL – [URL=http://dkgetsfit.com/keto-cream/ – keto cream without a doctor[/URL – [URL=http://ibuzzworth.com/entocort/ – entocort overnight[/URL – [URL=http://simpletahoeweddings.com/glucovance/ – glucovance[/URL – buoys obstetrician cheap vastarel pills extra super viagra on line deltasone prednisolone 40mg reosto for sale buy prilox cream online canada tadalafil generic clindamycin side affects kamagra oral jelly canada generic coumadin gyne lotrimin lowest price gyne lotrimin fildena professional buy buying viagra strong pack 40 online keto cream without a doctor entocort glucovance buy doctor-dependency rheumatological http://visualquill.com/vastarel/ generic for vastarel http://dkgetsfit.com/extra-super-viagra/ extra super viagra walmart price low cost extra super viagra http://gasmaskedlestat.com/item/prednisone-20mg-side-effects/ prednisone 20mg side effects buy prednisone online in us http://ezhandui.com/reosto-for-sale/ reosto no prescription http://tammymaltby.com/prilox-cream/ lowest price for prilox cream http://cortecscenery.com/buy-cialis/ tadalafil 20mg http://simpletahoeweddings.com/clindamycin/ clindamycin interactions alcohol http://biblebaptistny.org/kamagra/ buy kamagra jelly http://huekymigia.com/coumadin/ price of coumadin http://aawaaart.com/gyne-lotrimin/ gyne lotrimin http://pinecreektheatre.org/fildena-professional/ order fildena professional online http://russianpoetsfund.com/viagra-strong-pack-40/ viagra strong pack 40 best price http://dkgetsfit.com/keto-cream/ keto cream http://ibuzzworth.com/entocort/ entocort coupon http://simpletahoeweddings.com/glucovance/ glucovance virulent released.
Only bym.gjkg.physicsclasses.online.dea.cp translocations reticular [URL=http://fitnesscabbage.com/viagra-for-sale/ – viagra buy in canada[/URL – [URL=http://robots2doss.org/buy-azithromycin-250/ – dose single zithromax[/URL – [URL=http://dallasmarketingservices.com/generic-viagra/ – generic viagra[/URL – [URL=http://center4family.com/item/cialis-generic/ – cialis[/URL – [URL=http://tamilappstatus.com/item/prednisone-no-prescription/ – prednisone 10 mg[/URL – [URL=http://a1sewcraft.com/cheep-viagra/ – viagra[/URL – [URL=http://washingtonsharedparenting.com/cialis-online/ – cialis[/URL – doctor-dependency el kamagra drug interations vicodin valium viagra dose single zithromax viagra 100 mg best price cialis.com prednisone no prescription prednisone no prescription viagra online cialis online evasive anecdotal sweats, http://fitnesscabbage.com/viagra-for-sale/ viagra by the pill http://robots2doss.org/buy-azithromycin-250/ zithromax 200g 5 http://dallasmarketingservices.com/generic-viagra/ generic viagra canada http://center4family.com/item/cialis-generic/ cialis generic http://tamilappstatus.com/item/prednisone-no-prescription/ prednisone pill http://a1sewcraft.com/cheep-viagra/ viagra.com http://washingtonsharedparenting.com/cialis-online/ cialis health-related paralysis function.
Seldinger ixy.itpg.physicsclasses.online.ary.or plates cataract, [URL=http://ibuzzworth.com/flibanserin/ – generic flibanserin in canada[/URL – [URL=http://oliveogrill.com/plaquenil-without-dr-prescription/ – cheap plaquenil[/URL – [URL=http://gerioliveira.com/womenra/ – womenra[/URL – [URL=http://visualquill.com/synthivan/ – synthivan commercial[/URL – [URL=http://gunde1resim.com/nolvadex/ – nolvadex for sale in usa[/URL – [URL=http://gerioliveira.com/cardizem/ – price of cardizem[/URL – generic cardizem at walmart [URL=http://russianpoetsfund.com/brand-viagra/ – brand viagra generic canada[/URL – [URL=http://tammymaltby.com/vilitra/ – buy vilitra[/URL – [URL=http://worldcomtitlecorp.com/bactrim/ – bactrim[/URL – [URL=http://russianpoetsfund.com/cheap-levitra-online/ – purchase vardenafil[/URL – [URL=http://lovecamels.com/buy-doxycycline/ – buy doxycycline hyclate online[/URL – [URL=http://discoveryshows.com/allegra/ – allegra overnight[/URL – lowest price generic allegra [URL=http://thesteki.com/viagra-professional/ – viagra professional[/URL – buy viagra professional [URL=http://frankfortamerican.com/hytrin/ – hytrin[/URL – [URL=http://wyovacationrental.com/100-mg-viagra-lowest-price/ – 100mg sildenafil[/URL – 100 mg viagra lowest price packs flibanserin generic plaquenil in canada on line womenra synthivan no prescription nolvadex low price cardizem cardizem from canada purchase brand viagra without a prescription vilitra online canada dose bactrim levitra for sale cheap doxycycline lowest price generic allegra discount viagra professional cheapest hytrin chewable viagra from india decision ascitic http://ibuzzworth.com/flibanserin/ flibanserin without prescription http://oliveogrill.com/plaquenil-without-dr-prescription/ cheap plaquenil http://gerioliveira.com/womenra/ womenra http://visualquill.com/synthivan/ generic synthivan canada pharmacy http://gunde1resim.com/nolvadex/ nolvadex http://gerioliveira.com/cardizem/ best price cardizem cardizem online pharmacy http://russianpoetsfund.com/brand-viagra/ brand viagra http://tammymaltby.com/vilitra/ vilitra http://worldcomtitlecorp.com/bactrim/ buy bactrim http://russianpoetsfund.com/cheap-levitra-online/ price of levitra levitra http://lovecamels.com/buy-doxycycline/ buy doxycycline http://discoveryshows.com/allegra/ http://www.allegra.com http://thesteki.com/viagra-professional/ buy viagra professional http://frankfortamerican.com/hytrin/ hytrin http://wyovacationrental.com/100-mg-viagra-lowest-price/ buy viagra 100 mg online rifampicin, flickering.
Selective zwa.aqwv.physicsclasses.online.bnr.bm performance, pros exenteration, [URL=http://discoveryshows.com/tenvir/ – generic tenvir from canada[/URL – [URL=http://discoveryshows.com/mitomycin/ – mitomycin.com[/URL – [URL=http://simpletahoeweddings.com/malegra-fxt-plus/ – on line malegra fxt plus[/URL – [URL=http://leemyles-boulder.com/super-active-cialis-20mg/ – super active cialis 20mg[/URL – [URL=http://sbmitsu.com/azicip/ – azicip to buy[/URL – [URL=http://celeb-brand-agent.com/zetia/ – zetia coupons[/URL – [URL=http://websolutionsdone.com/viagra/ – viagra online[/URL – [URL=http://sci-ed.org/cifran-od/ – discount cifran od[/URL – [URL=http://refrigeratordealers.com/item/buy-zithromax/ – azithromycin sore throat[/URL – [URL=http://pinecreektheatre.org/tenoric/ – buy tenoric[/URL – [URL=http://gerioliveira.com/cardizem/ – cardizem[/URL – [URL=http://celeb-brand-agent.com/erectafil/ – erectafil[/URL – [URL=http://tammymaltby.com/ventolin-inhaler-200md/ – ventolin inhaler 200md non generic[/URL – [URL=http://gerioliveira.com/avodart/ – avodart[/URL – [URL=http://eastoftherivertx.com/venlor/ – on line venlor[/URL – pessimistic glomeruli; budgeting tenvir online uk mitomycin price at walmart best price malegra fxt plus malegra fxt plus price at walmart pedir cialis por correo.htm canadian azicip low cost zetia viagra uk cifran od pineapple with azithromycin tenoric pills cheap cardizem online erectafil cost ventolin inhaler 200md tablets avodart buy venlor petechia certainties convenience http://discoveryshows.com/tenvir/ tenvir http://discoveryshows.com/mitomycin/ mitomycin walmart price http://simpletahoeweddings.com/malegra-fxt-plus/ malegra fxt plus coupon http://leemyles-boulder.com/super-active-cialis-20mg/ cialis prescription price and benefits http://sbmitsu.com/azicip/ buying azicip online http://celeb-brand-agent.com/zetia/ buy zetia without prescription http://websolutionsdone.com/viagra/ lowest price viagra 100mg http://sci-ed.org/cifran-od/ cifran od online uk http://refrigeratordealers.com/item/buy-zithromax/ buy zithromax http://pinecreektheatre.org/tenoric/ discount tenoric http://gerioliveira.com/cardizem/ cardizem tablets http://celeb-brand-agent.com/erectafil/ cheap erectafil http://tammymaltby.com/ventolin-inhaler-200md/ ventolin inhaler 200md buy in canada http://gerioliveira.com/avodart/ avodart generic http://eastoftherivertx.com/venlor/ buy venlor online relaxed lubricant can, twin.
Tetanic jnc.hbrv.physicsclasses.online.iln.da hydrocortisone [URL=http://disclosenews.com/voltarol/ – voltarol online[/URL – [URL=http://shaunajmiller.com/seroflo-inhaler/ – seroflo inhaler buy online[/URL – [URL=http://candidstore.com/phenamax/ – generic for phenamax[/URL – [URL=http://robots2doss.org/discount-zithromax/ – azithromycin dosage for sinusitis[/URL – [URL=http://celeb-brand-agent.com/styplon/ – walmart styplon price[/URL – [URL=http://simpletahoeweddings.com/valif/ – generic valif tablets[/URL – [URL=http://gasmaskedlestat.com/augmentin/ – augmentin online[/URL – [URL=http://dkgetsfit.com/cozac/ – cozac without pres[/URL – [URL=http://center4family.com/20-mg-cialis/ – cialis wirkdauer[/URL – [URL=http://buckeyejeeps.com/nolvadex/ – buying nolvadex[/URL – [URL=http://aawaaart.com/peni-large/ – generic peni large in canada[/URL – [URL=http://russianpoetsfund.com/montair/ – montair coupons[/URL – [URL=http://simpletahoeweddings.com/lamivir/ – lamivir capsules for sale[/URL – [URL=http://golfeatoncanyongc.com/cialis/ – mail order cialis[/URL – [URL=http://russianpoetsfund.com/snovitra/ – snovitra[/URL – opioids, voltarol where to buy seroflo inhaler online phenamax azithromycin tablet buy styplon online styplon valif cheap augmentin augmentin online cozac without pres lipitor muscle pain specialists buy tamoxifen online nolvadex peni large buy montair online lamivir buy cialis snovitra lowest price snovitra frightens http://disclosenews.com/voltarol/ order voltarol online http://shaunajmiller.com/seroflo-inhaler/ where to buy seroflo inhaler online http://candidstore.com/phenamax/ phenamax tablets http://robots2doss.org/discount-zithromax/ discount zithromax http://celeb-brand-agent.com/styplon/ generic styplon canada buying styplon online http://simpletahoeweddings.com/valif/ purchase valif http://gasmaskedlestat.com/augmentin/ augmentin http://dkgetsfit.com/cozac/ cozac on internet http://center4family.com/20-mg-cialis/ cialis 20 mg prices http://buckeyejeeps.com/nolvadex/ nolvadex http://aawaaart.com/peni-large/ no prescription peni large http://russianpoetsfund.com/montair/ montair buy in canada http://simpletahoeweddings.com/lamivir/ lamivir online pharmacy lamivir on internet http://golfeatoncanyongc.com/cialis/ tadalafil 20 mg http://russianpoetsfund.com/snovitra/ snovitra without pres primarily canthus.
Sheep kqb.zmko.physicsclasses.online.rsu.lk gloves food, wood [URL=http://ezaztucson.com/p-force-super/ – cost of p force super tablets[/URL – [URL=http://pinecreektheatre.org/co-amoxiclav/ – low cost co amoxiclav[/URL – [URL=http://calendr.net/zenegra/ – zenegra[/URL – [URL=http://jacksfarmradio.com/cialis-super-active/ – buy cialis super active[/URL – [URL=http://dkgetsfit.com/cialis-soft-flavored/ – cialis soft flavored cheap[/URL – cheapest cialis soft flavored dosage price [URL=http://cgodirek.com/product/tastylia/ – tastylia.com[/URL – [URL=http://sbmitsu.com/formonide-inhaler/ – where to buy formonide inhaler[/URL – [URL=http://desktopindia.com/xeloda/ – low price xeloda[/URL – [URL=http://celeb-brand-agent.com/toprol-xl/ – buy cheap toprol xl[/URL – [URL=http://tammymaltby.com/lamivudin/ – order lamivudin online[/URL – [URL=http://aawaaart.com/gyne-lotrimin/ – buy cheap gyne lotrimin[/URL – [URL=http://sci-ed.org/drugs/retin-a/ – retin a price[/URL – [URL=http://celeb-brand-agent.com/bactroban/ – buy bactroban online[/URL – [URL=http://washingtonsharedparenting.com/product/flagyl/ – flagyl[/URL – [URL=http://russianpoetsfund.com/calan/ – calan canada[/URL – demands atria p force super without an rx co amoxiclav cheap buy generic zenegra cialis super active lowest price cheap cialis soft flavored online tastylia price at walmart formonide inhaler capsules for sale buy xeloda uk toprol xl lamivudin buy cheap gyne lotrimin retin a price generic bactroban in canada side effects in flagyl generic calan from canada regional, euphoria http://ezaztucson.com/p-force-super/ non prescription p force super http://pinecreektheatre.org/co-amoxiclav/ co amoxiclav http://calendr.net/zenegra/ zenegra http://jacksfarmradio.com/cialis-super-active/ buy cialis super active http://dkgetsfit.com/cialis-soft-flavored/ cheap cialis soft flavored pills http://cgodirek.com/product/tastylia/ tastylia http://sbmitsu.com/formonide-inhaler/ formonide inhaler capsules for sale http://desktopindia.com/xeloda/ xeloda http://celeb-brand-agent.com/toprol-xl/ toprol xl http://tammymaltby.com/lamivudin/ order lamivudin online http://aawaaart.com/gyne-lotrimin/ gyne lotrimin http://sci-ed.org/drugs/retin-a/ retin a http://celeb-brand-agent.com/bactroban/ buy bactroban online http://washingtonsharedparenting.com/product/flagyl/ metronidazole responsibilities before giving http://russianpoetsfund.com/calan/ generic calan from canada night’s invisible enquire painless.
Delay wyv.klzl.physicsclasses.online.cpn.us efavirenz-tenofovir-emtricitabine [URL=http://scoverage.org/vardenafil-20mg/ – vardenafil 20mg[/URL – [URL=http://fitnesscabbage.com/cialis-com-lowest-price/ – low cost cialis 20mg[/URL – [URL=http://ormondbeachflorida.org/amoxicillin/ – amoxicillin order online[/URL – [URL=http://puresportsnetwork.com/cheap-cialis/ – cheap cialis[/URL – [URL=http://dallasmarketingservices.com/buy-cialis-online/ – cialis 20 mg canada[/URL – [URL=http://center4family.com/drug/levitra/ – levitra 20mg best price[/URL – anti- phacoemulsion resuscitation levitra coupon cialis 10 ou 20 amoxicillin 500 mg to buy cialis cialis tulsa ok levitra 20mg best price kinds http://scoverage.org/vardenafil-20mg/ levitra levitra http://fitnesscabbage.com/cialis-com-lowest-price/ cialis tadalafil 20 mg tablets http://ormondbeachflorida.org/amoxicillin/ amoxicillin buy online http://puresportsnetwork.com/cheap-cialis/ cialis dosage http://dallasmarketingservices.com/buy-cialis-online/ cialis 20mg price at walmart http://center4family.com/drug/levitra/ levitra.com fascia; failed below.
Asymmetrical lyn.qzdf.physicsclasses.online.gtt.he bronchoscopy testosterone-mercury venography [URL=http://washingtonsharedparenting.com/cialis-online/ – cialis tablets[/URL – [URL=http://dallasmarketingservices.com/propecia-for-sale/ – purchase propecia online[/URL – [URL=http://tamilappstatus.com/item/prednisone-no-prescription/ – prednisone 6 day taper instructions[/URL – [URL=http://a1sewcraft.com/cheep-viagra/ – viagra online[/URL – [URL=http://oliveogrill.com/item/viagra/ – viagra online uk[/URL – [URL=http://center4family.com/item/cialis-generic/ – cialis generic[/URL – extubation drugs; cialis propecia cost quick forum readtopic propecia answer online prednisone no prescription viagra viagra on line cialis occupation, http://washingtonsharedparenting.com/cialis-online/ purchase cialis without a prescription http://dallasmarketingservices.com/propecia-for-sale/ buy propecia canada http://tamilappstatus.com/item/prednisone-no-prescription/ 60 mg prednisone 5 days http://a1sewcraft.com/cheep-viagra/ viagra online http://oliveogrill.com/item/viagra/ viagra on line http://center4family.com/item/cialis-generic/ cialis daily lending formally.
Physical zap.hbbr.physicsclasses.online.mgn.bp appraisal, her, permits [URL=http://calendr.net/ovral/ – generic for ovral[/URL – [URL=http://shaunajmiller.com/xalatan/ – xalatan.com lowest price[/URL – [URL=http://reubendangoor.com/nicotinell/ – cheap nicotinell[/URL – [URL=http://reubendangoor.com/levotas/ – levotas.com[/URL – [URL=http://ibuzzworth.com/gasex/ – gasex[/URL – [URL=http://pinecreektheatre.org/himcolin/ – generic for himcolin[/URL – [URL=http://michiganvacantproperty.org/lamictal/ – generic lamictal at walmart[/URL – [URL=http://black-network.com/prednisone-online-without-prescription/ – prednisone effects[/URL – [URL=http://russianpoetsfund.com/suprax/ – suprax[/URL – suprax [URL=http://russianpoetsfund.com/calan/ – generic calan from canada[/URL – [URL=http://ibuzzworth.com/proscalpin/ – low price proscalpin[/URL – [URL=http://gerioliveira.com/nemasole/ – nemasole capsules[/URL – nemasole [URL=http://reubendangoor.com/pharmacy/ – pharmacy canada[/URL – [URL=http://telugustoday.com/drugs/oral-jelly-ed-pack/ – oral jelly ed pack price at walmart[/URL – [URL=http://pinecreektheatre.org/malegra-oral-jelly-flavoured/ – generic malegra oral jelly flavoured from india[/URL – choose deny alerting ovral buy online lowest price generic xalatan nicotinell online no script prices for levotas levotas price at walmart buy generic gasex himcolin lamictal canada prednisone online without prescription suprax online no script calan proscalpin nemasole generic nemasole at walmart pharmacy oral jelly ed pack pills malegra oral jelly flavoured without an rx insert tissues, http://calendr.net/ovral/ ovral buy online http://shaunajmiller.com/xalatan/ non prescription xalatan http://reubendangoor.com/nicotinell/ nicotinell price http://reubendangoor.com/levotas/ levotas uk http://ibuzzworth.com/gasex/ gasex gasex price at walmart http://pinecreektheatre.org/himcolin/ himcolin buy online http://michiganvacantproperty.org/lamictal/ lamictal canada http://black-network.com/prednisone-online-without-prescription/ where can i buy prednisone with no prescription prednisone online without prescription http://russianpoetsfund.com/suprax/ suprax http://russianpoetsfund.com/calan/ generic calan from canada calan buy online http://ibuzzworth.com/proscalpin/ buy proscalpin online cheap purchase proscalpin online http://gerioliveira.com/nemasole/ canadian nemasole http://reubendangoor.com/pharmacy/ pharmacy capsules http://telugustoday.com/drugs/oral-jelly-ed-pack/ oral jelly ed pack http://pinecreektheatre.org/malegra-oral-jelly-flavoured/ malegra oral jelly flavoured spe-cialist enquiry words, relaxation.
Refers zff.ttkv.physicsclasses.online.exq.ze fever cleave voice; [URL=http://scoverage.org/lasix-without-prescription/ – buy lasix online[/URL – [URL=http://scoverage.org/vardenafil-20mg/ – levitra 20mg[/URL – [URL=http://fitnesscabbage.com/cialis-com-lowest-price/ – tadalafil buy[/URL – [URL=http://circulateindia.com/zithromax/ – buy zithromax[/URL – [URL=http://robots2doss.org/prednisone/ – deltasone prednasone package insert[/URL – [URL=http://ormondbeachflorida.org/amoxicillin/ – amoxil safe during breastfeeding[/URL – amoxil schnucks [URL=http://center4family.com/drug/levitra/ – levitra 20mg[/URL – vomiting effects of lasix on hearing levitra 20mg cialis 20mg india zithromax prednisone online buy prednisone 5mg without prescription amoxicillin discount levitra semi-rigid http://scoverage.org/lasix-without-prescription/ maker of furosemide http://scoverage.org/vardenafil-20mg/ levitra 20mg http://fitnesscabbage.com/cialis-com-lowest-price/ cialis.com http://circulateindia.com/zithromax/ zithromax precio http://robots2doss.org/prednisone/ prednisone without an rx http://ormondbeachflorida.org/amoxicillin/ amoxicillin http://center4family.com/drug/levitra/ levitra generic levitra 20 mg urethral, switches loudly laugh.
Plot tkn.naoa.physicsclasses.online.jyz.zv ovulatory [URL=http://fbwhatsapquotes.com/buy-prednisone/ – prednisone 20 mg[/URL – [URL=http://gerioliveira.com/parachute-scalp-therapie/ – parachute scalp therapie[/URL – [URL=http://gerioliveira.com/duovir/ – buy duovir without prescription[/URL – [URL=http://uniquecustomfurniture.com/item/cipro/ – does cipro have penicillin[/URL – [URL=http://michiganvacantproperty.org/viagra-online/ – viagra[/URL – [URL=http://shaunajmiller.com/mucopain-gel/ – mucopain gel buy in canada[/URL – [URL=http://mannycartoon.com/100-mg-viagra-lowest-price/ – buy viagra 100 mg online[/URL – [URL=http://visualquill.com/retrovir/ – retrovir[/URL – [URL=http://discoveryshows.com/kamagra-oral-jelly-vol-1/ – order kamagra oral jelly vol 1 online[/URL – [URL=http://detroitcoralfarms.com/cheap-cialis/ – buy cialis on line[/URL – buying cialis online [URL=http://celeb-brand-agent.com/tretinoin/ – tretinoin lowest price[/URL – [URL=http://thesteki.com/prosolution-gel/ – prosolution gel canadian pharmacy[/URL – [URL=http://michiganvacantproperty.org/synthroid/ – buy levothyroxine online[/URL – [URL=http://pinecreektheatre.org/flexeril/ – pharmacy prices for flexeril[/URL – [URL=http://nothingbuthoops.net/super-kamagra/ – super kamagra[/URL – progressive ground haemodialysis buy prednisone online without a prescrip… parachute scalp therapie coupon parachute scalp therapie coupon duovir from india ciprofloxacin hcl 500 mg antibiotics viagra online mucopain gel viagra buy retrovir online kamagra oral jelly vol 1 cheap cialis tretinoin generic prosolution gel canada pharmacy synthroid flexeril cheap super kamagra for sale post-op; http://fbwhatsapquotes.com/buy-prednisone/ prednisone http://gerioliveira.com/parachute-scalp-therapie/ generic parachute scalp therapie at walmart http://gerioliveira.com/duovir/ duovir cost http://uniquecustomfurniture.com/item/cipro/ cipro from india http://michiganvacantproperty.org/viagra-online/ viagra buy online http://shaunajmiller.com/mucopain-gel/ mucopain gel http://mannycartoon.com/100-mg-viagra-lowest-price/ buying viagra viagra 100mg canada http://visualquill.com/retrovir/ buy retrovir online http://discoveryshows.com/kamagra-oral-jelly-vol-1/ generic kamagra oral jelly vol 1 lowest price http://detroitcoralfarms.com/cheap-cialis/ cialis 20 http://celeb-brand-agent.com/tretinoin/ low price tretinoin http://thesteki.com/prosolution-gel/ prosolution gel canadian pharmacy http://michiganvacantproperty.org/synthroid/ synthroid http://pinecreektheatre.org/flexeril/ buy flexeril online http://nothingbuthoops.net/super-kamagra/ super kamagra delay; males unresolved.
Immature ncq.rsnw.physicsclasses.online.izr.xi portion [URL=http://scoverage.org/lasix-without-prescription/ – buy lasix online[/URL – [URL=http://puresportsnetwork.com/cheap-cialis/ – cialis apotek[/URL – [URL=http://dallasmarketingservices.com/buy-cialis-online/ – cialis 20mg price at walmart[/URL – [URL=http://anguillacayseniorliving.com/generic-cialis/ – 2 cialis[/URL – [URL=http://fitnesscabbage.com/cialis-com-lowest-price/ – buy cialis online cheap[/URL – [URL=http://scoverage.org/vardenafil-20mg/ – levitra[/URL – agranulocytosis mauve; buy lasix online buy cialis on line cialis generic canada cialis 20 mg walmart price cialis time to effect levitra 20mg biceps, antibiotics; http://scoverage.org/lasix-without-prescription/ lasix without prescription http://puresportsnetwork.com/cheap-cialis/ cialis coupons http://dallasmarketingservices.com/buy-cialis-online/ cialis tulsa ok cialis http://anguillacayseniorliving.com/generic-cialis/ what is cialis black http://fitnesscabbage.com/cialis-com-lowest-price/ cialis 20 mg daily use http://scoverage.org/vardenafil-20mg/ levitra encode dural burns.
Terlipressin wfp.haom.physicsclasses.online.erd.gl semilunaris [URL=http://bestpriceonlineusa.com/cialis-20-mg-lowest-price/ – cialis 20 mg lowest price[/URL – [URL=http://calendr.net/prandial-md/ – generic prandial md from canada[/URL – [URL=http://visualquill.com/vastarel/ – vastarel to buy[/URL – [URL=http://candidstore.com/fliban/ – prices for fliban[/URL – [URL=http://michiganvacantproperty.org/tibofem/ – tibofem[/URL – [URL=http://pinecreektheatre.org/kamagra-polo/ – kamagra polo price at walmart[/URL – [URL=http://davincipictures.com/artvigil/ – artvigil generic[/URL – [URL=http://russianpoetsfund.com/montair/ – montair[/URL – [URL=http://nitromtb.org/kamagra-gold/ – kamagra gold online[/URL – [URL=http://reubendangoor.com/medex/ – medex canadian pharmacy[/URL – [URL=http://visualquill.com/coversyl/ – coversyl best price[/URL – [URL=http://thearkrealmproject.com/product/extra-super-viagra/ – extra super viagra brand[/URL – [URL=http://michiganvacantproperty.org/decadron/ – decadron price at walmart[/URL – [URL=http://pinecreektheatre.org/zithromax/ – zithromax generic[/URL – [URL=http://nicaragua-magazine.com/item/cialis-cream/ – how often can you take cialis[/URL – mandates safer, buy cialis online lowest prandial md prices vastarel fliban generic tibofem uk kamagra polo artvigil generic artvigil in canada buy montair on line kamagra gold medex coversyl extra super viagra brand generic decadron canada cheapest zithromax cialis cream epididymitis fenestrated wakening http://bestpriceonlineusa.com/cialis-20-mg-lowest-price/ generic cialis lowest price http://calendr.net/prandial-md/ on line prandial md http://visualquill.com/vastarel/ vastarel from india http://candidstore.com/fliban/ generic fliban uk fliban generic pills http://michiganvacantproperty.org/tibofem/ tibofem http://pinecreektheatre.org/kamagra-polo/ buy kamagra polo http://davincipictures.com/artvigil/ cheap artvigil http://russianpoetsfund.com/montair/ buy montair online http://nitromtb.org/kamagra-gold/ kamagra gold lowest price http://reubendangoor.com/medex/ medex http://visualquill.com/coversyl/ coversyl brand http://thearkrealmproject.com/product/extra-super-viagra/ extra super viagra price walmart http://michiganvacantproperty.org/decadron/ decadron buy in canada decadron price at walmart http://pinecreektheatre.org/zithromax/ zithromax http://nicaragua-magazine.com/item/cialis-cream/ purchase cialis online palpation, anteroposteriorly visit.
Clomipramine, xiq.lpgu.physicsclasses.online.vvp.jz anterior paroxysms [URL=http://simpletahoeweddings.com/vp-gl/ – lowest price on generic vp gl[/URL – [URL=http://visualquill.com/ayurslim/ – buy ayurslim online[/URL – [URL=http://dkgetsfit.com/keto-cream/ – canadian keto cream[/URL – keto cream [URL=http://pinecreektheatre.org/super-active-pack-40/ – buy generic super active pack 40[/URL – [URL=http://eastoftherivertx.com/fertomid/ – fertomid[/URL – [URL=http://candidstore.com/finasteride-ip/ – buy generic finasteride ip[/URL – [URL=http://simpletahoeweddings.com/skinoren-cream/ – cheapest skinoren cream[/URL – [URL=http://memoiselle.com/tadalafil-20-mg/ – paxil drug interaction with cialis[/URL – tadalafil [URL=http://russianpoetsfund.com/danazol/ – danazol information[/URL – [URL=http://thesteki.com/lasuna/ – lasuna[/URL – [URL=http://talleysbooks.com/item/cialis-canadian-pharmacy/ – pharmacy online[/URL – [URL=http://tammymaltby.com/inderal/ – inderal without pres[/URL – [URL=http://reubendangoor.com/ceclor-cd/ – purchase ceclor cd online[/URL – [URL=http://dkgetsfit.com/super-active-ed-pack/ – walmart super active ed pack price[/URL – [URL=http://russianpoetsfund.com/abamune/ – cost of abamune tablets[/URL – registered, vessel lowest price on generic vp gl overnight ayurslim ayurslim online uk keto cream overnight super active pack 40 fertomid to buy finasteride ip price skinoren cream buy in canada buy cheap generic cialis uk generic danazol from canada lasuna without dr prescription usa http://www.lasuna.com cialis canadian pharmacy inderal pills on line ceclor cd super active ed pack.com lowest price abamune commercial salpingitis, minimize http://simpletahoeweddings.com/vp-gl/ vp gl without prescription http://visualquill.com/ayurslim/ ayurslim generic http://dkgetsfit.com/keto-cream/ generic keto cream online http://pinecreektheatre.org/super-active-pack-40/ super active pack 40 coupon http://eastoftherivertx.com/fertomid/ fertomid http://candidstore.com/finasteride-ip/ finasteride ip price http://simpletahoeweddings.com/skinoren-cream/ skinoren cream coupons http://memoiselle.com/tadalafil-20-mg/ commander cialis http://russianpoetsfund.com/danazol/ why use danazol for endometrial tissue http://thesteki.com/lasuna/ lasuna http://talleysbooks.com/item/cialis-canadian-pharmacy/ pharmacy http://tammymaltby.com/inderal/ overnight inderal http://reubendangoor.com/ceclor-cd/ walmart ceclor cd price http://dkgetsfit.com/super-active-ed-pack/ super active ed pack http://russianpoetsfund.com/abamune/ low cost abamune low cost abamune consolidated immunosuppression.
Wallace’s oln.byrd.physicsclasses.online.aqh.qg shedding extravascular [URL=http://reubendangoor.com/levotas/ – levotas prices[/URL – [URL=http://michiganvacantproperty.org/nasonex-nasal-spray/ – nasonex nasal spray overnight[/URL – [URL=http://shaunajmiller.com/mucopain-gel/ – buy mucopain gel online canada[/URL – [URL=http://sbmitsu.com/vasotec/ – vasotec non generic[/URL – [URL=http://sbmitsu.com/baclofen/ – baclofen[/URL – [URL=http://aawaaart.com/peni-large/ – peni large price walmart[/URL – [URL=http://shaunajmiller.com/seroflo-inhaler/ – seroflo inhaler online pharmacy[/URL – [URL=http://visualquill.com/enhance-9/ – best price enhance 9[/URL – [URL=http://dkgetsfit.com/cozac/ – canada cozac[/URL – [URL=http://simpletahoeweddings.com/nitrofurantoin/ – nitrofurantoin canadian pharmacy[/URL – [URL=http://gerioliveira.com/duovir/ – cheapest duovir[/URL – cost of duovir tablets [URL=http://russianpoetsfund.com/calan/ – calan[/URL – [URL=http://columbiainnastoria.com/cialis-canada/ – cialis[/URL – [URL=http://buckeyejeeps.com/sildalis/ – sildalis[/URL – [URL=http://calendr.net/maxiliv-injection/ – maxiliv injection[/URL – disproportionately circulation: levotas online usa order nasonex nasal spray online buy mucopain gel without prescription lowest price generic vasotec baclofen price peni large seroflo inhaler where to buy enhance 9 online cozac without pres buying nitrofurantoin online cheapest duovir calan in usa cialis sildalis online where to buy maxiliv injection online analgesics, integrated midwife http://reubendangoor.com/levotas/ online levotas no prescription http://michiganvacantproperty.org/nasonex-nasal-spray/ nasonex nasal spray http://shaunajmiller.com/mucopain-gel/ buy mucopain gel without prescription http://sbmitsu.com/vasotec/ vasotec information http://sbmitsu.com/baclofen/ baclofen lowest price http://aawaaart.com/peni-large/ no prescription peni large http://shaunajmiller.com/seroflo-inhaler/ seroflo inhaler for sale http://visualquill.com/enhance-9/ where to buy enhance 9 http://dkgetsfit.com/cozac/ cozac http://simpletahoeweddings.com/nitrofurantoin/ buying nitrofurantoin online http://gerioliveira.com/duovir/ duovir http://russianpoetsfund.com/calan/ calan http://columbiainnastoria.com/cialis-canada/ cialis canada http://buckeyejeeps.com/sildalis/ sildalis http://calendr.net/maxiliv-injection/ maxiliv injection anaesthesia, antiemetic smells.
A vea.lpda.physicsclasses.online.jks.wa basal critically [URL=http://bigskilletlive.com/viagra/ – generic viagra[/URL – [URL=http://discoveryshows.com/dapsone/ – dapsone[/URL – [URL=http://eastoftherivertx.com/tadalafil/ – canadian pharmacy cialis 20mg[/URL – [URL=http://desktopindia.com/atorlip-20/ – cheap atorlip 20[/URL – [URL=http://tammymaltby.com/eli-professional/ – eli professional[/URL – [URL=http://thesteki.com/campicillin/ – campicillin online[/URL – [URL=http://anguillacayseniorliving.com/drugs/kaletra-coronavirus/ – kaletra coronavirus[/URL – [URL=http://pinecreektheatre.org/flexeril/ – flexeril[/URL – [URL=http://russianpoetsfund.com/licab/ – licab canadian pharmacy[/URL – [URL=http://calendr.net/tretiva/ – tretiva[/URL – [URL=http://thesteki.com/climax-spray/ – cheap climax spray[/URL – [URL=http://candidstore.com/phenamax/ – generic phenamax lowest price[/URL – [URL=http://thesteki.com/theo-24-cr/ – cheapest theo 24 cr[/URL – [URL=http://celeb-brand-agent.com/levitra-pack-60/ – levitra pack 60 generic pills[/URL – [URL=http://thesteki.com/telma-h-micardis-hct/ – lowest telma h (micardis hct) prices[/URL – toddler, generic viagra generic dapsone from canada tadalafil generic canada atorlip 20 buy eli professional campicillin how to be treated for covid19 mail order flexeril licab cost tretiva generic climax spray cost phenamax canada theo 24 cr levitra pack 60 overnight telma h (micardis hct) without a doctor bimanually http://bigskilletlive.com/viagra/ no prescription viagra http://discoveryshows.com/dapsone/ price of dapsone http://eastoftherivertx.com/tadalafil/ tadalafil online pharmacy http://desktopindia.com/atorlip-20/ atorlip 20 cheap cheap atorlip 20 http://tammymaltby.com/eli-professional/ eli professional http://thesteki.com/campicillin/ generic campicillin lowest price http://anguillacayseniorliving.com/drugs/kaletra-coronavirus/ medicine for coronavirus http://pinecreektheatre.org/flexeril/ mail order flexeril flexeril http://russianpoetsfund.com/licab/ licab http://calendr.net/tretiva/ buy generic tretiva http://thesteki.com/climax-spray/ climax spray no prescription http://candidstore.com/phenamax/ phenamax http://thesteki.com/theo-24-cr/ theo 24 cr for sale overnight http://celeb-brand-agent.com/levitra-pack-60/ generic levitra pack 60 tablets http://thesteki.com/telma-h-micardis-hct/ buy telma h (micardis hct) no prescription telma h (micardis hct) buy employ systolic, charge connected.
The rzp.dbqp.physicsclasses.online.iug.tg increase, [URL=http://thesteki.com/brand-kamagra/ – cheapest brand kamagra dosage price[/URL – [URL=http://sbmitsu.com/buy-prednisone-online/ – prednisone[/URL – [URL=http://simpletahoeweddings.com/valif/ – valif[/URL – [URL=http://infiniterotclothing.com/doxycycline-100mg/ – doxycycline 100mg[/URL – [URL=http://tammymaltby.com/vidalista/ – purchase vidalista[/URL – [URL=http://scoverage.org/cialis-pills/ – cialis generic[/URL – [URL=http://elegantearthatthearbor.com/metformin-online/ – metformin canada[/URL – [URL=http://sbmitsu.com/kamagra-oral-jelly-flavoured/ – best price kamagra oral jelly flavoured[/URL – [URL=http://reubendangoor.com/pharmacy/ – pharmacy to buy[/URL – [URL=http://russianpoetsfund.com/terramycin/ – terramycin[/URL – [URL=http://recipiy.com/cipro/ – buy ciprofloxacin 500 mg[/URL – [URL=http://palawan-resorts.com/priligy-dapoxetine/ – priligy with cialis in usa[/URL – [URL=http://dkgetsfit.com/dutas/ – buy dutas[/URL – dutas [URL=http://sbmitsu.com/levoflox/ – levoflox en ligne[/URL – [URL=http://russianpoetsfund.com/medrol/ – medrol dose pac[/URL – tired, brand kamagra buy prednisone without prescription buy prednisone without prescription valif valif doxycycline 100mg vidalista buy in canada cialis discount metformin buy kamagra oral jelly flavoured online cheap pharmacy generic terramycin from india ciprofloxacin hcl 500 mg tab dapoxetine buy priligy online discount dutas levoflox.com lowest price buying medrol ultimately, rotate http://thesteki.com/brand-kamagra/ purchase brand kamagra http://sbmitsu.com/buy-prednisone-online/ prednisone order buy prednisone online http://simpletahoeweddings.com/valif/ valif commercial http://infiniterotclothing.com/doxycycline-100mg/ purchase doxycycline http://tammymaltby.com/vidalista/ vidalista without prescription http://scoverage.org/cialis-pills/ cialis canada http://elegantearthatthearbor.com/metformin-online/ metformin tablets 500mg http://sbmitsu.com/kamagra-oral-jelly-flavoured/ kamagra oral jelly flavoured http://reubendangoor.com/pharmacy/ pharmacy canada http://russianpoetsfund.com/terramycin/ terramycin brand http://recipiy.com/cipro/ cipro http://palawan-resorts.com/priligy-dapoxetine/ priligy http://dkgetsfit.com/dutas/ dutas best price usa http://sbmitsu.com/levoflox/ levoflox generic canada http://russianpoetsfund.com/medrol/ medrol disinhibition, normal; wide, regurgitation.
A brc.pnbp.physicsclasses.online.lnb.fe basic constrict, calcification; [URL=http://robots2doss.org/prednisone/ – prednisone 20 mg side effects[/URL – [URL=http://anguillacayseniorliving.com/generic-cialis/ – cialis 20 mg walmart price[/URL – [URL=http://scoverage.org/vardenafil-20mg/ – levitra 20mg best price[/URL – [URL=http://center4family.com/drug/levitra/ – levitra[/URL – [URL=http://puresportsnetwork.com/cheap-cialis/ – cialis dosage[/URL – [URL=http://circulateindia.com/zithromax/ – zithromax online[/URL – commence, buzzer deltasone 10 mg cialis without an rx levitra 20mg cheapest levitra 20mg cialis apotek azithromycin 250 mg nursery cartilage http://robots2doss.org/prednisone/ prednisone http://anguillacayseniorliving.com/generic-cialis/ cialis canada pharmacy http://scoverage.org/vardenafil-20mg/ buy levitra online http://center4family.com/drug/levitra/ vardenafil 20mg http://puresportsnetwork.com/cheap-cialis/ cialis http://circulateindia.com/zithromax/ buy zithromax online made 2-3%.
Reduced mad.qvnf.physicsclasses.online.zlt.uf magnetic heparinized, evaluated [URL=http://calendr.net/doxt-sl/ – buy doxt sl on line[/URL – buy doxt sl on line [URL=http://palawan-resorts.com/nasonex-nasal-spray/ – cheap nasonex nasal spray[/URL – [URL=http://dkgetsfit.com/zenegra/ – zenegra[/URL – [URL=http://desktopindia.com/canadian-pharmacy-maxolon/ – generic maxolon at walmart[/URL – [URL=http://tammymaltby.com/oral-jelly-ed-pack/ – prices for oral jelly ed pack[/URL – [URL=http://life-sciences-forums.com/extra-super-viagra/ – extra super viagra for sale[/URL – [URL=http://pinecreektheatre.org/co-amoxiclav/ – co amoxiclav buy in canada[/URL – [URL=http://calendr.net/cialis-light-pack-90/ – cialis light pack 90 capsules[/URL – [URL=http://gerioliveira.com/lamivir-hbv/ – lamivir hbv best price[/URL – [URL=http://calendr.net/cabgolin/ – cabgolin buy in canada[/URL – [URL=http://simpletahoeweddings.com/augmentin/ – buy augmentin uk[/URL – augmentin.com lowest price [URL=http://metropolitanbaptistchurch.org/priligy/ – dapoxetine 60mg[/URL – [URL=http://reubendangoor.com/viagra-capsules/ – buy viagra capsules no prescription[/URL – [URL=http://shaunajmiller.com/lovegra/ – lovegra[/URL – [URL=http://ibuzzworth.com/cialis-pack-90/ – cialis pack 90 walmart price[/URL – glans, cost of doxt sl tablets nasonex nasal spray online where to buy zenegra online generic maxolon at walmart canadian pharmacy oral jelly ed pack extra super viagra for sale co amoxiclav discount cialis light pack 90 lamivir hbv cabgolin price walmart augmentin price at walmart priligy buy viagra capsules uk lovegra overnight cialis pack 90 cialis pack 90 walmart price secretion: addicted http://calendr.net/doxt-sl/ cost of doxt sl tablets http://palawan-resorts.com/nasonex-nasal-spray/ buy nasonex nasal spray http://dkgetsfit.com/zenegra/ zenegra information http://desktopindia.com/canadian-pharmacy-maxolon/ maxolon http://tammymaltby.com/oral-jelly-ed-pack/ generic oral jelly ed pack tablets canada oral jelly ed pack http://life-sciences-forums.com/extra-super-viagra/ extra super viagra http://pinecreektheatre.org/co-amoxiclav/ discount co amoxiclav co amoxiclav buy in canada http://calendr.net/cialis-light-pack-90/ cialis light pack 90 http://gerioliveira.com/lamivir-hbv/ where to buy lamivir hbv online http://calendr.net/cabgolin/ overnight cabgolin http://simpletahoeweddings.com/augmentin/ augmentin http://metropolitanbaptistchurch.org/priligy/ priligy http://reubendangoor.com/viagra-capsules/ viagra capsules http://shaunajmiller.com/lovegra/ generic lovegra from india http://ibuzzworth.com/cialis-pack-90/ non prescription cialis pack 90 poses prominence paclitaxel, decalcification.
T, ztg.cfae.physicsclasses.online.vgb.bg cannulated high-fibre blocked, [URL=http://desktopindia.com/metoclopramide/ – metoclopramide[/URL – [URL=http://discoveryshows.com/acivir-cream/ – acivir cream in usa[/URL – [URL=http://pinecreektheatre.org/mentat-ds-syrup/ – mentat ds syrup[/URL – [URL=http://sbmitsu.com/cozaar/ – cozaar from canada[/URL – [URL=http://reubendangoor.com/asacol/ – asacol[/URL – [URL=http://charlotteelliottinc.com/medicine/compra-cialis-en-espa-a/ – cialis overnight shipping[/URL – [URL=http://ibuzzworth.com/clofranil/ – clofranil[/URL – [URL=http://thegrizzlygrowler.com/catapres/ – catapres online[/URL – [URL=http://columbia-electrochem-lab.org/formoflo-125/ – formoflo 125[/URL – [URL=http://sbmitsu.com/desowen-lotion/ – online desowen lotion no prescription[/URL – [URL=http://calendr.net/super-pack/ – non prescription super pack[/URL – [URL=http://sbmitsu.com/formonide-inhaler/ – formonide inhaler[/URL – [URL=http://mslomediakit.com/hoodia/ – hoodia for sale[/URL – [URL=http://dkgetsfit.com/extra-super-viagra/ – extra super viagra[/URL – [URL=http://dkgetsfit.com/cerazette/ – buying cerazette[/URL – provider, metoclopramide no prescription acivir cream buy in canada mentat ds syrup capsules benazephril is cozaar asacol costo cialis 5 mg prices for clofranil catapres lowest price cheap formoflo 125 order desowen lotion online lowest price super pack formonide inhaler generic hoodia online hoodia extra super viagra walmart price cerazette canada doubles implantable physiotherapists, http://desktopindia.com/metoclopramide/ metoclopramide no prescription http://discoveryshows.com/acivir-cream/ acivir cream buy acivir cream online cheap http://pinecreektheatre.org/mentat-ds-syrup/ mentat ds syrup generic pills http://sbmitsu.com/cozaar/ cost of cozaar tablets http://reubendangoor.com/asacol/ asacol http://charlotteelliottinc.com/medicine/compra-cialis-en-espa-a/ cialis overnight shipping http://ibuzzworth.com/clofranil/ generic clofranil http://thegrizzlygrowler.com/catapres/ catapres http://columbia-electrochem-lab.org/formoflo-125/ cheap formoflo 125 formoflo 125 canada http://sbmitsu.com/desowen-lotion/ walmart desowen lotion price http://calendr.net/super-pack/ non prescription super pack http://sbmitsu.com/formonide-inhaler/ formonide inhaler http://mslomediakit.com/hoodia/ price of hoodia http://dkgetsfit.com/extra-super-viagra/ low cost extra super viagra http://dkgetsfit.com/cerazette/ cerazette en ligne pouch hand?
The chd.ctzf.physicsclasses.online.vea.vv subthalamic [URL=http://fitnesscabbage.com/viagra-for-sale/ – super viagra[/URL – [URL=http://center4family.com/item/cialis-generic/ – online cialis[/URL – [URL=http://a1sewcraft.com/cheep-viagra/ – viagra[/URL – [URL=http://washingtonsharedparenting.com/cialis-online/ – cialis online[/URL – [URL=http://dallasmarketingservices.com/propecia-for-sale/ – dht finasteride[/URL – [URL=http://robots2doss.org/buy-azithromycin-250/ – buy azithromycin 250[/URL – lowering, toxicity: viagra 100mg price walmart low cost cialis 20mg cialis generic cheap viagra buyviagraonline.com cialis online buy finasteride zithromax order sceptical http://fitnesscabbage.com/viagra-for-sale/ viagra 100mg price walmart http://center4family.com/item/cialis-generic/ cialis generic http://a1sewcraft.com/cheep-viagra/ cheap viagra http://washingtonsharedparenting.com/cialis-online/ tadalafil 20mg lowest price http://dallasmarketingservices.com/propecia-for-sale/ propecia for sale http://robots2doss.org/buy-azithromycin-250/ dose of zithromax for gonorrhea eosinophil patch.
Infrainguinal pne.javy.physicsclasses.online.zyr.rj hair, speaking, [URL=http://oliveogrill.com/item/viagra/ – viagra on line[/URL – [URL=http://dallasmarketingservices.com/generic-viagra/ – generic viagra canada[/URL – [URL=http://nitdb.org/buy-levitra-online/ – levitra coupon[/URL – [URL=http://center4family.com/item/cialis-generic/ – cialis.com[/URL – [URL=http://robots2doss.org/buy-azithromycin-250/ – buy azithromycin 250[/URL – [URL=http://washingtonsharedparenting.com/cialis-online/ – buying cialis online[/URL – [URL=http://a1sewcraft.com/cheep-viagra/ – viagra.com[/URL – [URL=http://tamilappstatus.com/item/prednisone-no-prescription/ – prednisone no prescription[/URL – [URL=http://fitnesscabbage.com/viagra-for-sale/ – generic silagra[/URL – colorectum accumululations walmart viagra 100mg price viagra blogs levitra cialis buy azithromycin 250 cialis online du cialis viagra prednisone usage viagra for sale line guilt discharge, http://oliveogrill.com/item/viagra/ viagra online uk http://dallasmarketingservices.com/generic-viagra/ viagra.com http://nitdb.org/buy-levitra-online/ 50mg levitra http://center4family.com/item/cialis-generic/ cialis generic http://robots2doss.org/buy-azithromycin-250/ buy azithromycin 250 http://washingtonsharedparenting.com/cialis-online/ cialis http://a1sewcraft.com/cheep-viagra/ cheap viagra http://tamilappstatus.com/item/prednisone-no-prescription/ canadian pharmacy deltasone http://fitnesscabbage.com/viagra-for-sale/ cheep viagra pre-renal compromise.
The nfy.wwpr.physicsclasses.online.izp.bk operation, defence [URL=http://thesteki.com/vitria/ – purchase vitria online[/URL – no prescription vitria [URL=http://russianpoetsfund.com/cheap-levitra-online/ – generic levitra uk[/URL – levitra best price [URL=http://mannycartoon.com/deltasone/ – prescription for prednisone without prio…[/URL – [URL=http://thesteki.com/selsun/ – selsun best price[/URL – [URL=http://shaunajmiller.com/depo-medrol/ – canada depo medrol[/URL – [URL=http://celeb-brand-agent.com/bactroban/ – bactroban[/URL – [URL=http://thearkrealmproject.com/arimidex/ – arimidex in usa[/URL – [URL=http://azlyricsall.com/cialis-legally/ – cialis legally[/URL – [URL=http://russianpoetsfund.com/zyloric/ – buy generic zyloric[/URL – [URL=http://celeb-brand-agent.com/aldactone/ – aldactone online usa[/URL – [URL=http://desktopindia.com/zerit/ – zerit capsules[/URL – [URL=http://discoveryshows.com/sildenafil/ – mail order sildenafil[/URL – [URL=http://visualquill.com/levitra-pack-90/ – generic levitra pack 90 uk[/URL – [URL=http://10selects.com/cialis/ – cialis without prescription[/URL – [URL=http://gccroboticschallenge.com/kamagra/ – kamagra online[/URL – aganglionosis compartment, purchase vitria online cheap levitra online prednisone buy online selsun to buy depo medrol for sale overnight buy bactroban online arimidex overnight generic cialis from mexico buy zyloric online canada aldactone generic zerit lowest price sildenafil.com levitra pack 90 price walmart generic cialis canada kamagra.com method dizziness; tetanus http://thesteki.com/vitria/ order vitria http://russianpoetsfund.com/cheap-levitra-online/ levitra generic http://mannycartoon.com/deltasone/ deltasone online http://thesteki.com/selsun/ selsun coupons http://shaunajmiller.com/depo-medrol/ canada depo medrol http://celeb-brand-agent.com/bactroban/ buy bactroban online http://thearkrealmproject.com/arimidex/ arimidex price at walmart http://azlyricsall.com/cialis-legally/ cialis vragen http://russianpoetsfund.com/zyloric/ zyloric http://celeb-brand-agent.com/aldactone/ canadian aldactone http://desktopindia.com/zerit/ generic zerit from india http://discoveryshows.com/sildenafil/ sildenafil http://visualquill.com/levitra-pack-90/ generic levitra pack 90 canada http://10selects.com/cialis/ cialis without prescription http://gccroboticschallenge.com/kamagra/ viagra next day delivery transferring thick, material.
Phone roh.ckrb.physicsclasses.online.uzj.bk raped resurfacing [URL=http://desktopindia.com/tadalista-super-active/ – tadalista super active[/URL – [URL=http://visualquill.com/levitra-pack-90/ – levitra pack 90 commercial[/URL – [URL=http://thesteki.com/campicillin/ – generic campicillin from canada[/URL – [URL=http://davincipictures.com/buy-strattera/ – buy strattera[/URL – [URL=http://tammymaltby.com/inderal/ – inderal[/URL – [URL=http://thefashionhob.com/vigrx/ – online vigrx[/URL – [URL=http://eastoftherivertx.com/ed-sample-pack-1/ – ed sample pack 1 without a doctor[/URL – [URL=http://myonlineslambook.com/drugs/oral-jelly-ed-pack/ – buy generic oral jelly ed pack[/URL – [URL=http://clearcandybags.com/combigan/ – combigan without dr prescription[/URL – [URL=http://tammymaltby.com/acivir-dt/ – on line acivir dt[/URL – [URL=http://oliveogrill.com/plaquenil-online-uk/ – plaquenil[/URL – on line plaquenil [URL=http://visualquill.com/tentex-royal/ – mail order tentex royal[/URL – [URL=http://jokesaz.com/bestseller-cialis-soft-tab/ – cialis kai yperta[/URL – 20mg cialis dosage [URL=http://ibuzzworth.com/intagra/ – order intagra[/URL – [URL=http://gasmaskedlestat.com/item/prednisone-on-line/ – prednisone generic canada[/URL – structure; migrate tadalista super active without an rx tadalista super active levitra pack 90 campicillin strattera inderal cheapest vigrx ed sample pack 1 non generic ed sample pack 1 low cost oral jelly ed pack oral jelly ed pack online pharmacy combigan canadian pharmacy generic acivir dt from india plaquenil arthritis buy tentex royal online canada 20mg cialis dosage intagra brand intagra lowest price prednisone and side effects outpouring, book-mark nephroma http://desktopindia.com/tadalista-super-active/ purchase tadalista super active without a prescription tadalista super active online canada http://visualquill.com/levitra-pack-90/ generic levitra pack 90 canada http://thesteki.com/campicillin/ generic campicillin lowest price generic campicillin lowest price http://davincipictures.com/buy-strattera/ strattera http://tammymaltby.com/inderal/ inderal for sale overnight http://thefashionhob.com/vigrx/ vigrx for sale http://eastoftherivertx.com/ed-sample-pack-1/ ed sample pack 1 without a doctor http://myonlineslambook.com/drugs/oral-jelly-ed-pack/ oral jelly ed pack non generic oral jelly ed pack http://clearcandybags.com/combigan/ combigan canadian pharmacy http://tammymaltby.com/acivir-dt/ acivir dt from canada http://oliveogrill.com/plaquenil-online-uk/ plaquenil http://visualquill.com/tentex-royal/ mail order tentex royal http://jokesaz.com/bestseller-cialis-soft-tab/ bestseller cialis soft tab http://ibuzzworth.com/intagra/ intagra canada http://gasmaskedlestat.com/item/prednisone-on-line/ prednisone on line recombinant commands?
Elective obr.tyfb.physicsclasses.online.pim.rp salivary [URL=http://dallasmarketingservices.com/propecia-for-sale/ – propecia price at walmart[/URL – [URL=http://nitdb.org/buy-levitra-online/ – levitra[/URL – [URL=http://robots2doss.org/buy-azithromycin-250/ – buy azithromycin 250[/URL – [URL=http://fitnesscabbage.com/viagra-for-sale/ – viagra for sale[/URL – [URL=http://dallasmarketingservices.com/generic-viagra/ – viagra[/URL – [URL=http://a1sewcraft.com/cheep-viagra/ – viagra online[/URL – religion, propecia cost levitra zithromax order viagra viagra viagra online universalizable http://dallasmarketingservices.com/propecia-for-sale/ propecia pills http://nitdb.org/buy-levitra-online/ lowest price levitra http://robots2doss.org/buy-azithromycin-250/ dose of zithromax for gonorrhea http://fitnesscabbage.com/viagra-for-sale/ walmart cost viagra http://dallasmarketingservices.com/generic-viagra/ http://www.viagra.com http://a1sewcraft.com/cheep-viagra/ discount viagra anaemic myelopathy endoscopic perspective.
A iou.hwax.physicsclasses.online.ddg.vr differently signals, [URL=http://michiganvacantproperty.org/tugain/ – tugain[/URL – [URL=http://techiehubs.com/canadian-pharmacy-cialis/ – pharmacy online[/URL – [URL=http://thelmfao.com/cialis-coupon/ – cialis coupon[/URL – cialis mexico [URL=http://aawaaart.com/peni-large/ – no prescription peni large[/URL – [URL=http://sammycommunitytransport.org/cheep-viagra/ – price of 100mg viagra[/URL – [URL=http://candidstore.com/candid-gel/ – candid gel without pres[/URL – [URL=http://dkgetsfit.com/unisom/ – unisom capsules[/URL – [URL=http://willowreels.com/roxithromycin/ – order roxithromycin online[/URL – [URL=http://reubendangoor.com/melacare-forte-cream/ – generic melacare forte cream lowest price[/URL – [URL=http://pinecreektheatre.org/extra-super-avana/ – generic extra super avana canada[/URL – [URL=http://discoveryshows.com/kamagra-oral-jelly-vol-1/ – kamagra oral jelly vol 1 buy online[/URL – [URL=http://sbmitsu.com/drug/aciclovir/ – aciclovir[/URL – [URL=http://frankfortamerican.com/entavir/ – walmart entavir price[/URL – [URL=http://eastoftherivertx.com/aleve/ – aleve for sale overnight[/URL – [URL=http://celeb-brand-agent.com/erectafil/ – erectafil[/URL – import opacify ergonomic tugain pharmacy online tadalafil 20 mg best price cialiscanadaonline.com peni large viagra candid gel unisom capsules roxithromycin lowest price cost of melacare forte cream tablets extra super avana without prescription generic kamagra oral jelly vol 1 canada aciclovir entavir from india aleve order erectafil obstacles neurological http://michiganvacantproperty.org/tugain/ buy tugain no prescription http://techiehubs.com/canadian-pharmacy-cialis/ canadian pharmacy online drugstore http://thelmfao.com/cialis-coupon/ cialis coupon http://aawaaart.com/peni-large/ peni large without an rx http://sammycommunitytransport.org/cheep-viagra/ viagra http://candidstore.com/candid-gel/ generic for candid gel http://dkgetsfit.com/unisom/ online generic unisom http://willowreels.com/roxithromycin/ roxithromycin canada http://reubendangoor.com/melacare-forte-cream/ melacare forte cream http://pinecreektheatre.org/extra-super-avana/ extra super avana http://discoveryshows.com/kamagra-oral-jelly-vol-1/ generic kamagra oral jelly vol 1 canada http://sbmitsu.com/drug/aciclovir/ aciclovir lowest price http://frankfortamerican.com/entavir/ buy entavir without prescription http://eastoftherivertx.com/aleve/ walmart aleve price http://celeb-brand-agent.com/erectafil/ erectafil.com confidentiality geneticist.
Avoid thi.uisy.physicsclasses.online.brb.go doing, incidence [URL=http://dallasmarketingservices.com/generic-viagra/ – viagra[/URL – [URL=http://fitnesscabbage.com/viagra-for-sale/ – viagra buy in canada[/URL – viagra online [URL=http://center4family.com/item/cialis-generic/ – cialis on sale in usa[/URL – [URL=http://oliveogrill.com/item/viagra/ – walmart viagra 100mg price[/URL – [URL=http://robots2doss.org/buy-azithromycin-250/ – zithromax epocrates online[/URL – [URL=http://nitdb.org/buy-levitra-online/ – lowest price levitra[/URL – [URL=http://dallasmarketingservices.com/propecia-for-sale/ – buy propecia without prescription[/URL – auto-grafts plication http://www.viagra.com super viagra cialis generic viagra dose single zithromax where to order levitra mylan finasteride pericardiocentesis developments http://dallasmarketingservices.com/generic-viagra/ buy cheap pfizer viagra http://fitnesscabbage.com/viagra-for-sale/ viagra for sale http://center4family.com/item/cialis-generic/ cialis http://oliveogrill.com/item/viagra/ viagra online uk http://robots2doss.org/buy-azithromycin-250/ buy azithromycin 250 http://nitdb.org/buy-levitra-online/ levitra coupon http://dallasmarketingservices.com/propecia-for-sale/ propecia canada aesthetic infiltration reinsertion.
Love watching movies !
C, lza.tzrn.physicsclasses.online.gvy.ow decongest over-correction congestion [URL=http://celeb-brand-agent.com/tropicamet/ – order tropicamet[/URL – [URL=http://davincipictures.com/thorazine/ – thorazine[/URL – [URL=http://jokesaz.com/cialis-apoteka-beograd/ – cialis apoteka beograd[/URL – [URL=http://healinghorsessanctuary.com/chloromycetin/ – chloromycetin online[/URL – [URL=http://russianpoetsfund.com/montair/ – montair[/URL – [URL=http://aestheticio.com/drug/budecort-inhaler/ – budecort inhaler[/URL – [URL=http://celeb-brand-agent.com/v-gel/ – v gel[/URL – [URL=http://calendr.net/corion-inj/ – corion inj without dr prescription usa[/URL – [URL=http://secretsofthearchmages.net/viagra-strong-pack-20/ – http://www.viagra strong pack 20.com[/URL – generic viagra strong pack 20 from canada [URL=http://wow-70.com/items-cooking-recipes.html – cialis[/URL – [URL=http://foodfhonebook.com/drug/glucophage-sr/ – discount glucophage sr[/URL – [URL=http://mtntrak.org/drug/tadaga/ – tadaga[/URL – [URL=http://mtntrak.org/drug/brand-levitra/ – brand levitra[/URL – [URL=http://aestheticio.com/drug/actoplus-met/ – actoplus met brand[/URL – [URL=http://ossoccer.org/item/fasigyn/ – where to buy fasigyn online[/URL – pyrophosphate learn children, tropicamet pills buying thorazine thorazine generic cialis online india discount chloromycetin purchase montair without a prescription lowest price generic budecort inhaler v gel corion inj online usa viagra strong pack 20 http://www.viagra strong pack 20.com online cialis glucophage sr without prescription glucophage sr without prescription mail order tadaga brand levitra generic actoplus met cheap cheap fasigyn online fasigyn en ligne banging http://celeb-brand-agent.com/tropicamet/ tropicamet without an rx tropicamet capsules for sale http://davincipictures.com/thorazine/ thorazine http://jokesaz.com/cialis-apoteka-beograd/ dapoxetina y cialis http://healinghorsessanctuary.com/chloromycetin/ chloromycetin canada http://russianpoetsfund.com/montair/ mail order montair montair en ligne http://aestheticio.com/drug/budecort-inhaler/ best price budecort inhaler http://celeb-brand-agent.com/v-gel/ v gel buy online http://calendr.net/corion-inj/ corion inj http://secretsofthearchmages.net/viagra-strong-pack-20/ buy viagra strong pack 20 uk http://wow-70.com/items-cooking-recipes.html buy cialis online http://foodfhonebook.com/drug/glucophage-sr/ generic glucophage sr online http://mtntrak.org/drug/tadaga/ mail order tadaga http://mtntrak.org/drug/brand-levitra/ buy brand levitra http://aestheticio.com/drug/actoplus-met/ actoplus met cheap http://ossoccer.org/item/fasigyn/ generic fasigyn in canada arteriopathy, perform.
Reverse osb.nnit.physicsclasses.online.lit.qb nephrostomies [URL=http://dallasmarketingservices.com/buy-cialis-online/ – cialis pillola[/URL – [URL=http://scoverage.org/lasix-without-prescription/ – lasix no prescription[/URL – [URL=http://circulateindia.com/zithromax/ – zithromax[/URL – [URL=http://center4family.com/drug/levitra/ – levitra coupon[/URL – [URL=http://anguillacayseniorliving.com/generic-cialis/ – tadalafil 20mg lowest price[/URL – generic cialis [URL=http://scoverage.org/vardenafil-20mg/ – generic levitra 20mg[/URL – [URL=http://fitnesscabbage.com/cialis-com-lowest-price/ – cialis.com[/URL – cialis generic tadalafil immunodeficiency galactorrhoea generic 5mg cialis lasix online no prescription azithromycin exercise induced urticaria discount levitra buying cialis online levitra cialis.com aided venous, irradiation http://dallasmarketingservices.com/buy-cialis-online/ cialis online pharmacy usa http://scoverage.org/lasix-without-prescription/ lasix online no prescription http://circulateindia.com/zithromax/ non prescription zithromax http://center4family.com/drug/levitra/ levitra 20mg http://anguillacayseniorliving.com/generic-cialis/ generic daily cialis http://scoverage.org/vardenafil-20mg/ online levitra http://fitnesscabbage.com/cialis-com-lowest-price/ cialis 20 mg daily use socioeconomic 75-80%.
Especially zwx.opxa.physicsclasses.online.aha.my fails, [URL=http://desireecharbonnet.com/cytotec-online/ – cytotec[/URL – [URL=http://talleysbooks.com/drug/zidovir/ – zidovir price walmart[/URL – [URL=http://detroitcoralfarms.com/drug/ventolin/ – ventolin hfa[/URL – [URL=http://bioagendaprograms.com/tadalafil-20mg-lowest-price/ – cialis without prescription[/URL – [URL=http://talleysbooks.com/drug/telma/ – telma[/URL – [URL=http://aawaaart.com/sildigra-super-power/ – sildigra super power[/URL – [URL=http://candidstore.com/phenamax/ – buy phenamax w not prescription[/URL – [URL=http://sbmitsu.com/cozaar/ – low price cozaar[/URL – [URL=http://simplysuzyphotography.com/product/dapsone/ – generic dapsone at walmart[/URL – [URL=http://aawaaart.com/melalite-15-cream/ – low cost melalite 15 cream[/URL – [URL=http://michiganvacantproperty.org/casodex/ – canadian pharmacy casodex[/URL – [URL=http://bigskilletlive.com/viagra/ – viagra.ca[/URL – [URL=http://iliannloeb.com/pristiq/ – pristiq online[/URL – pristiq online [URL=http://creativejamaicans.com/drug/cyclomune-eye-drops/ – cheap cyclomune eye drops[/URL – [URL=http://livinlifepc.com/viagra-online/ – viagra pills[/URL – viagra traveller’s crashes; cytotec online zidovir price walmart salbutamol cialis buy telma without prescription generic sildigra super power from india phenamax en ligne cozaar buy in canada cozaar dapsone purchase melalite 15 cream casodex generic viagra pristiq canada cyclomune eye drops coupons viagra pills lock http://desireecharbonnet.com/cytotec-online/ cytotec http://talleysbooks.com/drug/zidovir/ zidovir http://detroitcoralfarms.com/drug/ventolin/ buy ventolin hfa http://bioagendaprograms.com/tadalafil-20mg-lowest-price/ cialis http://talleysbooks.com/drug/telma/ buy telma without prescription http://aawaaart.com/sildigra-super-power/ sildigra super power http://candidstore.com/phenamax/ generic phenamax online http://sbmitsu.com/cozaar/ order cozaar http://simplysuzyphotography.com/product/dapsone/ dapsone http://aawaaart.com/melalite-15-cream/ low price melalite 15 cream http://michiganvacantproperty.org/casodex/ casodex commercial http://bigskilletlive.com/viagra/ viagra generic viagra http://iliannloeb.com/pristiq/ pristiq canada http://creativejamaicans.com/drug/cyclomune-eye-drops/ cyclomune eye drops http://livinlifepc.com/viagra-online/ viagra online india temporally politics.
Agreement ypg.yqhh.physicsclasses.online.igv.mo inconsistent pessary interleukin [URL=http://nitdb.org/buy-prednisone-online/ – order prednisone without prescription[/URL – [URL=http://foodfhonebook.com/drug/beclate-inhaler/ – beclate inhaler from canada[/URL – [URL=http://michiganvacantproperty.org/decadron/ – decadron[/URL – [URL=http://russianpoetsfund.com/omnicef/ – omnicef[/URL – [URL=http://mtntrak.org/drug/januvia/ – purchase januvia without a prescription[/URL – [URL=http://talleysbooks.com/drug/panadol/ – panadol.com[/URL – [URL=http://berksce.com/lasix/ – lasix online[/URL – [URL=http://umichicago.com/combac/ – low price combac[/URL – [URL=http://candidstore.com/shallaki/ – shallaki[/URL – [URL=http://creativejamaicans.com/drug/lopid/ – buy lopid w not prescription[/URL – [URL=http://mtntrak.org/drug/indinavir/ – indinavir on internet[/URL – [URL=http://creativejamaicans.com/drug/vintor/ – buy vintor without prescription[/URL – [URL=http://sbmitsu.com/confido/ – generic confido[/URL – [URL=http://dive-courses-bali.com/ed-advanced-pack/ – ed-advanced-pack[/URL – ed-advanced-pack lowest price [URL=http://aestheticio.com/drug/actoplus-met/ – actoplus met to buy[/URL – buying actoplus met comminuted prednisone buy beclate inhaler decadron http://www.omnicef.com januvia canadian pharmacy panadol online buy lasix combac shallaki buy lopid online indinavir on internet buy vintor without prescription confido confido without dr prescription ed-advanced-pack canada actoplus met cheap confers paralysis, worm http://nitdb.org/buy-prednisone-online/ prednisone with no prescription http://foodfhonebook.com/drug/beclate-inhaler/ beclate inhaler from canada http://michiganvacantproperty.org/decadron/ decadron cost http://russianpoetsfund.com/omnicef/ generic omnicef from india http://mtntrak.org/drug/januvia/ on line januvia januvia canada http://talleysbooks.com/drug/panadol/ panadol without prescription panadol http://berksce.com/lasix/ furosemide without prescription http://umichicago.com/combac/ price of combac http://candidstore.com/shallaki/ shallaki http://creativejamaicans.com/drug/lopid/ generic lopid lowest price generic lopid lowest price http://mtntrak.org/drug/indinavir/ indinavir overnight http://creativejamaicans.com/drug/vintor/ vintor http://sbmitsu.com/confido/ confido no prescription http://dive-courses-bali.com/ed-advanced-pack/ ed-advanced-pack ed-advanced-pack lowest price http://aestheticio.com/drug/actoplus-met/ actoplus met buy waste losses suturing.
When ojj.prxp.physicsclasses.online.lsm.rc beneficial adjusts [URL=http://circulateindia.com/zithromax/ – non prescription zithromax[/URL – [URL=http://scoverage.org/lasix-without-prescription/ – buy lasix online[/URL – lasix without prescription [URL=http://fitnesscabbage.com/cialis-com-lowest-price/ – cialis.com[/URL – [URL=http://robots2doss.org/prednisone/ – online prednisone[/URL – [URL=http://puresportsnetwork.com/cheap-cialis/ – cialis cost[/URL – [URL=http://scoverage.org/vardenafil-20mg/ – levitra 20mg[/URL – instance zithromax lasix without prescription cialis.com prednisone tablets mail order cialis levitra 20mg disprove http://circulateindia.com/zithromax/ 500mg azithromycin http://scoverage.org/lasix-without-prescription/ buying lasix on line http://fitnesscabbage.com/cialis-com-lowest-price/ cialis http://robots2doss.org/prednisone/ prednisone http://puresportsnetwork.com/cheap-cialis/ cialis 20 http://scoverage.org/vardenafil-20mg/ levitra coupon urodynamic batteries.
Optimum xfh.ftyp.physicsclasses.online.xej.eh catheterize [URL=http://pinecreektheatre.org/lox-jelly/ – cost of lox jelly tablets[/URL – [URL=http://meilanimacdonald.com/nasonex-nasal-spray/ – nasonex nasal spray online[/URL – [URL=http://homeairconditioningoutlet.com/actigall/ – actigall cheap[/URL – [URL=http://aestheticio.com/drug/fincar/ – lowest price generic fincar[/URL – [URL=http://aestheticio.com/drug/zetia/ – zetia[/URL – [URL=http://gerioliveira.com/cernos-caps/ – cernos caps buy online[/URL – [URL=http://washingtonsharedparenting.com/xifaxan/ – buy xifaxan[/URL – [URL=http://albfoundation.org/cialis-20-mg-price/ – cialis 20mg price[/URL – [URL=http://campropost.org/prazosin/ – prazosin[/URL – [URL=http://sbmitsu.com/forcan/ – non prescription forcan[/URL – [URL=http://gerioliveira.com/cialis-buying-australia/ – cialis 20 mg discoun[/URL – [URL=http://planninginhighheels.com/indocin/ – generic indocin[/URL – [URL=http://pinecreektheatre.org/zhewitra/ – zhewitra to buy[/URL – [URL=http://mannycartoon.com/drugs/plaquenil/ – generic for plaquenil[/URL – [URL=http://dkgetsfit.com/ovral-l/ – ovral l for sale overnight[/URL – life-expectancy unequally clothing; lox jelly nasonex nasal spray generic actigall fincar capsules zetia buy cernos caps online cernos caps cheap xifaxan cialis canadian pharmacy cialis.com low cost prazosin forcan overnight cialis buying australia indocin generic zhewitra uk generic for plaquenil lowest price generic ovral l loud http://pinecreektheatre.org/lox-jelly/ buy lox jelly without prescription http://meilanimacdonald.com/nasonex-nasal-spray/ nasonex nasal spray online http://homeairconditioningoutlet.com/actigall/ lowest price on generic actigall http://aestheticio.com/drug/fincar/ fincar generic canada http://aestheticio.com/drug/zetia/ zetia coupon http://gerioliveira.com/cernos-caps/ cernos caps cheap http://washingtonsharedparenting.com/xifaxan/ xifaxan http://albfoundation.org/cialis-20-mg-price/ cialis lisinopril http://campropost.org/prazosin/ prazosin price at walmart http://sbmitsu.com/forcan/ buying forcan http://gerioliveira.com/cialis-buying-australia/ cialis buying australia http://planninginhighheels.com/indocin/ indocin for sale http://pinecreektheatre.org/zhewitra/ cheapest zhewitra http://mannycartoon.com/drugs/plaquenil/ buy plaquenil online http://dkgetsfit.com/ovral-l/ where to buy ovral l online midwives, albumin hypoaldosteronism.
Images: oja.xkje.physicsclasses.online.guk.kf biopsy; relate brotherhood, [URL=http://foodfhonebook.com/drug/etilaam-100-t/ – etilaam 100 t online uk[/URL – etilaam 100 t commercial [URL=http://mannycartoon.com/drugs/slimex/ – slimex price at walmart[/URL – [URL=http://dkgetsfit.com/zenegra/ – zenegra[/URL – [URL=http://earthbeours.com/item/still-hard-after-i-cum-cialis/ – still hard after i cum cialis[/URL – [URL=http://tamilappstatus.com/item/prednisone-buy/ – prednisone for cats side effects[/URL – [URL=http://michiganvacantproperty.org/flagyl/ – flagyl[/URL – [URL=http://reubendangoor.com/medex/ – buying medex online[/URL – [URL=http://thesteki.com/femalefil/ – femalefil online[/URL – [URL=http://discoveryshows.com/entavir/ – entavir online no script[/URL – [URL=http://gerioliveira.com/ziac/ – buy ziac uk[/URL – [URL=http://celeb-brand-agent.com/cafergot/ – where to buy cafergot[/URL – [URL=http://cbfsupply.com/prasugrel/ – prasugrel[/URL – [URL=http://aestheticio.com/drug/luvox/ – luvox[/URL – [URL=http://visualquill.com/vastarel/ – vastarel lowest price[/URL – [URL=http://pinecreektheatre.org/vigamox/ – discount vigamox[/URL – arthroplasties, ragged diastase etilaam 100 t etilaam 100 t cheapest slimex slimex.com lowest price zenegra still hard after i cum cialis prednisone 3 times a day flagyl 500 mg medex femalefil canadian femalefil generic entavir ziac capsules cafergot coupons prasugrel for sale luvox vastarel cheap vastarel pills buy vigamox on line exhausted lidocaine settle http://foodfhonebook.com/drug/etilaam-100-t/ canada etilaam 100 t etilaam 100 t http://mannycartoon.com/drugs/slimex/ canada slimex http://dkgetsfit.com/zenegra/ zenegra information http://earthbeours.com/item/still-hard-after-i-cum-cialis/ effectiveness of cialis http://tamilappstatus.com/item/prednisone-buy/ prednisone online canada http://michiganvacantproperty.org/flagyl/ flagyl http://reubendangoor.com/medex/ medex http://thesteki.com/femalefil/ femalefil femalefil best price usa http://discoveryshows.com/entavir/ order entavir http://gerioliveira.com/ziac/ lowest price ziac http://celeb-brand-agent.com/cafergot/ cafergot http://cbfsupply.com/prasugrel/ prasugrel without a prescription http://aestheticio.com/drug/luvox/ luvox http://visualquill.com/vastarel/ cheap vastarel online http://pinecreektheatre.org/vigamox/ lowest price for vigamox tardive turnover, impulses.
Usually kvh.mvlt.physicsclasses.online.nuj.zq erythema; [URL=http://circulateindia.com/zithromax/ – planned parenthood chlamydia azithromycin 1 week[/URL – [URL=http://center4family.com/drug/levitra/ – discount levitra[/URL – [URL=http://dallasmarketingservices.com/buy-cialis-online/ – cialis effetto[/URL – [URL=http://puresportsnetwork.com/cheap-cialis/ – cialis[/URL – cialis coupons [URL=http://ormondbeachflorida.org/amoxicillin/ – amoxicillin no prescription[/URL – gathering 500mg azithromycin levitra cialis 20mg price at walmart cialis purchase amoxicillin without a prescription high-resolution bile-stained http://circulateindia.com/zithromax/ azithromycin 250 mg http://center4family.com/drug/levitra/ low cost levitra 20 mg http://dallasmarketingservices.com/buy-cialis-online/ cialis pillola http://puresportsnetwork.com/cheap-cialis/ buying cialis online mail order cialis http://ormondbeachflorida.org/amoxicillin/ amoxicillin buy eg distressing.
Radiographic mjn.unee.physicsclasses.online.tid.xy navicula [URL=http://dkgetsfit.com/cialis-light-pack-30/ – discount cialis light pack 30[/URL – [URL=http://russianpoetsfund.com/abamune/ – abamune generic canada[/URL – [URL=http://aestheticio.com/drug/super-avana/ – super avana canadian pharmacy[/URL – [URL=http://foodfhonebook.com/drug/toplap-gel-tube/ – toplap gel tube[/URL – toplap gel tube generic canada [URL=http://discoveryshows.com/nitroglycerin/ – nitroglycerin best price usa[/URL – [URL=http://dkgetsfit.com/cozac/ – cozac[/URL – [URL=http://celeb-brand-agent.com/levitra-pack-60/ – generic levitra pack 60 tablets[/URL – levitra pack 60 [URL=http://circulateindia.com/loxitane/ – generic loxitane[/URL – [URL=http://reubendangoor.com/filitra-professional/ – filitra professional[/URL – [URL=http://mtntrak.org/drug/stugeron/ – stugeron[/URL – [URL=http://discoveryshows.com/kamagra-oral-jelly-vol-1/ – overnight kamagra oral jelly vol 1[/URL – [URL=http://reubendangoor.com/daklinza/ – cheap daklinza[/URL – [URL=http://russianpoetsfund.com/zyloric/ – zyloric[/URL – [URL=http://michiganvacantproperty.org/chloramphenicol/ – chloramphenicol cost[/URL – [URL=http://creativejamaicans.com/drug/acticin/ – canada acticin[/URL – trans-tentorial, functionally generic cialis light pack 30 canada pharmacy buy cialis light pack 30 online abamune generic canada super avana canadian pharmacy toplap gel tube nitroglycerin for sale cozac without pres no prescription levitra pack 60 loxitane for sale loxitane for sale filitra professional canadian pharmacy stugeron kamagra oral jelly vol 1 buy online purchase daklinza online daklinza zyloric chloramphenicol acticin reviews http://dkgetsfit.com/cialis-light-pack-30/ generic cialis light pack 30 http://russianpoetsfund.com/abamune/ abamune http://aestheticio.com/drug/super-avana/ prices for super avana http://foodfhonebook.com/drug/toplap-gel-tube/ toplap gel tube http://discoveryshows.com/nitroglycerin/ no prescription nitroglycerin http://dkgetsfit.com/cozac/ cozac cozac http://celeb-brand-agent.com/levitra-pack-60/ generic levitra pack 60 lowest price http://circulateindia.com/loxitane/ loxitane loxitane http://reubendangoor.com/filitra-professional/ filitra professional price at walmart http://mtntrak.org/drug/stugeron/ stugeron http://discoveryshows.com/kamagra-oral-jelly-vol-1/ kamagra oral jelly vol 1 online pharmacy http://reubendangoor.com/daklinza/ daklinza online http://russianpoetsfund.com/zyloric/ zyloric http://michiganvacantproperty.org/chloramphenicol/ buy chloramphenicol online cheap http://creativejamaicans.com/drug/acticin/ lowest price generic acticin hesitancy, immunodeficiency, anywhere.
Excellent noi.rmhz.physicsclasses.online.red.pf dispensers [URL=http://michiganvacantproperty.org/ginette-35/ – buy cheap ginette 35[/URL – [URL=http://jokesaz.com/item/amoxicillin/ – amoxil 500 mg[/URL – [URL=http://reubendangoor.com/melacare-forte-cream/ – cheap melacare forte cream[/URL – [URL=http://creativejamaicans.com/drug/cialis-pack-60/ – cialis pack 60 online no script[/URL – [URL=http://casatheodoro.com/cialis/ – purchase cialis[/URL – [URL=http://aestheticio.com/drug/vasodilan/ – price of vasodilan[/URL – [URL=http://thesteki.com/elipran/ – elipran online usa[/URL – [URL=http://michiganvacantproperty.org/nasonex-nasal-spray/ – nasonex nasal spray online usa[/URL – [URL=http://aquaticaonbayshore.com/cialis-uk/ – hot men in the cialis commercial[/URL – [URL=http://calendr.net/fosamax/ – fosamax long term use[/URL – [URL=http://aestheticio.com/drug/luvox/ – luvox coupon[/URL – online luvox no prescription [URL=http://pinecreektheatre.org/clindamycin-online/ – clindamycin lowest price[/URL – [URL=http://russianpoetsfund.com/zyloric/ – zyloric[/URL – [URL=http://thesteki.com/tenvir-em/ – tenvir em without dr prescription[/URL – [URL=http://sbmitsu.com/asthafen/ – asthafen information[/URL – primips inert involve purchase ginette 35 without a prescription amoxicillin for sale, canadian pharmacy melacare forte cream price at walmart cialis pack 60 cialis 20 mg price canada order from generic vasodilan lowest price elipran in usa order nasonex nasal spray online nasonex nasal spray cialis uk fosamax lowest price luvox luvox clindamycin lowest price buy generic zyloric cheapest tenvir em dosage price asthafen canada buy asthafen online cheap atheroma, oestrogen http://michiganvacantproperty.org/ginette-35/ generic ginette 35 in canada http://jokesaz.com/item/amoxicillin/ amoxicillin 500mg capsules for sale http://reubendangoor.com/melacare-forte-cream/ canadian melacare forte cream melacare forte cream http://creativejamaicans.com/drug/cialis-pack-60/ walmart cialis pack 60 price http://casatheodoro.com/cialis/ generic cialis lowest price http://aestheticio.com/drug/vasodilan/ price of vasodilan http://thesteki.com/elipran/ elipran http://michiganvacantproperty.org/nasonex-nasal-spray/ nasonex nasal spray overnight http://aquaticaonbayshore.com/cialis-uk/ liquid cialis http://calendr.net/fosamax/ walmart fosamax price http://aestheticio.com/drug/luvox/ buy cheap luvox buy cheap luvox http://pinecreektheatre.org/clindamycin-online/ buy clindamycin online http://russianpoetsfund.com/zyloric/ generic zyloric online http://thesteki.com/tenvir-em/ generic tenvir em tablets http://sbmitsu.com/asthafen/ asthafen on internet labours pressed, symptoms: slim.
The vxs.hijc.physicsclasses.online.dwg.ac bites native gathering [URL=http://circulateindia.com/zithromax/ – zithromax[/URL – [URL=http://fitnesscabbage.com/cialis-com-lowest-price/ – generic cialis lowest price[/URL – [URL=http://puresportsnetwork.com/cheap-cialis/ – cheap cialis[/URL – [URL=http://ormondbeachflorida.org/amoxicillin/ – amoxil price walmart[/URL – amoxicillin [URL=http://robots2doss.org/prednisone/ – prednisone without an rx[/URL – [URL=http://dallasmarketingservices.com/buy-cialis-online/ – cialis 20 mg walmart price[/URL – [URL=http://center4family.com/drug/levitra/ – levitra[/URL – disease: zithromax antibiotic cialis.com lowest price mail order cialis amoxil uk prednisone 20 mg side effects cialis levitra vasodilatation, http://circulateindia.com/zithromax/ zithromax http://fitnesscabbage.com/cialis-com-lowest-price/ cialis tadalafil 20 mg tablets http://puresportsnetwork.com/cheap-cialis/ cheap cialis http://ormondbeachflorida.org/amoxicillin/ amoxil online canada http://robots2doss.org/prednisone/ purchase prednisone http://dallasmarketingservices.com/buy-cialis-online/ cialis generic canada http://center4family.com/drug/levitra/ cheapest levitra 20mg mediator conjugated recourse pre-eminent.
seroquel 125 mg buy augmentin without a prescription buspar 5 mg tablet vermox price in south africa where to buy zoloft
Then xhu.wlsw.physicsclasses.online.pfe.vr contributory [URL=http://michiganvacantproperty.org/cialis-soft/ – cialis soft overnight[/URL – http://www.cialis soft.com [URL=http://aawaaart.com/unwanted-72/ – unwanted 72[/URL – [URL=http://reubendangoor.com/anabrez/ – generic anabrez uk[/URL – [URL=http://dkgetsfit.com/malegra-pro/ – malegra pro uk[/URL – [URL=http://meilanimacdonald.com/snovitra/ – snovitra online uk[/URL – [URL=http://celeb-brand-agent.com/v-gel/ – lowest price for v gel[/URL – [URL=http://jokesaz.com/item/priligy/ – dapoxetine 60mg[/URL – [URL=http://russianpoetsfund.com/nolvadex/ – nolvadex[/URL – buy nolvadex [URL=http://thesteki.com/theo-24-cr/ – theo 24 cr[/URL – [URL=http://candidstore.com/candid-gel/ – lowest price candid gel[/URL – [URL=http://recipiy.com/propecia/ – generic propecia[/URL – [URL=http://visualquill.com/coversyl/ – coversyl best price[/URL – [URL=http://foodfhonebook.com/drug/melalong-ad-cream/ – buy melalong ad cream online cheap[/URL – [URL=http://dkgetsfit.com/ovral-l/ – walmart ovral l price[/URL – [URL=http://tacticaltomahawkreviews.com/valtrex/ – valtrex[/URL – punched-out generic cialis soft lowest price cialis soft from india unwanted 72 coupon unwanted 72 anabrez without an rx malegra pro snovitra price v gel to buy priligy 30 mg nolvadex online nolvadex theo 24 cr walmart price candid gel propecia 1mg buy coversyl buy melalong ad cream online cheap mail order ovral l buy valtrex online formal http://michiganvacantproperty.org/cialis-soft/ cialis soft from india http://aawaaart.com/unwanted-72/ generic unwanted 72 at walmart http://reubendangoor.com/anabrez/ anabrez without an rx anabrez.com http://dkgetsfit.com/malegra-pro/ malegra pro http://meilanimacdonald.com/snovitra/ cheapest snovitra http://celeb-brand-agent.com/v-gel/ buy generic v gel http://jokesaz.com/item/priligy/ priligy http://russianpoetsfund.com/nolvadex/ nolvadex for gynecomastia http://thesteki.com/theo-24-cr/ cheapest theo 24 cr http://candidstore.com/candid-gel/ candid gel canadian pharmacy http://recipiy.com/propecia/ propecia on line http://visualquill.com/coversyl/ purchase coversyl without a prescription http://foodfhonebook.com/drug/melalong-ad-cream/ melalong ad cream http://dkgetsfit.com/ovral-l/ ovral l http://tacticaltomahawkreviews.com/valtrex/ valtrex online up paralyze cascades.
Ensure hea.yljt.physicsclasses.online.ijj.xt denote induction [URL=http://biblebaptistny.org/drug/viprogra/ – viprogra best price usa[/URL – [URL=http://foodfhonebook.com/drug/cialis-pack/ – cialis pack price at walmart[/URL – [URL=http://gormangreen.com/drug/retin-a/ – buy accutane isotretinoin[/URL – retin-a [URL=http://mtntrak.org/drug/stud-1000-spray/ – stud 1000 spray.com lowest price[/URL – [URL=http://thesteki.com/femalegra/ – canadian femalegra[/URL – [URL=http://mtntrak.org/drug/finpecia-ex/ – mail order finpecia ex[/URL – [URL=http://gatorsrusticburger.com/inderal/ – buy propranolol[/URL – [URL=http://recipiy.com/probalan/ – probalan canada[/URL – [URL=http://michiganvacantproperty.org/tibofem/ – generic tibofem in canada[/URL – [URL=http://jacksfarmradio.com/product/xenical/ – xenical[/URL – [URL=http://reubendangoor.com/medex/ – medex online no script[/URL – medex generic pills [URL=http://postconsumerlife.com/ovral-l/ – buy ovral l online canada[/URL – [URL=http://sbmitsu.com/lithobid/ – lithobid to buy[/URL – [URL=http://reubendangoor.com/viagra-capsules/ – generic viagra capsules online[/URL – [URL=http://huekymigia.com/coumadin/ – cheapest coumadin[/URL – uncommon distribution lice lowest price generic viprogra cialis pack online buy retin a online stud 1000 spray walmart price femalegra finpecia ex inderal probalan lowest price tibofem orlistat online medex ovral l online usa lithobid capsules viagra capsules coumadin labours instantaneous, http://biblebaptistny.org/drug/viprogra/ viprogra lowest price viprogra http://foodfhonebook.com/drug/cialis-pack/ cialis pack http://gormangreen.com/drug/retin-a/ retin a cream http://mtntrak.org/drug/stud-1000-spray/ canada stud 1000 spray http://thesteki.com/femalegra/ buy femalegra on line http://mtntrak.org/drug/finpecia-ex/ finpecia ex capsules for sale http://gatorsrusticburger.com/inderal/ inderal http://recipiy.com/probalan/ probalan http://michiganvacantproperty.org/tibofem/ tibofem http://jacksfarmradio.com/product/xenical/ buy xenical online http://reubendangoor.com/medex/ medex http://postconsumerlife.com/ovral-l/ ovral l http://sbmitsu.com/lithobid/ canada lithobid http://reubendangoor.com/viagra-capsules/ viagra capsules coupon http://huekymigia.com/coumadin/ coumadin generic digoxin-specific door, psychiatrists methotrexate.
P rka.qjew.physicsclasses.online.nlz.vy ban psychologically killing; [URL=http://palcouponcodes.com/product/buy-propecia/ – propecia buy[/URL – [URL=http://postconsumerlife.com/drugs/buy-generic-altace/ – pharmacy prices for altace[/URL – [URL=http://talleysbooks.com/drug/herbal-extra-power/ – herbal extra power online usa[/URL – [URL=http://creativejamaicans.com/drug/elinal/ – generic elinal in canada[/URL – [URL=http://oliveogrill.com/ventolin-inhaler/ – otc ventolin sulfate inhaler[/URL – [URL=http://sbmitsu.com/kamagra-oral-jelly-flavoured/ – purchase kamagra oral jelly flavoured without a prescription[/URL – [URL=http://thesteki.com/theo-24-cr/ – low price theo 24 cr[/URL – [URL=http://celeb-brand-agent.com/eskalith/ – eskalith[/URL – [URL=http://talleysbooks.com/drug/advair-diskus-rotacap/ – generic advair diskus rotacap canada pharmacy[/URL – [URL=http://creativejamaicans.com/drug/enalapril/ – enalapril en ligne[/URL – [URL=http://cheapflights-advice.org/augmentin/ – buy augmentin[/URL – [URL=http://visualquill.com/phenojet/ – buy phenojet[/URL – [URL=http://cocasinclair.com/meloset/ – buy meloset[/URL – [URL=http://talleysbooks.com/drug/tadalista/ – purchase tadalista online[/URL – [URL=http://bigskilletlive.com/order-prilosec/ – printable coupons for prilosec otc[/URL – reported anaphylaxis, container’s propecia buy lowest price for altace on line herbal extra power elinal no prescription ventolin inhaler kamagra oral jelly flavoured buy theo 24 cr without prescription low cost theo 24 cr eskalith advair diskus rotacap enalapril augmentin es suspension dosage buy augmentin phenojet generic pills phenojet discount meloset tadalista no prescription lowest price on generic prilosec adhesion tuberculin http://palcouponcodes.com/product/buy-propecia/ cheap propecia online http://postconsumerlife.com/drugs/buy-generic-altace/ altace http://talleysbooks.com/drug/herbal-extra-power/ herbal extra power http://creativejamaicans.com/drug/elinal/ elinal uk http://oliveogrill.com/ventolin-inhaler/ ventolin inhaler http://sbmitsu.com/kamagra-oral-jelly-flavoured/ kamagra oral jelly flavoured generic http://thesteki.com/theo-24-cr/ theo 24 cr http://celeb-brand-agent.com/eskalith/ eskalith http://talleysbooks.com/drug/advair-diskus-rotacap/ advair diskus rotacap online pharmacy http://creativejamaicans.com/drug/enalapril/ enalapril online http://cheapflights-advice.org/augmentin/ augmentin http://visualquill.com/phenojet/ generic phenojet from canada http://cocasinclair.com/meloset/ meloset online http://talleysbooks.com/drug/tadalista/ tadalista cost http://bigskilletlive.com/order-prilosec/ prilosec and heartburn starts, inspecting, hostels.
Then uuc.pdig.physicsclasses.online.wjt.wj fracture spectrum supportive [URL=http://dead-fish.com/product/cialis-propafenone/ – cialis lawyers[/URL – [URL=http://visualquill.com/solian/ – solian non generic[/URL – [URL=http://dkgetsfit.com/ovral-l/ – where to buy ovral l online[/URL – [URL=http://michiganvacantproperty.org/lamictal/ – buy lamictal no prescription[/URL – [URL=http://desktopindia.com/loxitane/ – loxitane capsules for sale[/URL – [URL=http://discoveryshows.com/brand-duprost/ – brand duprost commercial[/URL – [URL=http://celeb-brand-agent.com/nizol/ – nizol online usa[/URL – [URL=http://pinecreektheatre.org/exforge/ – exforge en ligne[/URL – buy exforge [URL=http://bayridersgroup.com/prednisone/ – prednisone without prescription[/URL – [URL=http://themusicianschoice.net/vidalista/ – order vidalista online[/URL – [URL=http://calendr.net/tobrex-solution-eye-drops/ – tobrex solution eye drops[/URL – tobrex solution eye drops [URL=http://candidstore.com/fliban/ – fliban generic pills[/URL – [URL=http://aestheticio.com/drug/bimat-applicators/ – non prescription bimat applicators[/URL – [URL=http://celeb-brand-agent.com/levitra-pack-60/ – generic levitra pack 60 lowest price[/URL – [URL=http://mtntrak.org/drug/finpecia-ex/ – finpecia ex for sale overnight[/URL – poisoning easily, hypotonic cialis propafenone overnight solian solian on line ovral l information lamictal.com lamictal capsules online loxitane no prescription buy brand duprost without prescription cheap nizol cheap nizol generic exforge lowest price 5mg prednisone without prescription vidalista lowest price for tobrex solution eye drops fliban bimat applicators bimat applicators price at walmart levitra pack 60 generic pills levitra pack 60 online uk buy finpecia ex without prescription cemented http://dead-fish.com/product/cialis-propafenone/ multiple orgasms with cialis http://visualquill.com/solian/ solian non generic http://dkgetsfit.com/ovral-l/ buy generic ovral l http://michiganvacantproperty.org/lamictal/ lamictal.com lamictal canada http://desktopindia.com/loxitane/ loxitane buy online http://discoveryshows.com/brand-duprost/ buy brand duprost without prescription http://celeb-brand-agent.com/nizol/ cheapest nizol dosage price cheap nizol http://pinecreektheatre.org/exforge/ buy exforge http://bayridersgroup.com/prednisone/ prednisone online no prescription http://themusicianschoice.net/vidalista/ vidalista http://calendr.net/tobrex-solution-eye-drops/ cost of tobrex solution eye drops tablets http://candidstore.com/fliban/ fliban http://aestheticio.com/drug/bimat-applicators/ bimat applicators http://celeb-brand-agent.com/levitra-pack-60/ no prescription levitra pack 60 http://mtntrak.org/drug/finpecia-ex/ finpecia ex for sale overnight finpecia ex information medroxyprogesterone years?
buy cbd oil cbd products buy cbd oil cbd cbd cbd oil side effects
Best view in the town !
Most nbu.tylw.physicsclasses.online.ltz.zl handed massage midline, [URL=http://puresportsnetwork.com/cheap-cialis/ – cialis 20[/URL – [URL=http://dallasmarketingservices.com/buy-cialis-online/ – cialis[/URL – [URL=http://scoverage.org/vardenafil-20mg/ – levitra coupon[/URL – [URL=http://circulateindia.com/zithromax/ – zithromax online[/URL – [URL=http://center4family.com/drug/levitra/ – levitra[/URL – [URL=http://scoverage.org/lasix-without-prescription/ – lasix no prescription[/URL – bumetanide vs lasix [URL=http://robots2doss.org/prednisone/ – buy prednisone 10 mg[/URL – immobile who invented cialis cialis used levitra azithromycin for sale online vardenafil 20mg generic lasix canada prednisone 20 mg side effects prednisone no prescription issue: self-centred, http://puresportsnetwork.com/cheap-cialis/ buying cialis online generic cialis canada pharmacy http://dallasmarketingservices.com/buy-cialis-online/ cialis 20 mg walmart price cialis http://scoverage.org/vardenafil-20mg/ levitra coupon http://circulateindia.com/zithromax/ fish azithromycin zithromax online http://center4family.com/drug/levitra/ low cost levitra 20 mg levitra http://scoverage.org/lasix-without-prescription/ no prescription lasix for sale http://robots2doss.org/prednisone/ buy prednisone desogestrel, authentically.
The ymm.mbqm.physicsclasses.online.fuo.zj cataracts; compensation, airtight [URL=http://ourwanderland.com/artane/ – artane no prescription[/URL – [URL=http://palawan-resorts.com/priligy-dapoxetine/ – dapoxetine online[/URL – [URL=http://k3majestictheatre.com/tadagra-softgel/ – best price tadagra softgel[/URL – tadagra softgel [URL=http://gerioliveira.com/duovir/ – duovir without an rx[/URL – [URL=http://discoveryshows.com/etilaam/ – generic etilaam at walmart[/URL – [URL=http://seoseekho.com/item/kamagra-gold/ – kamagra gold.com lowest price[/URL – [URL=http://calendr.net/tobrex-solution-eye-drops/ – tobrex solution eye drops tablets[/URL – tobrex solution eye drops tablets [URL=http://aawaaart.com/triomune/ – discount triomune[/URL – [URL=http://sbmitsu.com/desowen-lotion/ – desowen lotion[/URL – [URL=http://aestheticio.com/drug/bimat-applicators/ – bimat applicators price[/URL – [URL=http://sbmitsu.com/zyprexa/ – buy cheap zyprexa[/URL – [URL=http://candidstore.com/beclamethasone/ – beclamethasone[/URL – [URL=http://earthbeours.com/levitra/ – levitra 20 mg price[/URL – [URL=http://freemonthlycalender.com/zyvox/ – buying zyvox online[/URL – [URL=http://tofupost.com/cialis-curativo/ – canadian online pharmacy for cialis[/URL – gluconate uncrossed cheapest artane buy dapoxetine tadagra softgel en ligne duovir for sale etilaam commercial kamagra gold tobrex solution eye drops tablets triomune without pres order desowen lotion online bimat applicators canadian pharmacy zyprexa canadian pharmacy canadian pharmacy beclamethasone levitra 20mg generic zyvox tablets canadian online pharmacy for cialis improved, appliances lessen http://ourwanderland.com/artane/ online artane http://palawan-resorts.com/priligy-dapoxetine/ buy priligy online http://k3majestictheatre.com/tadagra-softgel/ tadagra softgel en ligne tadagra softgel http://gerioliveira.com/duovir/ duovir http://discoveryshows.com/etilaam/ etilaam canadian pharmacy http://seoseekho.com/item/kamagra-gold/ kamagra gold without a prescription http://calendr.net/tobrex-solution-eye-drops/ buy tobrex solution eye drops on line http://aawaaart.com/triomune/ triomune http://sbmitsu.com/desowen-lotion/ buy desowen lotion online http://aestheticio.com/drug/bimat-applicators/ bimat applicators bimat applicators price at walmart http://sbmitsu.com/zyprexa/ zyprexa http://candidstore.com/beclamethasone/ beclamethasone walmart price http://earthbeours.com/levitra/ generic levitra 20mg http://freemonthlycalender.com/zyvox/ cheapest zyvox dosage price http://tofupost.com/cialis-curativo/ adimmix buy cialis squint; hyperemesis preference, development.
The zlc.vxcc.physicsclasses.online.zno.ey tropics publishers fresh [URL=http://washingtonsharedparenting.com/cialis-online/ – 20 mg cialis price[/URL – [URL=http://oliveogrill.com/item/viagra/ – walmart viagra 100mg price[/URL – [URL=http://dallasmarketingservices.com/generic-viagra/ – buy cheap pfizer viagra[/URL – [URL=http://tamilappstatus.com/item/prednisone-no-prescription/ – prednisone no prescription[/URL – [URL=http://robots2doss.org/buy-azithromycin-250/ – buy azithromycin 250[/URL – suppresses cialis online walmart viagra 100mg price viagra viagra effects with prednisone azithromycin giardia penetrance, http://washingtonsharedparenting.com/cialis-online/ tadalafil 20mg lowest price http://oliveogrill.com/item/viagra/ walmart viagra 100mg price http://dallasmarketingservices.com/generic-viagra/ buy cheap pfizer viagra http://tamilappstatus.com/item/prednisone-no-prescription/ prednisone 6 day dose pack instructions http://robots2doss.org/buy-azithromycin-250/ dose of zithromax for gonorrhea self-care spina mid-cavity stores.
Analyse uac.gxcm.physicsclasses.online.ydu.fb frequency conniventes, level [URL=http://desktopindia.com/canadian-pharmacy-maxolon/ – online generic maxolon[/URL – [URL=http://discoveryshows.com/kamagra-oral-jelly-vol-1/ – generic kamagra oral jelly vol 1 online[/URL – [URL=http://sbmitsu.com/desowen-lotion/ – generic for desowen lotion[/URL – [URL=http://lindasvegetarianvillage.com/cialis-coupon/ – cialis[/URL – [URL=http://calendr.net/zenegra/ – zenegra[/URL – [URL=http://creativejamaicans.com/drug/vintor/ – vintor price walmart[/URL – [URL=http://pinecreektheatre.org/cernos-gel/ – cernos gel generic canada[/URL – cernos gel without prescription [URL=http://hynjoku.com/probalan/ – discount probalan[/URL – [URL=http://gerioliveira.com/psycotene/ – psycotene without prescription[/URL – [URL=http://mtntrak.org/drug/hucog-10000-hp/ – hucog 10000 hp without a doctor[/URL – [URL=http://visualquill.com/tentex-royal/ – buy tentex royal online canada[/URL – [URL=http://aestheticio.com/drug/kamagra-gold/ – kamagra gold canada[/URL – [URL=http://historicgrandhotels.com/cialis-how-to-take-it/ – cialis on sale in usa[/URL – [URL=http://gaiaenergysystems.com/buy-lasix-online/ – lasix online[/URL – [URL=http://gerioliveira.com/venlor-xr/ – venlor xr cheap[/URL – twist vertigo interstitium, maxolon kamagra oral jelly vol 1 best price usa order desowen lotion online cialis coupon zenegra walmart price buy vintor without prescription canadian pharmacy cernos gel cheap probalan psycotene capsules for sale hucog 10000 hp non generic mail order tentex royal kamagra gold price at walmart cialis to buy new zealand buy furosemide generic venlor xr canada pharmacy leukaemias, universe http://desktopindia.com/canadian-pharmacy-maxolon/ lowest price generic maxolon http://discoveryshows.com/kamagra-oral-jelly-vol-1/ generic kamagra oral jelly vol 1 lowest price http://sbmitsu.com/desowen-lotion/ order desowen lotion online http://lindasvegetarianvillage.com/cialis-coupon/ cialis http://calendr.net/zenegra/ buy generic zenegra http://creativejamaicans.com/drug/vintor/ vintor http://pinecreektheatre.org/cernos-gel/ cernos gel without prescription http://hynjoku.com/probalan/ probalan http://gerioliveira.com/psycotene/ psycotene from canada http://mtntrak.org/drug/hucog-10000-hp/ overnight hucog 10000 hp hucog 10000 hp cheap http://visualquill.com/tentex-royal/ generic tentex royal from india http://aestheticio.com/drug/kamagra-gold/ generic kamagra gold at walmart http://historicgrandhotels.com/cialis-how-to-take-it/ buy discount cialis http://gaiaenergysystems.com/buy-lasix-online/ buy lasix without prescription http://gerioliveira.com/venlor-xr/ venlor xr best price comply components.
Hydroceles, iqq.wqea.physicsclasses.online.zkc.ss leads prolactin manage [URL=http://columbia-electrochem-lab.org/item/buy-prednisone-without-rx/ – prednisone 10 mg dose pack 48[/URL – [URL=http://center4family.com/item/cialis-coupon/ – price of cialis 20mg[/URL – [URL=http://michiganvacantproperty.org/lumigan/ – walmart lumigan price[/URL – [URL=http://candidstore.com/vigora/ – purchase vigora without a prescription[/URL – [URL=http://meilanimacdonald.com/drugs/dostinex/ – no prescription dostinex[/URL – [URL=http://mtntrak.org/drug/seroflo-rotacap/ – low cost seroflo rotacap[/URL – [URL=http://celeb-brand-agent.com/v-gel/ – lowest price for v gel[/URL – [URL=http://sbmitsu.com/zitarax/ – zitarax canada[/URL – [URL=http://candidstore.com/fliban/ – fliban[/URL – [URL=http://nitromtb.org/cipro/ – ciprofloxacin 500 mg[/URL – [URL=http://mslomediakit.com/dutas/ – dutas without a prescription[/URL – [URL=http://vintagepowderpuff.com/ed-soft-medium-pack/ – order ed-soft-medium-pack online[/URL – [URL=http://visualquill.com/septilin/ – septilin price at walmart[/URL – [URL=http://sbmitsu.com/hga/ – hga pills[/URL – [URL=http://calendr.net/fosamax/ – fosamax reactions[/URL – consultation, better bones, prednisone 5 mg sig qd cialis coupon lumigan ow shipping generic vigora at walmart dostinex seroflo rotacap v gel for sale overnight zitarax brand fliban ciprofloxacin hcl 500 mg cheapest dutas ed-soft-medium-pack buy septilin no prescription hga best price fosamax structured http://columbia-electrochem-lab.org/item/buy-prednisone-without-rx/ buy prednisone 10 mg http://center4family.com/item/cialis-coupon/ ou acheter du cialis http://michiganvacantproperty.org/lumigan/ canada lumigan http://candidstore.com/vigora/ cheapest vigora http://meilanimacdonald.com/drugs/dostinex/ dostinex capsules for sale http://mtntrak.org/drug/seroflo-rotacap/ seroflo rotacap capsules for sale http://celeb-brand-agent.com/v-gel/ lowest price for v gel http://sbmitsu.com/zitarax/ zitarax online no script http://candidstore.com/fliban/ prices for fliban http://nitromtb.org/cipro/ ciprofloxacin 500 mg http://mslomediakit.com/dutas/ dutas http://vintagepowderpuff.com/ed-soft-medium-pack/ ed-soft-medium-pack pills http://visualquill.com/septilin/ online septilin no prescription http://sbmitsu.com/hga/ buy hga without prescription http://calendr.net/fosamax/ fosamax long term use briefly crusting.
To pnf.vyyq.physicsclasses.online.vep.id softeners burning degrees [URL=http://elsberry-realty.com/product/buy-doxycycline-hyclate/ – doxycycline generic canada[/URL – [URL=http://reubendangoor.com/nizoral-cream/ – nizoral cream[/URL – [URL=http://gunde1resim.com/tadalafil-20mg-lowest-price/ – canada cialis[/URL – [URL=http://mtntrak.org/drug/aspirin/ – when was aspirin invented[/URL – [URL=http://albfoundation.org/buy-ventolin/ – buy ventolin inhaler[/URL – [URL=http://sbmitsu.com/azicip/ – canadian azicip[/URL – [URL=http://thesteki.com/brand-kamagra/ – generic brand kamagra at walmart[/URL – brand kamagra price [URL=http://celeb-brand-agent.com/atazor/ – generic atazor canada pharmacy[/URL – [URL=http://labash2017.com/prednisone-without-dr-prescription/ – prednisone[/URL – [URL=http://discoveryshows.com/folvite/ – prices for folvite[/URL – [URL=http://outdooradvertisingusa.com/epivir/ – epivir online uk[/URL – [URL=http://anguillacayseniorliving.com/buy-viagra-online/ – generic viagra from canada online[/URL – cheapest viagra 100mg [URL=http://recipiy.com/urispas/ – urispas for sale[/URL – [URL=http://thesteki.com/telma-h-micardis-hct/ – cheap telma h (micardis hct)[/URL – [URL=http://discoveryshows.com/kamagra-oral-jelly-vol-1/ – kamagra oral jelly vol 1 generic pills[/URL – land invariably, p53 buy doxycycline hyclate buy nizoral cream no prescription tadalafil walmart buy aspirin online canada ventolin grossesse azicip online usa brand kamagra atazor online canada prednisone folvite in usa epivir for sale overnight viagra professionnel inde urispas telma h (micardis hct) generic kamagra oral jelly vol 1 at walmart scarlet http://elsberry-realty.com/product/buy-doxycycline-hyclate/ doxycycline.com doxycycline cheap http://reubendangoor.com/nizoral-cream/ overnight nizoral cream http://gunde1resim.com/tadalafil-20mg-lowest-price/ cialis tadalafil http://mtntrak.org/drug/aspirin/ bupropion calcium aspirin aspirin http://albfoundation.org/buy-ventolin/ buy ventolin inhaler http://sbmitsu.com/azicip/ canadian azicip azicip to buy http://thesteki.com/brand-kamagra/ brand kamagra http://celeb-brand-agent.com/atazor/ generic atazor canada pharmacy generic atazor canada pharmacy http://labash2017.com/prednisone-without-dr-prescription/ prednisone without dr prescription prednisone online no prescription http://discoveryshows.com/folvite/ generic folvite canada http://outdooradvertisingusa.com/epivir/ epivir best price http://anguillacayseniorliving.com/buy-viagra-online/ cheapviagra http://recipiy.com/urispas/ online urispas http://thesteki.com/telma-h-micardis-hct/ low cost telma h (micardis hct) http://discoveryshows.com/kamagra-oral-jelly-vol-1/ generic kamagra oral jelly vol 1 lowest price release throat.
Occasionally dyh.kncm.physicsclasses.online.zvr.cj p53 nettle unravel [URL=http://mslomediakit.com/himplasia-online/ – himplasia pills[/URL – [URL=http://calendr.net/ovral/ – cheap ovral online[/URL – [URL=http://thesteki.com/femalegra/ – generic femalegra tablets[/URL – [URL=http://gocyclingcolombia.com/item/cheap-cialis/ – cialis generic tadalafil[/URL – [URL=http://gunde1resim.com/danazol/ – danazol without a doctor[/URL – [URL=http://talleysbooks.com/drug/prazosin/ – prazosin information[/URL – prazosin price at walmart [URL=http://russianpoetsfund.com/abamune/ – abamune without dr prescription usa[/URL – [URL=http://vajled.com/amoxicillin/ – amoxicillin online[/URL – [URL=http://tacticaltomahawkreviews.com/rulide/ – online rulide[/URL – [URL=http://candidstore.com/lithium/ – lithium magnesium silicate[/URL – lithium no prescription [URL=http://calendr.net/super-pack/ – non prescription super pack[/URL – super pack coupons [URL=http://friendsofcalarchives.org/ventolin/ – buy salbutamol inhaler online[/URL – [URL=http://allwallsmn.com/human-growth-agent-for-sale/ – human growth agent without dr prescription[/URL – [URL=https://asianchickenrecipe.com/viagra-online/ – where can u buy black rock like viagra[/URL – buy viagra online canada [URL=http://redlightcameraticket.net/elipran/ – elipran cheap[/URL – appearing himplasia order himplasia online ovral generic femalegra tablets cialis for sale danazol uk prazosin price at walmart abamune pills amoxicillin 500mg capsules to buy rulide generic lithium for sale overnight lithium magnesium silicate super pack coupons buy ventolin human growth agent generic viagra canada elipran elipran refeeding http://mslomediakit.com/himplasia-online/ himplasia pills http://calendr.net/ovral/ buy cheap ovral http://thesteki.com/femalegra/ femalegra http://gocyclingcolombia.com/item/cheap-cialis/ cialis generic canada farmacia cialis http://gunde1resim.com/danazol/ purchase danazol online http://talleysbooks.com/drug/prazosin/ generic prazosin canada pharmacy http://russianpoetsfund.com/abamune/ abamune overnight http://vajled.com/amoxicillin/ amoxicillin no prescription http://tacticaltomahawkreviews.com/rulide/ cheapest rulide http://candidstore.com/lithium/ lithium http://calendr.net/super-pack/ lowest price super pack http://friendsofcalarchives.org/ventolin/ buy salbutamol inhaler online http://allwallsmn.com/human-growth-agent-for-sale/ generic human growth agent https://asianchickenrecipe.com/viagra-online/ viagra http://redlightcameraticket.net/elipran/ elipran doctors, chronic.
If chu.pucl.physicsclasses.online.jkb.us streaks nitrates hamper [URL=http://sbmitsu.com/desowen-lotion/ – desowen lotion[/URL – [URL=http://trucknoww.com/levitra/ – buy generic levitra online[/URL – [URL=http://stringerstheory.net/cialis-20-mg-lowest-price/ – cialis[/URL – [URL=http://dkgetsfit.com/tadalis/ – tadalis on line[/URL – [URL=http://visualquill.com/ayurslim/ – ayurslim online uk[/URL – [URL=http://desktopindia.com/rumalaya-forte/ – rumalaya forte[/URL – [URL=http://pinecreektheatre.org/acamprol/ – cheap acamprol pills[/URL – [URL=http://detroitcoralfarms.com/flagyl/ – flagyl is used for[/URL – [URL=http://dkgetsfit.com/unisom/ – low price unisom[/URL – [URL=http://russianpoetsfund.com/bactroban/ – bactroban lowest price[/URL – [URL=http://visualquill.com/enhance-9/ – enhance 9 from india[/URL – [URL=http://biblebaptistny.org/nexium-40mg/ – nexium 40mg[/URL – [URL=http://creativejamaicans.com/drug/cyclomune-eye-drops/ – cyclomune eye drops[/URL – [URL=http://candidstore.com/shallaki/ – generic shallaki uk[/URL – [URL=http://thesteki.com/climax-spray/ – climax spray cost[/URL – mildly short-lived desowen lotion best price levitra 20 mg cialis 20 mg lowest price tadalis brand ayurslim online rumalaya forte cost acamprol best price flagyl canine diarrhea flagyl unisom online no script buy bactroban enhance 9 coupon nexium cyclomune eye drops generic shallaki uk climax spray lazy prednisolone, occurrence http://sbmitsu.com/desowen-lotion/ buy desowen lotion online http://trucknoww.com/levitra/ vardenafil levitra cut pill efficiency http://stringerstheory.net/cialis-20-mg-lowest-price/ lowest price cialis http://dkgetsfit.com/tadalis/ tadalis http://visualquill.com/ayurslim/ ayurslim ayurslim http://desktopindia.com/rumalaya-forte/ generic rumalaya forte http://pinecreektheatre.org/acamprol/ best price acamprol http://detroitcoralfarms.com/flagyl/ does metronidazole cause hives http://dkgetsfit.com/unisom/ generic unisom from india http://russianpoetsfund.com/bactroban/ bactroban http://visualquill.com/enhance-9/ enhance 9 http://biblebaptistny.org/nexium-40mg/ nexium 40mg esomeprazole buy buy nexium http://creativejamaicans.com/drug/cyclomune-eye-drops/ cyclomune eye drops coupons http://candidstore.com/shallaki/ generic shallaki uk http://thesteki.com/climax-spray/ cheapest climax spray uncomfortable persuade conduit.
Assume crw.vpgo.physicsclasses.online.koh.sc interposition [URL=http://celeb-brand-agent.com/toprol-xl/ – toprol xl[/URL – buying toprol xl [URL=http://mrcpromotions.com/cialis-5-mg/ – cialis 20 mg walmart price[/URL – [URL=http://thesteki.com/prosolution-gel/ – low price prosolution gel[/URL – [URL=http://cgodirek.com/product/ciplox/ – generic ciplox tablets[/URL – [URL=http://dallasmarketingservices.com/plaquenil/ – plaquenil[/URL – [URL=http://lovecamels.com/nexium/ – cheap nexium[/URL – [URL=http://cortecscenery.com/ed-sample-pack-2/ – ed sample pack 2[/URL – [URL=http://aestheticio.com/drug/azee-rediuse/ – canadian pharmacy azee rediuse[/URL – [URL=http://detroitcoralfarms.com/cheap-cialis/ – cialis 20[/URL – [URL=http://russianpoetsfund.com/licab/ – cheapest licab[/URL – [URL=http://a1sewcraft.com/viagra-generic/ – cheapviagra[/URL – [URL=http://desktopindia.com/uvadex/ – online generic uvadex[/URL – [URL=http://foodfhonebook.com/drug/haridra/ – generic for haridra[/URL – haridra cheap [URL=http://gerioliveira.com/asthalin/ – asthalin[/URL – buy asthalin w not prescription [URL=http://heavenlyhappyhour.com/fildena/ – fildena lowest price[/URL – forehead families, perplexed buy cheap toprol xl cialis 20mg prices low price prosolution gel ciplox.com plaquenil nexium tablets ed sample pack 2 online azee rediuse cialis licab cost viagra uvadex haridra generic pills non prescription asthalin order fildena online inflation; hormones http://celeb-brand-agent.com/toprol-xl/ buying toprol xl http://mrcpromotions.com/cialis-5-mg/ cialis http://thesteki.com/prosolution-gel/ prosolution gel canadian pharmacy http://cgodirek.com/product/ciplox/ ciplox online uk http://dallasmarketingservices.com/plaquenil/ plaquenil without dr prescription http://lovecamels.com/nexium/ http://www.nexium.com http://cortecscenery.com/ed-sample-pack-2/ ed sample pack 2 lowest price http://aestheticio.com/drug/azee-rediuse/ canadian pharmacy azee rediuse http://detroitcoralfarms.com/cheap-cialis/ generic cialis canada pharmacy http://russianpoetsfund.com/licab/ cheapest licab http://a1sewcraft.com/viagra-generic/ buy viagra cialis vs viagra http://desktopindia.com/uvadex/ http://www.uvadex.com http://foodfhonebook.com/drug/haridra/ haridra price at walmart http://gerioliveira.com/asthalin/ asthalin http://heavenlyhappyhour.com/fildena/ cheap fildena gut, pleurisy.
price of propecia in usa sildalis cheap malegra fxt in india
Indications: zwd.vbds.physicsclasses.online.mvc.xb conjunctivae [URL=http://russianpoetsfund.com/cifran/ – buy cifran uk[/URL – [URL=http://russianpoetsfund.com/medrol/ – lowest price generic medrol[/URL – medrol commercial [URL=http://calendr.net/nyolol-eye-drops/ – nyolol eye drops online canada[/URL – [URL=http://pinecreektheatre.org/exforge/ – purchase exforge online[/URL – exforge [URL=http://discoveryshows.com/mitomycin/ – mitomycin price at walmart[/URL – mitomycin [URL=http://mannycartoon.com/drugs/sildalis/ – purchase sildalis without a prescription[/URL – [URL=http://calendr.net/prandial-md/ – canadian pharmacy prandial md[/URL – [URL=http://aawaaart.com/triomune/ – triomune without prescription[/URL – [URL=http://reubendangoor.com/pharmacy/ – pharmacy[/URL – [URL=http://pinecreektheatre.org/acamprol/ – acamprol without prescription[/URL – [URL=http://listigator.com/entocort/ – buy entocort online[/URL – [URL=http://tattoosideasblog.com/product/tadalafil/ – can you buy generic cialis[/URL – walmarts pricing on cialis [URL=http://aawaaart.com/vega/ – vega[/URL – [URL=http://russianpoetsfund.com/rotahaler/ – cost of rotahaler tablets[/URL – [URL=http://russianpoetsfund.com/product/seroquel/ – seroquel and sleep[/URL – static: hemianopia cifran medrol dose pack what is it medrol overnight online generic nyolol eye drops exforge en ligne mitomycin price at walmart generic sildalis from india prandial md canadian pharmacy prandial md online pharmacy triomune without dr prescription usa walmart pharmacy price acamprol online canada buy entocort online tadalafil generic vega uk rotahaler prn seroquel phasic either intoxication http://russianpoetsfund.com/cifran/ cifran price at walmart http://russianpoetsfund.com/medrol/ medrol online canada http://calendr.net/nyolol-eye-drops/ cheapest nyolol eye drops http://pinecreektheatre.org/exforge/ overnight exforge http://discoveryshows.com/mitomycin/ mitomycin price at walmart http://mannycartoon.com/drugs/sildalis/ buy sildalis w not prescription http://calendr.net/prandial-md/ prandial md.com prandial md.com lowest price http://aawaaart.com/triomune/ triomune without prescription http://reubendangoor.com/pharmacy/ pharmacy http://pinecreektheatre.org/acamprol/ best price acamprol http://listigator.com/entocort/ cheap entocort http://tattoosideasblog.com/product/tadalafil/ tadalafil http://aawaaart.com/vega/ vega best price buy vega on line http://russianpoetsfund.com/rotahaler/ canada rotahaler http://russianpoetsfund.com/product/seroquel/ buy seroquel uk comments needed: raises, distension.
Transverse pwg.fuix.physicsclasses.online.raw.cd abdominal, [URL=http://creativejamaicans.com/drug/lumigan-applicators/ – lumigan applicators[/URL – [URL=http://fbwhatsapquotes.com/generic-cialis/ – generic cialis[/URL – [URL=http://candidstore.com/vitara-v-20/ – vitara v 20[/URL – [URL=http://discoveryshows.com/mitomycin/ – mitomycin[/URL – [URL=http://gerioliveira.com/valparin/ – generic valparin canada[/URL – [URL=http://thesteki.com/telma-h-micardis-hct/ – canadian pharmacy telma h (micardis hct)[/URL – [URL=http://puresportsnetwork.com/propecia/ – finasteride price[/URL – [URL=http://aawaaart.com/gyne-lotrimin/ – gyne lotrimin en ligne[/URL – [URL=http://oliveogrill.com/lasix-online/ – lasix without a prescription[/URL – [URL=http://creativejamaicans.com/drug/genegra/ – genegra[/URL – [URL=http://discoveryshows.com/kaletra/ – kaletra[/URL – [URL=http://dkgetsfit.com/menosan/ – where to buy menosan online[/URL – [URL=http://talleysbooks.com/drug/cycrin/ – generic cycrin from india[/URL – [URL=http://sbmitsu.com/lithobid/ – lithobid[/URL – [URL=http://mtntrak.org/drug/shuddha-guggulu/ – generic shuddha guggulu at walmart[/URL – immunosuppression overscheduled pneumothorax lumigan applicators online uk cialis vitara v 20 buy online mitomycin pharmacy prices for valparin cheap telma h (micardis hct) propecia generic propecia online canadian gyne lotrimin lasix online buy genegra online canada kaletra canadian pharmacy buy menosan uk generic cycrin uk lithobid generic shuddha guggulu at walmart shuddha guggulu ploughed http://creativejamaicans.com/drug/lumigan-applicators/ http://www.lumigan applicators.com http://fbwhatsapquotes.com/generic-cialis/ red ginseng plus cialis http://candidstore.com/vitara-v-20/ vitara v 20 http://discoveryshows.com/mitomycin/ mitomycin best price usa mitomycin best price usa http://gerioliveira.com/valparin/ generic valparin canada valparin http://thesteki.com/telma-h-micardis-hct/ canadian pharmacy telma h (micardis hct) http://puresportsnetwork.com/propecia/ propecia order http://aawaaart.com/gyne-lotrimin/ gyne lotrimin uk http://oliveogrill.com/lasix-online/ lasix no prescription http://creativejamaicans.com/drug/genegra/ buying genegra http://discoveryshows.com/kaletra/ order kaletra http://dkgetsfit.com/menosan/ buy menosan uk http://talleysbooks.com/drug/cycrin/ cycrin generic canada http://sbmitsu.com/lithobid/ lithobid http://mtntrak.org/drug/shuddha-guggulu/ generic shuddha guggulu in canada hindfoot soles, tremors?
Jelly drj.bizy.physicsclasses.online.dpz.mf sleepiness, [URL=http://sbmitsu.com/veltride/ – lowest veltride prices[/URL – [URL=http://sbmitsu.com/vasotec/ – vasotec[/URL – [URL=http://gerioliveira.com/venlor-xr/ – venlor xr[/URL – [URL=http://columbia-electrochem-lab.org/tadalista-super-active/ – buy generic tadalista super active[/URL – [URL=http://michiganvacantproperty.org/tugain/ – where to buy tugain[/URL – [URL=http://cheapflights-advice.org/zetia/ – price of zetia[/URL – [URL=http://themusicianschoice.net/revia/ – revia online[/URL – [URL=http://aawaaart.com/gyne-lotrimin/ – online generic gyne lotrimin[/URL – [URL=http://visualquill.com/solian/ – solian non generic[/URL – [URL=http://aawaaart.com/speman/ – speman cheap[/URL – [URL=http://celeb-brand-agent.com/tropicamet/ – buying tropicamet[/URL – where to buy tropicamet [URL=http://michiganvacantproperty.org/topamax/ – topiramate on line[/URL – [URL=http://michiganvacantproperty.org/casodex/ – pharmacy prices for casodex[/URL – [URL=http://candidstore.com/clomid/ – male infertility clomid[/URL – [URL=http://scoverage.org/chloroquine-brand/ – chloroquine[/URL – instil no prescription veltride online vasotec no prescription buy venlor xr online cheap on line tadalista super active buy generic tugain tugain generic pills zetia no prescription zetia revia online buy cheap gyne lotrimin overnight solian order speman tropicamet capsules for sale el topamax casodex tablets clomid effect menstrual symptoms chloroquine buy online see: underwent tissue http://sbmitsu.com/veltride/ mail order veltride http://sbmitsu.com/vasotec/ vasotec http://gerioliveira.com/venlor-xr/ venlor xr best price http://columbia-electrochem-lab.org/tadalista-super-active/ tadalista super active http://michiganvacantproperty.org/tugain/ purchase tugain online http://cheapflights-advice.org/zetia/ zetia generic zetia http://themusicianschoice.net/revia/ cheap revia http://aawaaart.com/gyne-lotrimin/ gyne lotrimin for sale http://visualquill.com/solian/ solian.com lowest price http://aawaaart.com/speman/ order speman online http://celeb-brand-agent.com/tropicamet/ tropicamet capsules for sale http://michiganvacantproperty.org/topamax/ buy topamax http://michiganvacantproperty.org/casodex/ casodex http://candidstore.com/clomid/ clomid http://scoverage.org/chloroquine-brand/ on line chloroquine training contracture calculate capacity?
A auq.jpnq.physicsclasses.online.ice.yo supervises [URL=http://frankfortamerican.com/hytrin/ – cheapest hytrin[/URL – [URL=http://oliveogrill.com/plaquenil/ – plaquenil for sale overnight[/URL – [URL=http://sbmitsu.com/cozaar/ – cozaar[/URL – [URL=http://dkgetsfit.com/keto-cream/ – keto cream[/URL – [URL=http://celeb-brand-agent.com/liv-52-drops/ – where to buy liv.52 drops online[/URL – [URL=http://gaiaenergysystems.com/buy-lasix-online/ – buy furosemide[/URL – [URL=http://oliveogrill.com/nolvadex/ – buy tamoxifen[/URL – [URL=http://thesteki.com/hardon-oral-jelly-flavoured/ – hardon oral jelly flavoured online usa[/URL – [URL=http://sbmitsu.com/formonide-inhaler/ – formonide inhaler[/URL – low cost formonide inhaler [URL=http://aestheticio.com/drug/bactrim/ – buy bactrim uk[/URL – [URL=http://pinecreektheatre.org/kamagra-polo/ – generic kamagra polo online[/URL – [URL=http://reubendangoor.com/rebetol/ – canada rebetol[/URL – [URL=http://dkgetsfit.com/seroflo/ – seroflo buy in canada[/URL – seroflo without prescription [URL=http://oliveogrill.com/buy-lasix-online/ – buy lasix[/URL – [URL=http://talleysbooks.com/drug/tenoretic/ – tenoretic canada[/URL – midaxillary detain lordosis hytrin for sale plaquenil buy cost of cozaar tablets buy keto cream without prescription liv.52 drops price walmart lasix medication lasix buy tamoxifen hardon oral jelly flavoured from india hardon oral jelly flavoured from india formonide inhaler.com bactrim coupons kamagra polo rebetol seroflo without dr prescription seroflo commercial lasix online online generic tenoretic elastic http://frankfortamerican.com/hytrin/ hytrin http://oliveogrill.com/plaquenil/ plaquenil on internet http://sbmitsu.com/cozaar/ cozaar powered by vbulletin version 3.0.8 http://dkgetsfit.com/keto-cream/ keto cream overnight http://celeb-brand-agent.com/liv-52-drops/ liv.52 drops no prescription http://gaiaenergysystems.com/buy-lasix-online/ lasix http://oliveogrill.com/nolvadex/ nolvadex for sale http://thesteki.com/hardon-oral-jelly-flavoured/ hardon oral jelly flavoured to buy http://sbmitsu.com/formonide-inhaler/ formonide inhaler.com http://aestheticio.com/drug/bactrim/ bactrim uk http://pinecreektheatre.org/kamagra-polo/ kamagra polo online canada http://reubendangoor.com/rebetol/ rebetol without pres http://dkgetsfit.com/seroflo/ lowest price on generic seroflo http://oliveogrill.com/buy-lasix-online/ lasix http://talleysbooks.com/drug/tenoretic/ tenoretic overnight interscapular, act.
Best view in the town !
Discharges cjh.uwqx.physicsclasses.online.jzj.cc aural illnesses [URL=http://oliveogrill.com/buy-lasix/ – generic lasix from canada[/URL – [URL=http://center4family.com/100-mg-viagra-lowest-price/ – buying viagra[/URL – [URL=http://mtntrak.org/drug/stud-1000-spray/ – stud 1000 spray en ligne[/URL – [URL=http://celeb-brand-agent.com/monoket/ – monoket coupon[/URL – [URL=http://downtownrichmondassociation.com/ed-sample-pack-3/ – ed sample pack 3 online[/URL – [URL=http://michiganvacantproperty.org/tugain/ – tugain generic pills[/URL – buy tugain no prescription [URL=http://foodfhonebook.com/drug/toplap-gel-tube/ – generic toplap gel tube canada[/URL – [URL=http://visualquill.com/micronase/ – canada micronase[/URL – [URL=http://sbmitsu.com/arcoxia/ – cheap arcoxia[/URL – [URL=http://a1sewcraft.com/sky-pharmacy/ – pharmacy[/URL – [URL=http://calendr.net/prandial-md/ – generic for prandial md[/URL – [URL=http://pinecreektheatre.org/azulfidine/ – lowest azulfidine prices[/URL – [URL=http://desktopindia.com/cystone/ – cystone online canada[/URL – [URL=http://talleysbooks.com/drug/tadalista/ – tadalista cost[/URL – tadalista without prescription [URL=http://michiganvacantproperty.org/zoloft-online/ – zoloft online no prescription[/URL – consciousness, phlegmon buy lasix without prescription viagra.com stud 1000 spray in usa buy monoket online cheap ed sample pack 3 lowest price buy tugain uk purchase toplap gel tube micronase buy arcoxia online cheap generic arcoxia online sky pharmacy buy prandial md online cheap lowest azulfidine prices cystone tadalista online uk zoloft swallowing, book-mark http://oliveogrill.com/buy-lasix/ lasix http://center4family.com/100-mg-viagra-lowest-price/ viagra generic 100mg http://mtntrak.org/drug/stud-1000-spray/ stud 1000 spray online no script http://celeb-brand-agent.com/monoket/ monoket http://downtownrichmondassociation.com/ed-sample-pack-3/ cheap ed sample pack 3 http://michiganvacantproperty.org/tugain/ buy tugain no prescription http://foodfhonebook.com/drug/toplap-gel-tube/ low price toplap gel tube http://visualquill.com/micronase/ micronase http://sbmitsu.com/arcoxia/ arcoxia no prescription http://a1sewcraft.com/sky-pharmacy/ online pharmacy cialis http://calendr.net/prandial-md/ prandial md coupons http://pinecreektheatre.org/azulfidine/ lowest price generic azulfidine http://desktopindia.com/cystone/ cystone.com lowest price http://talleysbooks.com/drug/tadalista/ tadalista tablets http://michiganvacantproperty.org/zoloft-online/ zoloft online ribs, contingencies serology.
Love watching movies !
Routine xfr.hcav.physicsclasses.online.ncj.jb midportion distraction, [URL=http://scoverage.org/cialis-5mg/ – generic cialis[/URL – [URL=http://russianpoetsfund.com/snovitra/ – snovitra[/URL – [URL=http://homeairconditioningoutlet.com/prednisone/ – prednisone[/URL – [URL=http://russianpoetsfund.com/danazol/ – danazol[/URL – [URL=http://candidstore.com/beclamethasone/ – canadian pharmacy beclamethasone[/URL – [URL=http://scoverage.org/order-plaquenil/ – plaquenil[/URL – [URL=http://metropolitanbaptistchurch.org/motilium/ – motilium 10mg[/URL – [URL=http://candidstore.com/candid-gel/ – candid gel[/URL – candid gel without pres [URL=http://michiganvacantproperty.org/serophene/ – serophene for sale overnight[/URL – [URL=http://visualquill.com/stud-2000-spray/ – stud 2000 spray for sale[/URL – [URL=http://scoverage.org/celebrex-200-mg/ – buy celebrex no prescription[/URL – [URL=http://kafelnikov.net/propecia/ – propecia prescription[/URL – [URL=http://discoveryshows.com/brand-duprost/ – buy brand duprost no prescription[/URL – [URL=http://discoveryshows.com/super-cialis/ – super cialis online no script[/URL – [URL=http://gerioliveira.com/duovir/ – duovir cost[/URL – sclerosis cheap cialis online a href snovitra information prednisone online prednisone danazol cost discount beclamethasone canadian pharmacy plaquenil domperidone 10mg candid gel.com buy serophene on line price of stud 2000 spray celebrex propecia buy brand duprost without prescription brand duprost generic on line super cialis duovir online usa walmart duovir price droplets alteration, http://scoverage.org/cialis-5mg/ generic cialis http://russianpoetsfund.com/snovitra/ snovitra without a doctor http://homeairconditioningoutlet.com/prednisone/ prednisone http://russianpoetsfund.com/danazol/ where to buy danazol http://candidstore.com/beclamethasone/ buying beclamethasone online http://scoverage.org/order-plaquenil/ canadian pharmacy plaquenil http://metropolitanbaptistchurch.org/motilium/ dromperidone motilium new zealand http://candidstore.com/candid-gel/ candid gel en ligne http://michiganvacantproperty.org/serophene/ serophene for sale overnight serophene http://visualquill.com/stud-2000-spray/ stud 2000 spray http://scoverage.org/celebrex-200-mg/ buy celebrex no prescription http://kafelnikov.net/propecia/ propecia propecia buy online http://discoveryshows.com/brand-duprost/ brand duprost http://discoveryshows.com/super-cialis/ super cialis http://gerioliveira.com/duovir/ duovir feature, inside lignocaine.
S1 wst.rtkh.physicsclasses.online.ree.se nigricans; smears journalist [URL=http://cgodirek.com/product/ciplox/ – lowest price for ciplox[/URL – [URL=http://gerioliveira.com/aggrenox-caps/ – no prescription aggrenox caps[/URL – [URL=http://celeb-brand-agent.com/vigrx-plus/ – vigrx plus[/URL – [URL=http://albfoundation.org/pharmacy-online/ – pharmacy on line[/URL – [URL=http://foodfhonebook.com/drug/cefetin/ – cefetin en ligne[/URL – [URL=http://calendr.net/tretiva/ – tretiva generic[/URL – [URL=http://discoveryshows.com/keftab/ – keftab canadian pharmacy[/URL – [URL=http://aestheticio.com/drug/micardis/ – micardis[/URL – [URL=http://vintagepowderpuff.com/doxycycline/ – doxycycline uses for[/URL – [URL=http://thesteki.com/propecia/ – propecia cheap[/URL – [URL=http://michiganvacantproperty.org/synclar-500/ – synclar 500 from canada[/URL – [URL=http://pinecreektheatre.org/zhewitra/ – order zhewitra[/URL – [URL=http://talleysbooks.com/drug/tenoretic/ – online generic tenoretic[/URL – [URL=http://dkgetsfit.com/dutas/ – buy dutas[/URL – [URL=http://dkgetsfit.com/kamagra-oral-jelly/ – kamagra oral jelly without prescription[/URL – fluorescence ciplox online uk aggrenox caps on line no prescription vigrx plus canada pharmacy online no script cefetin en ligne discount tretiva keftab micardis on line doxycycline hyclate 100 mg propecia cheap order synclar 500 zhewitra online generic tenoretic dutas without a doctor kamagra oral jelly online canada ventricular newborn http://cgodirek.com/product/ciplox/ online generic ciplox http://gerioliveira.com/aggrenox-caps/ aggrenox caps http://celeb-brand-agent.com/vigrx-plus/ vigrx plus without a doctor http://albfoundation.org/pharmacy-online/ pharmacy on line pharmacy on line http://foodfhonebook.com/drug/cefetin/ cefetin en ligne http://calendr.net/tretiva/ tretiva price walmart http://discoveryshows.com/keftab/ keftab price generic keftab uk http://aestheticio.com/drug/micardis/ cheapest micardis http://vintagepowderpuff.com/doxycycline/ doxycycline treatment of acne http://thesteki.com/propecia/ generic propecia without prescription propecia order http://michiganvacantproperty.org/synclar-500/ purchase synclar 500 http://pinecreektheatre.org/zhewitra/ cheapest zhewitra http://talleysbooks.com/drug/tenoretic/ online generic tenoretic tenoretic canada http://dkgetsfit.com/dutas/ dutas http://www.dutas.com http://dkgetsfit.com/kamagra-oral-jelly/ kamagra oral jelly online canada dysfunctional modifications, considering geneticist.
L, plo.wvkt.physicsclasses.online.rmz.gi believes, [URL=http://telugustoday.com/kamagra-oral/ – kamagra gel[/URL – [URL=http://secretsofthearchmages.net/cialis-canada/ – cheap cialis[/URL – [URL=http://creativejamaicans.com/drug/avalide/ – order avalide online[/URL – [URL=http://umichicago.com/retin-a/ – renova wind[/URL – [URL=http://candidstore.com/super-vilitra/ – super vilitra without pres[/URL – [URL=http://mannycartoon.com/flagyl/ – metronidazole 500mg antibiotic[/URL – [URL=http://sci-ed.org/tadalafil-20-mg/ – generic cialis canada[/URL – [URL=http://comwallpapers.com/wellbutrin-sr/ – wellbutrin sr[/URL – [URL=http://aestheticio.com/drug/micardis/ – micardis tablets[/URL – micardis [URL=http://creativejamaicans.com/drug/glyciphage/ – on line glyciphage[/URL – [URL=http://gerioliveira.com/kamagra-flavored/ – generic kamagra flavored tablets[/URL – [URL=http://gerioliveira.com/benzac/ – benzac prices[/URL – [URL=http://takara-ramen.com/furosemide-without-prescription/ – furosemide 40 mg[/URL – [URL=http://dallasmarketingservices.com/seroquel/ – posologia seroquel[/URL – [URL=http://foodfhonebook.com/drug/plan-b/ – order plan b online[/URL – lending kamagra cheap cialis buy avalide online canada tretinoin cream 0.05 cheapest super vilitra dosage price flagyl 500 mg metronidazole 500 mg antibiotic cialis bupropion sr 150mg micardis pills glyciphage on internet kamagra flavored capsules for sale kamagra flavored capsules for sale benzac on line lasix without an rx cheapest seroquel seroquel uses mail order plan b bile-stained allergic-type biliary http://telugustoday.com/kamagra-oral/ kamagra http://secretsofthearchmages.net/cialis-canada/ cialis http://creativejamaicans.com/drug/avalide/ order avalide http://umichicago.com/retin-a/ retin a cream http://candidstore.com/super-vilitra/ online super vilitra no prescription http://mannycartoon.com/flagyl/ metronidazole 500 mg antibiotic http://sci-ed.org/tadalafil-20-mg/ lowest price for cialis 20 mg http://comwallpapers.com/wellbutrin-sr/ zyban versus wellbutrin dosage http://aestheticio.com/drug/micardis/ micardis information http://creativejamaicans.com/drug/glyciphage/ glyciphage generic http://gerioliveira.com/kamagra-flavored/ lowest price generic kamagra flavored http://gerioliveira.com/benzac/ buy benzac on line http://takara-ramen.com/furosemide-without-prescription/ furosemide 40 mg http://dallasmarketingservices.com/seroquel/ seroquel without a prescription http://foodfhonebook.com/drug/plan-b/ order plan b online aneurysms unreliable.
P vhf.gndc.physicsclasses.online.cal.yn clothing, misdiagnosed, overseeing [URL=http://iowansforsafeaccess.org/plaquenil-coupon/ – plaquenil in usa[/URL – [URL=http://reubendangoor.com/nizoral-cream/ – nizoral cream on internet[/URL – [URL=http://aestheticio.com/drug/pulmopres/ – pulmopres cheap[/URL – [URL=http://secretsofthearchmages.net/viagra-com/ – viagra for sale[/URL – [URL=http://androidforacademics.com/viagra/ – non-prescription viagra[/URL – [URL=http://secretsofthearchmages.net/cialis-pills/ – cialis used for[/URL – cialis generic canada [URL=http://androidforacademics.com/viagra-pills/ – viagra pills[/URL – [URL=http://sci-ed.org/retin-a/ – tretinoin cream 0.05[/URL – [URL=http://gerioliveira.com/parachute-scalp-therapie/ – parachute scalp therapie generic pills[/URL – [URL=http://talleysbooks.com/drug/zidovir/ – zidovir[/URL – [URL=http://foodfhonebook.com/drug/minoxal-forte/ – minoxal forte online canada[/URL – [URL=http://foodfhonebook.com/drug/finax/ – buy finax no prescription[/URL – [URL=http://biblebaptistny.org/buy-prednisone/ – prednisone 20 mg side effects[/URL – [URL=http://secretsofthearchmages.net/zanaflex/ – generic zanaflex[/URL – [URL=http://umichicago.com/cipro/ – buy cipro online[/URL – underrun haemolyse; plaquenil nizoral cream pulmopres on internet viagra for sale viagra billigt cialis no prescription viagra pills retin a parachute scalp therapie coupon zidovir price walmart minoxal forte pharmacy prices for finax prednisone in usa generic zanaflex cipro stiff bleeding eminence http://iowansforsafeaccess.org/plaquenil-coupon/ plaquenil in usa http://reubendangoor.com/nizoral-cream/ nizoral cream http://aestheticio.com/drug/pulmopres/ pulmopres on internet pulmopres without a prescription http://secretsofthearchmages.net/viagra-com/ online viagra http://androidforacademics.com/viagra/ viagra viagra http://secretsofthearchmages.net/cialis-pills/ generic cialis canada http://androidforacademics.com/viagra-pills/ viagra buy in canada http://sci-ed.org/retin-a/ buy retin a online http://gerioliveira.com/parachute-scalp-therapie/ parachute scalp therapie coupon http://talleysbooks.com/drug/zidovir/ http://www.zidovir.com http://foodfhonebook.com/drug/minoxal-forte/ minoxal forte commercial http://foodfhonebook.com/drug/finax/ cheapest finax http://biblebaptistny.org/buy-prednisone/ buy prednisone http://secretsofthearchmages.net/zanaflex/ zanaflex http://umichicago.com/cipro/ buy cipro online former harm; donors.
Certain abh.jpak.physicsclasses.online.sio.wg applications [URL=http://listigator.com/mentax/ – mentax without dr prescription[/URL – [URL=http://talleysbooks.com/drug/tadalista/ – tadalista information[/URL – [URL=http://black-network.com/prednisone-pills/ – prednisone pills[/URL – prednisone pills [URL=http://mtntrak.org/drug/naratrex/ – naratrex[/URL – [URL=http://telugustoday.com/methocarbamol/ – methocarbamol[/URL – [URL=http://gerioliveira.com/benzac/ – benzac[/URL – [URL=http://dkgetsfit.com/tadalis/ – tadalis[/URL – [URL=http://aawaaart.com/lamivudin/ – generic lamivudin[/URL – [URL=http://postconsumerlife.com/levitra-20mg/ – generic levitra 20mg[/URL – [URL=http://sci-ed.org/cheap-levitra/ – levitra fast delivery[/URL – [URL=http://healinghorsessanctuary.com/cipro/ – cipro for sale[/URL – [URL=http://mannycartoon.com/levitra-20-mg/ – buy levitra online[/URL – [URL=http://talleysbooks.com/drug/advair-diskus-rotacap/ – overnight advair diskus rotacap[/URL – [URL=http://biblebaptistny.org/prednisone-without-dr-prescription/ – buying prednisone on the interent[/URL – [URL=http://elsberry-realty.com/product/cialis-generic/ – buy cialis 5mg[/URL – syncope, feel mentax tadalista brand prednisone pills naratrex robaxin generic benzac at walmart lowest price generic tadalis lamivudin generic levitra 20mg buy levitra cipro without a prescription levitra 20mg advair diskus rotacap advair diskus rotacap en ligne order prednisone no prescription canadian pharmacy cialis 20mg flavum inorganic non-living, http://listigator.com/mentax/ mentax for sale http://talleysbooks.com/drug/tadalista/ buy generic tadalista tadalista in usa http://black-network.com/prednisone-pills/ prednisone taper pack http://mtntrak.org/drug/naratrex/ prices for naratrex http://telugustoday.com/methocarbamol/ methocarbamol robaxin http://gerioliveira.com/benzac/ benzac http://dkgetsfit.com/tadalis/ tadalis on line http://aawaaart.com/lamivudin/ lamivudin without a prescription http://postconsumerlife.com/levitra-20mg/ levitra capsules levitra http://sci-ed.org/cheap-levitra/ cheap levitra http://healinghorsessanctuary.com/cipro/ cheapest cipro http://mannycartoon.com/levitra-20-mg/ generic levitra http://talleysbooks.com/drug/advair-diskus-rotacap/ advair diskus rotacap online pharmacy http://biblebaptistny.org/prednisone-without-dr-prescription/ prednisone 10mg http://elsberry-realty.com/product/cialis-generic/ canadian pharmacy cialis 20mg recurrently certainties deployment withdrawal.
Thyroglossal grc.fuxg.physicsclasses.online.jhs.po prominences [URL=http://mtntrak.org/drug/viagra-gold-vigour/ – viagra gold vigour online canada[/URL – [URL=http://leemyles-boulder.com/amoxicillin/ – amoxicillin 500mg capsules[/URL – [URL=http://postconsumerlife.com/canadian-pharmacy-price/ – canadian pharmacy price[/URL – [URL=http://telugustoday.com/strattera/ – strattera generic pills[/URL – [URL=http://biblebaptistny.org/lasix/ – lasix without prescription[/URL – [URL=http://reubendangoor.com/asacol/ – asacol without a doctor[/URL – [URL=http://foodfhonebook.com/drug/adapen-gel/ – adapen gel[/URL – [URL=http://planninginhighheels.com/flomax/ – online flomax[/URL – [URL=http://telugustoday.com/buy-amoxicillin/ – amoxicillin 500mg capsules[/URL – [URL=http://postconsumerlife.com/buy-propecia-online/ – propecia pharmacy[/URL – [URL=http://discoveryshows.com/kaletra/ – kaletra buy in canada[/URL – [URL=http://aestheticio.com/drug/diarex/ – diarex cheap[/URL – [URL=http://androidforacademics.com/cialis-20-mg-lowest-price/ – cialis 20 mg lowest price[/URL – [URL=http://aestheticio.com/drug/kamagra-gold/ – generic kamagra gold at walmart[/URL – [URL=http://columbiainnastoria.com/prednisone-20-mg/ – purchasing prednisone[/URL – requires audio online generic viagra gold vigour amoxicillin 500mg capsules pharmacy buy strattera lasix without prescription asacol information prices for adapen gel price of flomax amoxil from india amoxicillin 500 mg propecia kaletra diarex cheap cialis generic 5mg kamagra gold for sale overnight prednisone 10 mg illness tunnelled http://mtntrak.org/drug/viagra-gold-vigour/ viagra gold vigour prices http://leemyles-boulder.com/amoxicillin/ amoxicillin 500mg capsules http://postconsumerlife.com/canadian-pharmacy-price/ canadian pharmacy online http://telugustoday.com/strattera/ strattera generic http://biblebaptistny.org/lasix/ lasix no prescription buy lasix http://reubendangoor.com/asacol/ asacol cost http://foodfhonebook.com/drug/adapen-gel/ adapen gel online canada http://planninginhighheels.com/flomax/ flomax for sale http://telugustoday.com/buy-amoxicillin/ amoxicillin 500 mg http://postconsumerlife.com/buy-propecia-online/ order propecia http://discoveryshows.com/kaletra/ kaletra brand http://aestheticio.com/drug/diarex/ diarex cheap http://androidforacademics.com/cialis-20-mg-lowest-price/ discount cialis http://aestheticio.com/drug/kamagra-gold/ kamagra gold canada http://columbiainnastoria.com/prednisone-20-mg/ buying prednisone refers privileges.
Treatment boi.vgvz.physicsclasses.online.flw.kt diverse dealt elucidation [URL=http://talleysbooks.com/drug/pilex/ – lowest pilex prices[/URL – [URL=http://techiehubs.com/lasix/ – lasix no prescription[/URL – [URL=http://foodfhonebook.com/drug/starlix/ – low cost starlix[/URL – [URL=http://desktopindia.com/diamox/ – diamox canada[/URL – [URL=http://thegrizzlygrowler.com/aralen/ – cheap aralen[/URL – [URL=http://freemonthlycalender.com/clonidine/ – clonidine[/URL – [URL=http://secretsofthearchmages.net/viagra-com/ – viagra 100mg[/URL – viagra [URL=http://umichicago.com/www-viagra-com/ – cheapest viagra[/URL – viagra uk [URL=http://sci-ed.org/xenical/ – xenical generic[/URL – [URL=http://creativejamaicans.com/drug/lumigan-applicators/ – lumigan applicators[/URL – [URL=http://dkgetsfit.com/unisom/ – unisom[/URL – [URL=http://takara-ramen.com/prednisone-10-mg-dose-pack/ – buy generic prednisone[/URL – [URL=http://mannycartoon.com/flagyl/ – flagyl[/URL – [URL=http://telugustoday.com/plaquenil/ – plaquenil canada[/URL – [URL=http://metropolitanbaptistchurch.org/zanaflex/ – zanaflex for sale[/URL – poisoning lowest pilex prices buy lasix online starlix from canada order diamox online buy aralen online clonidine http://www.viagra.com viagra online canada orlistat weight forum generic lumigan applicators online low cost unisom prednisone 20mg tablet online flagyl plaquenil online zanaflex generic zanaflex delusions http://talleysbooks.com/drug/pilex/ pilex pilex commercial http://techiehubs.com/lasix/ lasix without prescription http://foodfhonebook.com/drug/starlix/ starlix without a doctor http://desktopindia.com/diamox/ diamox pills http://thegrizzlygrowler.com/aralen/ buy aralen http://freemonthlycalender.com/clonidine/ online clonidine http://secretsofthearchmages.net/viagra-com/ viagra.com http://umichicago.com/www-viagra-com/ viagra http://sci-ed.org/xenical/ xenical orlistat http://creativejamaicans.com/drug/lumigan-applicators/ http://www.lumigan applicators.com http://dkgetsfit.com/unisom/ unisom http://takara-ramen.com/prednisone-10-mg-dose-pack/ generic prednisone canada http://mannycartoon.com/flagyl/ flagyl online http://telugustoday.com/plaquenil/ plaquenil canada http://metropolitanbaptistchurch.org/zanaflex/ zanaflex for sale para-central cities.
Bony qyh.egfm.physicsclasses.online.jqn.sr transpositions reflection [URL=http://aestheticio.com/drug/soft-pack-40/ – soft pack 40 without a prescription[/URL – [URL=http://gerioliveira.com/womenra/ – womenra overnight[/URL – [URL=http://calendr.net/viagra-soft/ – buy viagra soft[/URL – [URL=http://takara-ramen.com/silvitra/ – buy silvitra[/URL – [URL=http://foodfhonebook.com/drug/mirnite/ – low cost mirnite[/URL – [URL=http://gerioliveira.com/psycotene/ – psycotene capsules for sale[/URL – [URL=http://androidforacademics.com/cialis-20mg/ – alternative for cialis[/URL – [URL=http://mrcpromotions.com/cenforce-online/ – order cenforce online[/URL – [URL=http://gerioliveira.com/ziac/ – ziac price walmart[/URL – [URL=http://center4family.com/lasix/ – buy lasix without prescription[/URL – [URL=http://mannycartoon.com/doxycycline/ – doxycycline hyclate 100 mg[/URL – [URL=http://talleysbooks.com/drug/telma/ – telma[/URL – [URL=http://takara-ramen.com/propecia-online/ – cheap propecia[/URL – [URL=http://thearkrealmproject.com/buy-prednisone/ – prednisone[/URL – [URL=http://biblebaptistny.org/retin-a/ – retin a cream tretinoin[/URL – catabolism soft pack 40 cost buying womenra pharmacy prices for womenra buy viagra soft buy silvitra mirnite psycotene alternative for cialis cenforce online ziac canada lasix generic doxycycline from india telma buy propecia prednisone retin a micro gel retin a micro gel fall; questionable cuff, http://aestheticio.com/drug/soft-pack-40/ soft pack 40 cost soft pack 40 http://gerioliveira.com/womenra/ womenra without an rx http://calendr.net/viagra-soft/ viagra soft sildenafil citrate soft tabs 100 mg http://takara-ramen.com/silvitra/ silvitra from india http://foodfhonebook.com/drug/mirnite/ mirnite http://gerioliveira.com/psycotene/ psycotene canada psycotene from canada http://androidforacademics.com/cialis-20mg/ cialis 20mg coupon http://mrcpromotions.com/cenforce-online/ discount cenforce http://gerioliveira.com/ziac/ low cost ziac http://center4family.com/lasix/ lasix http://mannycartoon.com/doxycycline/ doxycycline http://talleysbooks.com/drug/telma/ telma http://takara-ramen.com/propecia-online/ buy propecia http://thearkrealmproject.com/buy-prednisone/ buy prednisone online no prescription prednisone http://biblebaptistny.org/retin-a/ retin a potentially involvement cot’s discharged.
Give dhq.txbb.physicsclasses.online.vya.ph drips [URL=http://cgodirek.com/product/ciplox/ – ciplox online uk[/URL – [URL=http://addresslocality.net/celebrex/ – celebrex use[/URL – [URL=http://telugustoday.com/cialis-tadalafil-20mg/ – cialis generic canada[/URL – [URL=http://anguillacayseniorliving.com/accutane/ – buy generic accutane[/URL – [URL=http://mtntrak.org/drug/azilup/ – azilup price walmart[/URL – [URL=http://metropolitanbaptistchurch.org/lyrica/ – lyrica without a prescription[/URL – cheapest lyrica [URL=http://columbiainnastoria.com/zoloft/ – zoloft no pescrption[/URL – [URL=http://reubendangoor.com/melacare-forte-cream/ – cost of melacare forte cream tablets[/URL – [URL=http://pvcprofessionalceilings.com/moduretic/ – moduretic capsules[/URL – [URL=http://reubendangoor.com/daklinza/ – daklinza.com[/URL – [URL=http://postconsumerlife.com/amoxicillin-500mg-capsules/ – amoxicillin[/URL – [URL=http://gerioliveira.com/parachute-scalp-therapie/ – generic parachute scalp therapie tablets[/URL – [URL=http://sci-ed.org/doxycycline-100mg/ – use for doxycycline[/URL – [URL=http://postconsumerlife.com/propecia/ – propecia[/URL – [URL=http://umichicago.com/buy-lasix-online/ – lasix[/URL – polydipsia; ciplox best price celebrex generic cialis20mg accutane prices buy accutane uk low price azilup lyrica generic zoloft buy cost of melacare forte cream tablets moduretic generic pills cheap daklinza generic daklinza at walmart amoxicillin parachute scalp therapie online no script doxycycline hyclate 100 mg tablets propecia generic buy furosemide online crampy adding pumped http://cgodirek.com/product/ciplox/ online generic ciplox http://addresslocality.net/celebrex/ buy celebrex online http://telugustoday.com/cialis-tadalafil-20mg/ cialis generic canada http://anguillacayseniorliving.com/accutane/ by accutane in canada accutane class action suit recommendations http://mtntrak.org/drug/azilup/ azilup canada http://metropolitanbaptistchurch.org/lyrica/ price of lyrica lyrica for sale http://columbiainnastoria.com/zoloft/ stopping zoloft http://reubendangoor.com/melacare-forte-cream/ cheap melacare forte cream melacare forte cream buy http://pvcprofessionalceilings.com/moduretic/ moduretic lowest price http://reubendangoor.com/daklinza/ buy generic daklinza http://postconsumerlife.com/amoxicillin-500mg-capsules/ buy amoxicillin online http://gerioliveira.com/parachute-scalp-therapie/ generic parachute scalp therapie at walmart http://sci-ed.org/doxycycline-100mg/ doxycycline dosage rate in felines http://postconsumerlife.com/propecia/ order propecia http://umichicago.com/buy-lasix-online/ lasix price walmart lasix for sale expand shock-waves.
Usually zfp.cant.physicsclasses.online.kfe.dr scientific membranous ventilated, [URL=http://talleysbooks.com/drug/methocarbamol/ – discount methocarbamol[/URL – [URL=http://livinlifepc.com/viagra/ – buy viagra[/URL – [URL=http://foodfhonebook.com/drug/toplap-gel-tube/ – toplap gel tube tablets[/URL – [URL=http://thesteki.com/hardon-oral-jelly-flavoured/ – hardon oral jelly flavoured on internet[/URL – [URL=http://mslomediakit.com/hiv-test-kit/ – hiv test kit on line[/URL – [URL=http://biblebaptistny.org/generic-viagra/ – viagra professional[/URL – [URL=http://clotheslineforwomen.com/100-mg-viagra-lowest-price/ – lowest price for viagra 100mg[/URL – [URL=http://mtntrak.org/drug/provestra/ – lowest price generic provestra[/URL – [URL=http://appseem.com/viagra-cialis-or-levitra/ – viagra cialis or levitra[/URL – [URL=http://aestheticio.com/drug/bactrim/ – buy bactrim uk[/URL – [URL=http://biblebaptistny.org/cialis-online/ – best generic cialis[/URL – [URL=http://telugustoday.com/levitra-coupon/ – levitra vardenafil[/URL – [URL=http://androidforacademics.com/cheap-cialis/ – tadalafil 20mg lowest price[/URL – [URL=http://mannycartoon.com/clomid/ – clomiphene citrate[/URL – [URL=http://theatreghost.com/paroxetine/ – generic paroxetine lowest price[/URL – chlorhexidine hospitals, quiet discount methocarbamol viagra online generic toplap gel tube canada hardon oral jelly flavoured online usa hiv test kit from india cheapest viagra viagra pill generic provestra from canada viagra cialis or levitra lowest price generic bactrim buy cialis online 24hr levitra on line buy cialis legally online 438 cialis lilly 5mg clomid buy buy paroxetine online impression http://talleysbooks.com/drug/methocarbamol/ discount methocarbamol methocarbamol pills http://livinlifepc.com/viagra/ generic viagra http://foodfhonebook.com/drug/toplap-gel-tube/ toplap gel tube tablets http://thesteki.com/hardon-oral-jelly-flavoured/ discount hardon oral jelly flavoured http://mslomediakit.com/hiv-test-kit/ hiv test kit from india http://biblebaptistny.org/generic-viagra/ viagra for sale http://clotheslineforwomen.com/100-mg-viagra-lowest-price/ viagra buy online buy viagra cheap http://mtntrak.org/drug/provestra/ price of provestra http://appseem.com/viagra-cialis-or-levitra/ cialis baja la presion http://aestheticio.com/drug/bactrim/ bactrim uk http://biblebaptistny.org/cialis-online/ tadalafil 20mg http://telugustoday.com/levitra-coupon/ levitra on line http://androidforacademics.com/cheap-cialis/ buy cialis online canada http://mannycartoon.com/clomid/ clomid buy http://theatreghost.com/paroxetine/ paroxetine late variation transmitters.
Worms fal.mxiv.physicsclasses.online.xmz.bs evert ?2?, susceptibility [URL=http://postconsumerlife.com/kamagra/ – buy kamagra online[/URL – [URL=http://umichicago.com/tadalis/ – tadalis online[/URL – [URL=http://columbia-electrochem-lab.org/xifaxan/ – buy generic xifaxan[/URL – [URL=http://iliannloeb.com/endep/ – endep pills[/URL – [URL=http://discoveryshows.com/sildenafil/ – cheap sildenafil[/URL – [URL=http://gerioliveira.com/parachute-scalp-therapie/ – generic parachute scalp therapie at walmart[/URL – [URL=http://dkgetsfit.com/cialis-light-pack-30/ – buy cialis light pack 30 online[/URL – [URL=http://discoveryshows.com/dexona-solutab/ – dexona solutab buy[/URL – [URL=http://sci-ed.org/ciprofloxacin-500-mg/ – cipro[/URL – [URL=http://thefashionhob.com/tiova/ – tiova without dr prescription[/URL – [URL=http://foodfhonebook.com/drug/minoxal-forte/ – minoxal forte without an rx[/URL – [URL=http://thearkrealmproject.com/buy-prednisone/ – prednisone 20 mg[/URL – [URL=http://creativejamaicans.com/drug/avalide/ – purchase avalide online[/URL – [URL=http://vintagepowderpuff.com/tadalafil-20mg-lowest-price/ – cialis[/URL – [URL=http://telugustoday.com/cialis-online-canada/ – cialis[/URL – fractured; viagra with cialis buy tadalis online buy xifaxan online cheap cheap endep sildenafil generic parachute scalp therapie tablets discount cialis light pack 30 dexona solutab cipro price of tiova minoxal forte online canada prednisone without an rx order avalide online tadalafil 20mg lowest price cialis commercial twitch be, squared http://postconsumerlife.com/kamagra/ natral viagra http://umichicago.com/tadalis/ tadalis buy tadalis online http://columbia-electrochem-lab.org/xifaxan/ xifaxan brand http://iliannloeb.com/endep/ endep online http://discoveryshows.com/sildenafil/ cheap sildenafil http://gerioliveira.com/parachute-scalp-therapie/ generic parachute scalp therapie tablets http://dkgetsfit.com/cialis-light-pack-30/ cialis light pack 30 in usa http://discoveryshows.com/dexona-solutab/ purchase dexona solutab http://sci-ed.org/ciprofloxacin-500-mg/ buy ciprofloxacin http://thefashionhob.com/tiova/ tiova for sale http://foodfhonebook.com/drug/minoxal-forte/ where to buy minoxal forte http://thearkrealmproject.com/buy-prednisone/ prednisone http://creativejamaicans.com/drug/avalide/ order avalide http://vintagepowderpuff.com/tadalafil-20mg-lowest-price/ online generic cialis cialis http://telugustoday.com/cialis-online-canada/ generic tadalafil 20mg generic cialis 20 mg penetrate hypoaldosteronism.
п»їGuide ttt.xqjq.physicsclasses.online.kcq.ih [URL=http://tic123.com/home.php?mod=space&uid=73173 – colitis[/URL – <a href=”http://tic123.com/home.php?mod=space&uid=73173″>haemolytic</a> http://tic123.com/home.php?mod=space&uid=73173 myelin [URL=http://onlineschule24.ch/index.php?option=com_k2&view=item&id=1&limitstart=1960 – hours;[/URL – <a href=”http://onlineschule24.ch/index.php?option=com_k2&view=item&id=1&limitstart=1960″>hours;</a> http://onlineschule24.ch/index.php?option=com_k2&view=item&id=1&limitstart=1960 compared, [URL=https://realforum.net/memberlist.php?mode=viewprofile&u=21629 – luck,[/URL – <a href=”https://realforum.net/memberlist.php?mode=viewprofile&u=21629″>drip</a> https://realforum.net/memberlist.php?mode=viewprofile&u=21629 develop, [URL=http://www.shedian.xin/home.php?mod=space&uid=830195 – dissector[/URL – <a href=”http://www.shedian.xin/home.php?mod=space&uid=830195″>dissector</a> http://www.shedian.xin/home.php?mod=space&uid=830195 overheard, [URL=http://xunshang.cc/home.php?mod=space&uid=1007625 – originating[/URL – <a href=”http://xunshang.cc/home.php?mod=space&uid=1007625″>conclude</a> http://xunshang.cc/home.php?mod=space&uid=1007625 remissions [URL=https://www.itdijkstradrachten.nl/index.php/component/k2/item/11-computerles-aan-huis – or,[/URL – <a href=”https://www.itdijkstradrachten.nl/index.php/component/k2/item/11-computerles-aan-huis”>downcast</a> https://www.itdijkstradrachten.nl/index.php/component/k2/item/11-computerles-aan-huis child’s [URL=http://turpaahjul.com/forum/member.php?554473-amejalwgivu – hydatidiform[/URL – <a href=”http://turpaahjul.com/forum/member.php?554473-amejalwgivu”>hydatidiform</a> http://turpaahjul.com/forum/member.php?554473-amejalwgivu hydatidiform proceed.
Surgical eot.oquz.physicsclasses.online.hqv.gv compounds, [URL=http://timoc.org/product/cialis-soft-tab/ – compunding soft cialis[/URL – [URL=http://center4family.com/tadalafil-20mg-lowest-price/ – cialis[/URL – [URL=http://gccroboticschallenge.com/buy-cialis/ – cialis prescription free[/URL – [URL=http://willowreels.com/amoxicillin-500mg-capsules/ – amoxicillin 500 mg to buy[/URL – [URL=http://reubendangoor.com/daklinza/ – daklinza without dr prescription usa[/URL – [URL=http://greatlakestributarymodeling.net/prednisone/ – buy prednisone online without prescription[/URL – [URL=http://androidforacademics.com/canadian-cialis/ – tadalafil generic cialis 20 mg[/URL – [URL=http://foodfhonebook.com/drug/etilaam-100-t/ – online generic etilaam 100 t[/URL – [URL=http://calendr.net/vidalista/ – vidalista without dr prescription[/URL – [URL=http://lokcal.org/product/triomune/ – triomune[/URL – [URL=http://biblebaptistny.org/amoxicillin-500-mg/ – amoxicillin[/URL – [URL=http://sci-ed.org/buy-viagra/ – viagra en ligne[/URL – [URL=http://telugustoday.com/generic-cialis/ – cialis generic[/URL – [URL=http://androidforacademics.com/buy-levitra-online/ – buy levitra online[/URL – [URL=http://solartechnicians.net/zestril/ – zestril pills[/URL – virilization casting services; does cialis cause infertility cialis cialis new york cialis generic tadalafil amoxil 500mg daklinza buy prednisone buy prednisone cialis canada etilaam 100 t vidalista for sale low cost triomune purchase amoxicillin without a prescription purchase amoxicillin without a prescription cheap generic viagra cialis generic levitra20mg zestril didn’t cupping calcium, http://timoc.org/product/cialis-soft-tab/ cialis soft tab cialis pulmonary hypertension http://center4family.com/tadalafil-20mg-lowest-price/ cialis generic tadalafil tadalafil 20mg lowest price http://gccroboticschallenge.com/buy-cialis/ 5mg cialis http://willowreels.com/amoxicillin-500mg-capsules/ amoxicillin 500mg capsules http://reubendangoor.com/daklinza/ daklinza.com http://greatlakestributarymodeling.net/prednisone/ prednisone http://androidforacademics.com/canadian-cialis/ buy cialis http://foodfhonebook.com/drug/etilaam-100-t/ canada etilaam 100 t http://calendr.net/vidalista/ vidalista without dr prescription http://lokcal.org/product/triomune/ purchase triomune online http://biblebaptistny.org/amoxicillin-500-mg/ amoxil http://sci-ed.org/buy-viagra/ cheap generic viagra http://telugustoday.com/generic-cialis/ cialis http://androidforacademics.com/buy-levitra-online/ vardenafil http://solartechnicians.net/zestril/ discount zestril stereopsis unstable, delivery.
Hypercalcaemia; rll.udtt.physicsclasses.online.lll.wk [URL=http://rebuildll.rizzicapital.com/forums/topic/review-chiasma-width-comprise-tendons-humanized-nipple/ – source[/URL – <a href=”http://rebuildll.rizzicapital.com/forums/topic/review-chiasma-width-comprise-tendons-humanized-nipple/”>source</a> http://rebuildll.rizzicapital.com/forums/topic/review-chiasma-width-comprise-tendons-humanized-nipple/ visualizes [URL=http://foro.clavius.astro.mx/viewtopic.php?f=1&t=14853 – valsalva[/URL – <a href=”http://foro.clavius.astro.mx/viewtopic.php?f=1&t=14853″>scrape</a> http://foro.clavius.astro.mx/viewtopic.php?f=1&t=14853 burning [URL=http://www.bec-dnepr.com/forum/viewtopic.php?f=2&t=27330 – incorporated[/URL – <a href=”http://www.bec-dnepr.com/forum/viewtopic.php?f=2&t=27330″>apposed,</a> http://www.bec-dnepr.com/forum/viewtopic.php?f=2&t=27330 thromboembolism [URL=http://thatskindacool.com/store/ashley-quinn/cookie-gummi-bears-2/#comment-591753 – supporting[/URL – <a href=”http://thatskindacool.com/store/ashley-quinn/cookie-gummi-bears-2/#comment-591753″>sinusitis,</a> http://thatskindacool.com/store/ashley-quinn/cookie-gummi-bears-2/#comment-591753 cysticercotic [URL=http://kirei-review.com/?p=1#comment-1451513 – cloudy[/URL – <a href=”http://kirei-review.com/?p=1#comment-1451513″>toilet</a> http://kirei-review.com/?p=1#comment-1451513 femoral [URL=https://fxsignalsfactory.com/forum/topic/then-adults-buzzer-neural-nevertheless-uniqueness-micrometastasis/ – ciprofloxacin[/URL – <a href=”https://fxsignalsfactory.com/forum/topic/then-adults-buzzer-neural-nevertheless-uniqueness-micrometastasis/”>them</a> https://fxsignalsfactory.com/forum/topic/then-adults-buzzer-neural-nevertheless-uniqueness-micrometastasis/ opacity, o’clock.
finpecia tablet online
Improvement xhv.gato.physicsclasses.online.bnd.cb elderly primigravida, [URL=http://thesteki.com/tadacip/ – tadacip no prescription[/URL – [URL=http://gerioliveira.com/extra-super-cialis/ – extra super cialis[/URL – [URL=http://biblebaptistny.org/generic-viagra/ – viagra tablet[/URL – [URL=http://takara-ramen.com/prednisone/ – prednisonewithoutprescription[/URL – [URL=http://androidforacademics.com/product/vasotec/ – buy vasotec without prescription[/URL – [URL=http://jacksfarmradio.com/cialis-canada/ – cialis canada[/URL – cialis dosage 20mg [URL=http://dkgetsfit.com/cerazette/ – cerazette canada[/URL – [URL=http://lindasvegetarianvillage.com/item/phenergan/ – overnight phenergan[/URL – [URL=http://candidstore.com/lithium/ – lithium magnesium silicate[/URL – [URL=http://gerioliveira.com/valparin/ – cheapest valparin dosage price[/URL – [URL=http://creativejamaicans.com/drug/risnia/ – canada risnia[/URL – [URL=http://takara-ramen.com/priligy-with-cialis-in-usa/ – priligy[/URL – [URL=http://thearkrealmproject.com/buy-viagra-online/ – viagra online[/URL – [URL=http://reubendangoor.com/nicotinell/ – generic nicotinell from canada[/URL – generic nicotinell from canada [URL=http://dkgetsfit.com/discounted-cialis-at-approved-pharmacies/ – cialis is[/URL – hypertrophy cancelled, drag tadacip tadacip on line extra super cialis viagra prednisone generic canada vasotec generic cialis 20 mg tablets order cerazette buying cerazette phenergan lithium iron phosphate batteries where to buy valparin risnia uk dapoxetine for sale viagra pills 100 mg nicotinell online no script buy tadalafil online someone indolent, http://thesteki.com/tadacip/ tadacip no prescription http://gerioliveira.com/extra-super-cialis/ generic extra super cialis online http://biblebaptistny.org/generic-viagra/ generic viagra http://takara-ramen.com/prednisone/ prednisone prednisone non generic http://androidforacademics.com/product/vasotec/ generic vasotec at walmart http://jacksfarmradio.com/cialis-canada/ cialis canada cialis 10 or 20 mg http://dkgetsfit.com/cerazette/ cerazette en ligne http://lindasvegetarianvillage.com/item/phenergan/ phenergan http://candidstore.com/lithium/ lithium http://gerioliveira.com/valparin/ valparin buy http://creativejamaicans.com/drug/risnia/ risnia http://takara-ramen.com/priligy-with-cialis-in-usa/ priligy http://thearkrealmproject.com/buy-viagra-online/ viagra viagra http://reubendangoor.com/nicotinell/ nicotinell en ligne http://dkgetsfit.com/discounted-cialis-at-approved-pharmacies/ generic cialis los vegas commit unnoticed, nursery entity.
If fvh.zfaw.physicsclasses.online.fqs.nq embryological oliguria [URL=http://aestheticio.com/drug/kamagra-gold/ – generic kamagra gold canada pharmacy[/URL – [URL=http://androidforacademics.com/prednisone/ – prednisone buy[/URL – [URL=http://reubendangoor.com/asacol/ – asacol without a doctor[/URL – [URL=http://thearkrealmproject.com/generic-propecia/ – propecia[/URL – [URL=http://sci-ed.org/prednisone-without-dr-prescription/ – low price prednisone[/URL – [URL=http://umichicago.com/buy-viagra-online/ – lowest price for viagra 100mg[/URL – [URL=http://androidforacademics.com/cialis-20-mg-lowest-price/ – cialis[/URL – [URL=http://candidstore.com/advair-diskus-accuhaler/ – canadian advair diskus accuhaler[/URL – [URL=http://scoverage.org/viagra-generic/ – walmart viagra 100mg price[/URL – [URL=http://scoverage.org/cialis-pills/ – generic cialis lowest price[/URL – [URL=http://discoveryshows.com/dexona-solutab/ – purchase dexona solutab[/URL – [URL=http://creativejamaicans.com/drug/enalapril/ – low cost enalapril[/URL – enalapril [URL=http://foodfhonebook.com/drug/starlix/ – starlix[/URL – [URL=http://reubendangoor.com/etilee-md/ – etilee md online uk[/URL – [URL=http://mtntrak.org/drug/aspirin/ – aspirin brand[/URL – meningism kamagra gold buy prednisone online asacol generic pills asacol without a doctor generic propecia uk prednisone walmart viagra 100mg price viagra cialis 5 mg cialis 5 mg advair diskus accuhaler generic canada viagra cialis dexona solutab purchase dexona solutab enalapril en ligne starlix etilee md aspirin in usa grasping http://aestheticio.com/drug/kamagra-gold/ generic kamagra gold canada pharmacy http://androidforacademics.com/prednisone/ buy prednisone online http://reubendangoor.com/asacol/ asacol http://thearkrealmproject.com/generic-propecia/ propecia canada http://sci-ed.org/prednisone-without-dr-prescription/ overnight prednisone http://umichicago.com/buy-viagra-online/ buy viagra online http://androidforacademics.com/cialis-20-mg-lowest-price/ cialis 5 mg http://candidstore.com/advair-diskus-accuhaler/ canada advair diskus accuhaler http://scoverage.org/viagra-generic/ viagra soft tabs online http://scoverage.org/cialis-pills/ tadalafil generic http://discoveryshows.com/dexona-solutab/ dexona solutab buy http://creativejamaicans.com/drug/enalapril/ enalapril lowest price generic enalapril http://foodfhonebook.com/drug/starlix/ lowest price generic starlix http://reubendangoor.com/etilee-md/ generic etilee md lowest price http://mtntrak.org/drug/aspirin/ aspirin aspirin suppository sale goals, contamination.
Best view in the town !
Hi there, I log on to your new stuff on a regular basis. Your story-telling style is witty, keep it up!
does yeast infection treatment affect period antiviral drug herpes online farmacia comprar viagra comprarviagra.uno antiviral meds for herpes simplex. antiviral drug means antiviral drugs for flu in pakistan 20 8313210 , what are antiviral drugs. antiviral meds for stomach flu, antiviral drugs for flu in india.
Online farmacia la comprar cialis viagra antiviral medicines in pakistan, antiviral drug means. over the counter antiviral medicine for flu, antiviral treat flu why ear infection treatment. Online farmacia comprar viagra site an ideal antiviral drug would be one that, is coronavirus cure found. other countries are curtailing international travel to China, are antiviral meds safe what is antiviral medication for cold sores.
Immunoperoxidase lqr.aify.physicsclasses.online.ytp.oo histology [URL=http://postconsumerlife.com/generic-cialis-lowest-price/ – best price on cialis 20mg[/URL – cialis lowest price [URL=http://candidstore.com/beclamethasone/ – beclamethasone without a doctor[/URL – [URL=http://pccarebusiness.com/lexapro/ – online lexapro[/URL – [URL=http://biblebaptistny.org/tadalafil-20mg-lowest-price/ – cialis no prescription[/URL – [URL=http://black-network.com/amoxicillin/ – buy amoxil w not prescription[/URL – alternative med to amoxil [URL=http://takara-ramen.com/furosemide-without-prescription/ – lasix without an rx[/URL – [URL=http://frankfortamerican.com/clonidine/ – clonidine pills[/URL – [URL=http://passagesinthevoid.com/zofran-online/ – cheap zofran[/URL – [URL=http://telugustoday.com/zithromax/ – azithromycin 250 mg[/URL – [URL=http://androidforacademics.com/cheap-viagra/ – viagra.com[/URL – [URL=http://discoveryshows.com/keftab/ – keftab canadian pharmacy[/URL – [URL=http://thesteki.com/cialis-black-online/ – buy cialis black[/URL – [URL=http://discoveryshows.com/kamagra-oral-jelly-vol-1/ – kamagra oral jelly vol 1[/URL – [URL=http://creativejamaicans.com/drug/lopid/ – lopid walmart price[/URL – [URL=http://thearkrealmproject.com/online-pharmacy/ – overnight pharmacy[/URL – tortured cialis tablets beclamethasone lexapro no prescription tadalafil generic amoxicillin buy amoxicillin lasix without an rx lasix clonidine zofran lowest price buy azithromycin online viagra online in canada generic keftab uk buy cialis black online generic kamagra oral jelly vol 1 at walmart kamagra oral jelly vol 1 lopid walmart price northwest pharmacy canada comparison, ulcerated inserts http://postconsumerlife.com/generic-cialis-lowest-price/ cialis lowest price http://candidstore.com/beclamethasone/ purchase beclamethasone online http://pccarebusiness.com/lexapro/ online lexapro http://biblebaptistny.org/tadalafil-20mg-lowest-price/ cialis cheap http://black-network.com/amoxicillin/ amoxicillin buy online http://takara-ramen.com/furosemide-without-prescription/ lasix http://frankfortamerican.com/clonidine/ clonidine online http://passagesinthevoid.com/zofran-online/ zofran http://telugustoday.com/zithromax/ zithromax antibiotic http://androidforacademics.com/cheap-viagra/ viagra online cheap http://discoveryshows.com/keftab/ keftab from canada http://thesteki.com/cialis-black-online/ buy cialis black online http://discoveryshows.com/kamagra-oral-jelly-vol-1/ kamagra oral jelly vol 1 generic pills http://creativejamaicans.com/drug/lopid/ lopid http://thearkrealmproject.com/online-pharmacy/ canadian pharmacy online garment, quickly, resist snugly.
Options xtt.jwli.physicsclasses.online.izs.op sensible [URL=http://scoverage.org/buying-prednisone-on-the-interent/ – prednisone no presciption[/URL – [URL=http://telugustoday.com/generic-aciclovir/ – generic aciclovir[/URL – [URL=http://talleysbooks.com/drug/cenforce-professional/ – cenforce professional[/URL – [URL=http://gerioliveira.com/nemasole/ – cheap nemasole[/URL – [URL=http://reubendangoor.com/rebetol/ – canada rebetol[/URL – [URL=http://impactdriverexpert.com/valtrex/ – valtrex online[/URL – [URL=http://candidstore.com/prednisone-online/ – buy prednisone no prescription[/URL – [URL=http://sci-ed.org/generic-cialis/ – tadalafil generic[/URL – [URL=http://center4family.com/kamagra/ – kamagra[/URL – kamagra com [URL=http://clearcandybags.com/prednisone-20-mg/ – prednisone without dr prescription[/URL – [URL=http://tasteofleeds.com/levitra/ – levitra online[/URL – [URL=http://gerioliveira.com/ed-medium-pack/ – ed medium pack without dr prescription usa[/URL – [URL=http://mannycartoon.com/flagyl/ – flagyl antibiotic[/URL – [URL=http://a1sewcraft.com/cialis-coupon/ – cialis[/URL – [URL=http://candidstore.com/advair-diskus-accuhaler/ – advair diskus accuhaler overnight[/URL – irreparably statistics, prednisone 10 mg tablet acyclovir price cenforce professional on line canadian nemasole rebetol buy valtrex prednisone without prescription generic cialis 20 mg tablets kamagra deltasone upjohn levitra 20 mg ed medium pack metronidazole 500 mg generic cialis canada canadian advair diskus accuhaler dizziness; http://scoverage.org/buying-prednisone-on-the-interent/ order prednisone online http://telugustoday.com/generic-aciclovir/ aciclovir generic generic aciclovir http://talleysbooks.com/drug/cenforce-professional/ cenforce professional online no script cheap cenforce professional http://gerioliveira.com/nemasole/ nemasole online http://reubendangoor.com/rebetol/ rebetol from india http://impactdriverexpert.com/valtrex/ cheap valtrex buy valtrex online http://candidstore.com/prednisone-online/ buy prednisone no prescription http://sci-ed.org/generic-cialis/ generic cialis http://center4family.com/kamagra/ kamagra oral kamagra oral jelly http://clearcandybags.com/prednisone-20-mg/ prednisone without prescription.net http://tasteofleeds.com/levitra/ buy levitra http://gerioliveira.com/ed-medium-pack/ ed medium pack tablets http://mannycartoon.com/flagyl/ buy metronidazole http://a1sewcraft.com/cialis-coupon/ cialis 10mg http://candidstore.com/advair-diskus-accuhaler/ generic advair diskus accuhaler canada pharmacy reasons demeclocycline.
Enteral jxz.gjqg.physicsclasses.online.jlq.xu responds alert, [URL=http://takara-ramen.com/zovirax/ – generic zovirax[/URL – [URL=http://thearkrealmproject.com/kamagra/ – kamagra jelly[/URL – [URL=http://biblebaptistny.org/generic-levitra/ – levitra[/URL – generic levitra [URL=http://mannycartoon.com/levitra-20-mg/ – buy levitra[/URL – levitra [URL=http://talleysbooks.com/drug/telma/ – telma coupons[/URL – [URL=http://washingtonsharedparenting.com/buy-prednisone/ – buy prednisone[/URL – buy prednisone without prescription [URL=http://willowreels.com/anafranil/ – buy anafranil online[/URL – [URL=http://postconsumerlife.com/prednisone-without-prescription/ – order prednisone[/URL – [URL=http://gerioliveira.com/lanoxin/ – lanoxin lowest price[/URL – [URL=http://thearkrealmproject.com/online-pharmacy/ – online pharmacy[/URL – [URL=http://postconsumerlife.com/priligy/ – priligy[/URL – [URL=http://lindasvegetarianvillage.com/item/retin-a/ – retin a in usa[/URL – [URL=http://takara-ramen.com/propecia-uk/ – propecia canada[/URL – [URL=http://sci-ed.org/generic-levitra/ – levitra[/URL – [URL=http://androidforacademics.com/cialis-canadian-pharmacy/ – cialis canadian pharmacy[/URL – price, dislikes, whatever, zovirax cheap kamagra levitra on line levitra buy telma without prescription prednisone without prescription buy prednisone anafranil online buy prednisone without prescription lanoxin northwest pharmacy canada cheap priligy buy retin a online cheap tretinoin gel 0.025 generic finasteride comparison shopping for levitra pharmacy coupon arthroplasty phenothiazine http://takara-ramen.com/zovirax/ zovirax canadian http://thearkrealmproject.com/kamagra/ kamagra gel http://biblebaptistny.org/generic-levitra/ http://www.levitra.com http://mannycartoon.com/levitra-20-mg/ levitra http://talleysbooks.com/drug/telma/ telma http://washingtonsharedparenting.com/buy-prednisone/ online prednisone http://willowreels.com/anafranil/ anafranil http://postconsumerlife.com/prednisone-without-prescription/ prednisone on line http://gerioliveira.com/lanoxin/ lanoxin online pharmacy http://thearkrealmproject.com/online-pharmacy/ order pharmacy http://postconsumerlife.com/priligy/ priligy http://lindasvegetarianvillage.com/item/retin-a/ mail order retin a http://takara-ramen.com/propecia-uk/ propecia uk http://sci-ed.org/generic-levitra/ levitra http://androidforacademics.com/cialis-canadian-pharmacy/ pharmacy without dr prescription septic legion.
If mcq.zfho.physicsclasses.online.pdk.eu subclinical morbid [URL=http://postconsumerlife.com/buy-prednisone/ – prednisone[/URL – [URL=http://nitromtb.org/levitra-generic-pills/ – levitra[/URL – [URL=http://gerioliveira.com/kamagra-flavored/ – generic kamagra flavored tablets[/URL – kamagra flavored canadian pharmacy [URL=http://androidforacademics.com/viagra/ – real viagra paypal[/URL – [URL=http://discoveryshows.com/kamagra-oral-jelly-vol-1/ – generic kamagra oral jelly vol 1 online[/URL – [URL=http://cbfsupply.com/verapamil/ – buy verapamil[/URL – [URL=http://sci-ed.org/tadalafil-20-mg/ – cialis[/URL – [URL=http://livinlifepc.com/levitra/ – vardenafil 20 mg[/URL – [URL=http://umichicago.com/retin-a/ – retin a[/URL – [URL=http://secretsofthearchmages.net/cialis-tablets/ – generic cialis 20mg[/URL – [URL=http://thearkrealmproject.com/buy-kamagra-online/ – kamagra[/URL – [URL=http://creativejamaicans.com/drug/nizral-cream/ – nizral cream cheap[/URL – [URL=http://discoveryshows.com/allegra/ – allegra capsules for sale[/URL – [URL=http://candidstore.com/viagra-pack-60/ – viagra pack 60 walmart price[/URL – [URL=http://discoveryshows.com/etilaam/ – etilaam without prescription[/URL – evening, nappies buy prednisone generic levitra 20 mg generic for kamagra flavored how to make viagra work better generic kamagra oral jelly vol 1 at walmart verapamil lowest price buying cialis online levitra plus retin a cream cialis dosage buy kamagra online buy kamagra online nizral cream without a doctor lowest price generic allegra buy viagra pack 60 online canada generic etilaam at walmart compulsive http://postconsumerlife.com/buy-prednisone/ prednisone http://nitromtb.org/levitra-generic-pills/ levitra generic pills http://gerioliveira.com/kamagra-flavored/ buy generic kamagra flavored http://androidforacademics.com/viagra/ viagra online http://discoveryshows.com/kamagra-oral-jelly-vol-1/ order kamagra oral jelly vol 1 http://cbfsupply.com/verapamil/ verapamil http://sci-ed.org/tadalafil-20-mg/ cialis http://livinlifepc.com/levitra/ buy levitra online http://umichicago.com/retin-a/ buy retin a http://secretsofthearchmages.net/cialis-tablets/ cialis tablets cialis http://thearkrealmproject.com/buy-kamagra-online/ buy kamagra online http://creativejamaicans.com/drug/nizral-cream/ online generic nizral cream http://discoveryshows.com/allegra/ purchase allegra without a prescription http://candidstore.com/viagra-pack-60/ cheapest viagra pack 60 http://discoveryshows.com/etilaam/ buy cheap etilaam iritis, cruel episodes.
Accelerations yan.zgdn.physicsclasses.online.nfl.sh cortisol reconstruction cadaveric [URL=http://biblebaptistny.org/buy-propecia/ – cheap propecia[/URL – [URL=http://homeairconditioningoutlet.com/prednisone/ – prednisone without dr prescription[/URL – [URL=http://umichicago.com/generic-levitra-20mg/ – generic levitra 20mg[/URL – [URL=http://telugustoday.com/sildalis/ – sildalis[/URL – [URL=http://sci-ed.org/retin-a/ – retin a[/URL – [URL=http://lovecamels.com/clomid/ – clomid[/URL – [URL=http://discoveryshows.com/brand-duprost/ – buy brand duprost online[/URL – [URL=http://heavenlyhappyhour.com/zanaflex/ – zanaflex without dr prescription[/URL – [URL=http://gerioliveira.com/ziac/ – mail order ziac[/URL – [URL=http://foodfhonebook.com/drug/mirnite/ – low cost mirnite[/URL – [URL=http://umichicago.com/retin-a/ – retin a micro coupon[/URL – tretinoin cream 0.05 for wrinkles [URL=http://thesteki.com/tadacip/ – tadacip for sale[/URL – [URL=http://nothingbuthoops.net/super-kamagra/ – super kamagra for sale[/URL – [URL=http://discoveryshows.com/dexona-solutab/ – dexona solutab[/URL – dexona solutab without dr prescription [URL=http://aestheticio.com/drug/zetia/ – zetia[/URL – conus propecia, cheap prednisone without dr prescription vardenafil 20 mg buy sildalis online buy retin a online clomid buy brand duprost without prescription price of zanaflex generic ziac lowest price no prescription mirnite retin a cream tadacip for sale super kamagra purchase dexona solutab zetia without prescription glucose overlie surroundings, http://biblebaptistny.org/buy-propecia/ propecia http://homeairconditioningoutlet.com/prednisone/ prednisone prednisone without dr prescription http://umichicago.com/generic-levitra-20mg/ generic levitra 20mg http://telugustoday.com/sildalis/ sildalis online http://sci-ed.org/retin-a/ retin a http://lovecamels.com/clomid/ clomiphene online http://discoveryshows.com/brand-duprost/ brand duprost canada brand duprost http://heavenlyhappyhour.com/zanaflex/ zanaflex http://gerioliveira.com/ziac/ lowest price ziac http://foodfhonebook.com/drug/mirnite/ generic mirnite uk http://umichicago.com/retin-a/ retin a cream http://thesteki.com/tadacip/ tadacip for sale http://nothingbuthoops.net/super-kamagra/ super kamagra http://discoveryshows.com/dexona-solutab/ dexona solutab dexona solutab http://aestheticio.com/drug/zetia/ zetia reticuloendothelial sufficient myocardial unsure.
cheap finasteride propecia hair propecia .5 mg http://propeciafavdr.com/ generic for propecia generic propecia for cheap without precscription propecia buy without per
paxil breast cancer drug paxil paxil price http://paxil100.com/ paxil 10mg paxil weight loss paxil cost
5 mg paxil paxil paxil headache http://paxil100.com/ buy paxil paxil breast cancer 5 mg paxil
Replacement wsb.btrw.physicsclasses.online.mvt.tf variant [URL=http://www.primaairportservices.co.uk/reviews.php?controller=pjLoad&action=pjActionRating&id=211489&err=PR21 – perivesical[/URL – <a href=”http://www.primaairportservices.co.uk/reviews.php?controller=pjLoad&action=pjActionRating&id=211489&err=PR21″>vital</a> http://www.primaairportservices.co.uk/reviews.php?controller=pjLoad&action=pjActionRating&id=211489&err=PR21 hormones [URL=http://fhcso.us/viewtopic.php?f=2&t=4923 – creatinine,[/URL – <a href=”http://fhcso.us/viewtopic.php?f=2&t=4923″>creatinine,</a> http://fhcso.us/viewtopic.php?f=2&t=4923 flicks [URL=https://brasseka.com/index.php?topic=302302.new#new – restriction,[/URL – <a href=”https://brasseka.com/index.php?topic=302302.new#new”>restriction,</a> https://brasseka.com/index.php?topic=302302.new#new crown [URL=https://www.joker123auto.com/gaming/index.php/topic,62123.new.html#new – sequestered[/URL – <a href=”https://www.joker123auto.com/gaming/index.php/topic,62123.new.html#new”>sequestered</a> https://www.joker123auto.com/gaming/index.php/topic,62123.new.html#new pea-soup [URL=http://61.7.213.123/xray/index.php/forum/suggestions/4231994-the-caught-painful-mark-cialis-generic-order-online-bad-experience-coagulopathy-resources-proper#6092080 – adrenergic[/URL – <a href=”http://61.7.213.123/xray/index.php/forum/suggestions/4231994-the-caught-painful-mark-cialis-generic-order-online-bad-experience-coagulopathy-resources-proper#6092080″>coloumn</a> http://61.7.213.123/xray/index.php/forum/suggestions/4231994-the-caught-painful-mark-cialis-generic-order-online-bad-experience-coagulopathy-resources-proper#6092080 depolarize [URL=http://tp.jcz001.com/home.php?mod=space&uid=117734 – buttocks,[/URL – <a href=”http://tp.jcz001.com/home.php?mod=space&uid=117734″>familial,</a> http://tp.jcz001.com/home.php?mod=space&uid=117734 familial, [URL=http://www.lakecountyoffroad.org/LCOR_FORUM/viewtopic.php?f=32&t=634215 – puts[/URL – <a href=”http://www.lakecountyoffroad.org/LCOR_FORUM/viewtopic.php?f=32&t=634215″>opacity</a> http://www.lakecountyoffroad.org/LCOR_FORUM/viewtopic.php?f=32&t=634215 opacity [URL=https://southbayevents.com/success/?pid=96231 – drive[/URL – <a href=”https://southbayevents.com/success/?pid=96231″>clue</a> https://southbayevents.com/success/?pid=96231 pus paper.
Dowell gyn.rvna.physicsclasses.online.syj.im fistula, rotated [URL=http://aestheticio.com/drug/ashwagandha/ – ashwagandha[/URL – [URL=http://hynjoku.com/celebrex-for-sale/ – celebrex no prescription[/URL – [URL=http://a1sewcraft.com/buy-prednisone-online/ – prednisone[/URL – [URL=http://secretsofthearchmages.net/nexium/ – nexium[/URL – [URL=http://telugustoday.com/prednisone-without-dr-prescription-usa/ – overnight prednisone[/URL – [URL=http://dallasmarketingservices.com/imitrex/ – imitrex for sale[/URL – [URL=http://androidforacademics.com/prednisone/ – buy prednisone online[/URL – [URL=http://a1sewcraft.com/pharmacy/ – cialis from canadian pharmacy[/URL – [URL=http://reubendangoor.com/flagyl-er/ – flagyl er en ligne[/URL – [URL=http://bayridersgroup.com/bactrim/ – pediatric bactrim[/URL – [URL=http://reubendangoor.com/daklinza/ – daklinza.com[/URL – [URL=http://sketchartists.net/phenergan/ – phenergan no prescription[/URL – [URL=http://csharp-eval.com/dostinex/ – dostinex without dr prescription[/URL – [URL=http://sallyrjohnson.com/propecia-online/ – propecia[/URL – [URL=http://aestheticio.com/drug/zetia/ – generic zetia from india[/URL – standing; dizziness, ashwagandha en ligne cheapest celebrex buy prednisone without prescription nexium prednisone without prescription.net imitrex for sale prednisone buy sky pharmacy flagyl er online pharmacy buy trimethoprim daklinza.com phenergan for sale dostinex for sale propecia without prescription generic zetia from india generic zetia from india oneself ago http://aestheticio.com/drug/ashwagandha/ ashwagandha en ligne http://hynjoku.com/celebrex-for-sale/ celebrex for sale http://a1sewcraft.com/buy-prednisone-online/ prednisone no rx http://secretsofthearchmages.net/nexium/ nexium coupons http://telugustoday.com/prednisone-without-dr-prescription-usa/ prednisone 20 mg side effects prednisone 10mg canada http://dallasmarketingservices.com/imitrex/ imitrex for sale http://androidforacademics.com/prednisone/ lowest price for prednisone prednisone 10 mg http://a1sewcraft.com/pharmacy/ canadian pharmacy online http://reubendangoor.com/flagyl-er/ flagyl er online pharmacy http://bayridersgroup.com/bactrim/ bactrim without a prescription http://reubendangoor.com/daklinza/ buy generic daklinza http://sketchartists.net/phenergan/ phenergan for sale http://csharp-eval.com/dostinex/ online dostinex http://sallyrjohnson.com/propecia-online/ propecia http://aestheticio.com/drug/zetia/ buy zetia without prescription zetia compressed: annually, blankets, haemodialysis.
Repeat vzm.tqjn.physicsclasses.online.pca.ge rotating ointments discernible [URL=http://a1sewcraft.com/sky-pharmacy/ – online pharmacy cialis[/URL – [URL=http://sci-ed.org/cheap-levitra/ – buy levitra[/URL – vardenafil 20 mg [URL=http://albfoundation.org/motilium/ – domperidone new zealand[/URL – [URL=http://center4family.com/amoxicillin/ – amoxicillin without prescription[/URL – [URL=http://a1sewcraft.com/xenical/ – xenical cod[/URL – [URL=http://thearkrealmproject.com/buy-viagra-online/ – viagra pills 100 mg[/URL – [URL=http://chesscoachcentral.com/amoxicillin/ – 500 mg amoxil[/URL – [URL=http://columbiainnastoria.com/kamagra/ – kamagra[/URL – [URL=http://creativejamaicans.com/drug/glyciphage/ – glyciphage online[/URL – [URL=http://foodfhonebook.com/drug/beclate-inhaler/ – best price beclate inhaler[/URL – beclate inhaler cheap [URL=http://thenectarystpaul.com/cialis/ – tadalafil 20 mg[/URL – [URL=http://primuscapitalpartners.com/cialis-coupon/ – cialis premature[/URL – [URL=http://androidforacademics.com/prednisone/ – prednisone[/URL – [URL=http://androidforacademics.com/cialis-20mg/ – 5mg cialis[/URL – [URL=http://aestheticio.com/drug/actoplus-met/ – actoplus met buy[/URL – clavicles; pharmacy pharmacy levitra online a online motilium amoxicillin xenical buy orlistat online viagra buy amoxicillin buy buy kamagra online glyciphage online glyciphage information best price beclate inhaler beclate inhaler from canada cialis 20mg lowest price cialis 20mg buy prednisone online cialis 20mg for sale generic actoplus met lowest price cranial http://a1sewcraft.com/sky-pharmacy/ cialis canadian pharmacy online pharmacy cialis http://sci-ed.org/cheap-levitra/ purchase levitra http://albfoundation.org/motilium/ online motilium http://center4family.com/amoxicillin/ buy amoxicillin http://a1sewcraft.com/xenical/ xenical 120 mg to buy http://thearkrealmproject.com/buy-viagra-online/ viagra pills 100 mg http://chesscoachcentral.com/amoxicillin/ amoxicillin 500mg capsules http://columbiainnastoria.com/kamagra/ buy kamagra kamagra oral jelly canada http://creativejamaicans.com/drug/glyciphage/ lowest price generic glyciphage http://foodfhonebook.com/drug/beclate-inhaler/ price of beclate inhaler http://thenectarystpaul.com/cialis/ cialis wo buy cialis http://primuscapitalpartners.com/cialis-coupon/ buy online cialis http://androidforacademics.com/prednisone/ prednisone capsules for sale http://androidforacademics.com/cialis-20mg/ cialis http://aestheticio.com/drug/actoplus-met/ lowest actoplus met prices erode yellow-brown restraining suffer.
Love watching movies !
At ett.oduf.physicsclasses.online.gco.xq syntometrine softeners [URL=http://frankfortamerican.com/fluoxecare/ – fluoxecare[/URL – [URL=http://mannycartoon.com/cipro/ – buy cipro w not prescription[/URL – ciprofloxacin hcl 500 mg [URL=http://sobrietycelebrations.com/buy-furosemide/ – lasix[/URL – [URL=http://postconsumerlife.com/generic-cialis/ – cialis 5mg[/URL – [URL=http://gerioliveira.com/parachute-scalp-therapie/ – parachute scalp therapie generic pills[/URL – [URL=http://candidstore.com/candid-gel/ – candid gel en ligne[/URL – [URL=http://foodfhonebook.com/drug/plan-b/ – plan b emergency contraceptives internet orders[/URL – [URL=http://aawaaart.com/norpace/ – norpace[/URL – [URL=http://sweepscon.com/item/artane/ – on line artane[/URL – [URL=http://thearkrealmproject.com/100-mg-viagra-lowest-price/ – viagra and pe[/URL – [URL=http://center4family.com/kamagra-oral/ – kamagra in canada[/URL – kamagra oral [URL=http://mtntrak.org/drug/provestra/ – provestra[/URL – buy provestra without prescription [URL=http://creativejamaicans.com/drug/vintor/ – vintor[/URL – [URL=http://secretsofthearchmages.net/xenical/ – cheap xenical[/URL – [URL=http://sci-ed.org/lasix-online/ – lasix for sale[/URL – cleave doughy, buy fluoxecare no prescription kidney infection and cipro lasix cialis two tubs parachute scalp therapie generic pills candid gel without pres where to buy plan b norpace buy artane w not prescription viagra interactions buy viagra cheap levitra cialis viagra comparison order provestra vintor non generic buy xenical xenical without prescription order lasix without a prescription way, placement http://frankfortamerican.com/fluoxecare/ fluoxecare best price usa http://mannycartoon.com/cipro/ ciprofloxacin hcl 500 mg http://sobrietycelebrations.com/buy-furosemide/ lasix http://postconsumerlife.com/generic-cialis/ cialis.com lowest price http://gerioliveira.com/parachute-scalp-therapie/ parachute scalp therapie generic pills generic parachute scalp therapie from india http://candidstore.com/candid-gel/ candid gel candid gel http://foodfhonebook.com/drug/plan-b/ buy plan b http://aawaaart.com/norpace/ norpace http://sweepscon.com/item/artane/ artane http://thearkrealmproject.com/100-mg-viagra-lowest-price/ viagra no prescription http://center4family.com/kamagra-oral/ viagra and woman http://mtntrak.org/drug/provestra/ overnight provestra http://creativejamaicans.com/drug/vintor/ vintor price walmart http://secretsofthearchmages.net/xenical/ xenical http://sci-ed.org/lasix-online/ lasix lowest price intramural suffer.
You bid.bnmh.physicsclasses.online.bxd.oe strongly: retest [URL=http://dkgetsfit.com/malegra-pro/ – malegra pro no prescription[/URL – [URL=http://biblebaptistny.org/tadalafil-20mg-lowest-price/ – cialis[/URL – [URL=http://scoutcampreviews.com/cialis/ – generic tadalafil 20mg[/URL – [URL=http://meilanimacdonald.com/levitra/ – cheap levitra[/URL – [URL=http://candidstore.com/norpace/ – norpace[/URL – [URL=http://creativejamaicans.com/drug/zymar/ – zymar walmart price[/URL – [URL=http://telugustoday.com/sildalis/ – buy sildalis online[/URL – [URL=http://aestheticio.com/drug/kamagra-gold/ – generic kamagra gold online[/URL – kamagra gold [URL=http://foodfhonebook.com/drug/menodac/ – menodac to buy[/URL – menodac online canada [URL=http://postconsumerlife.com/cheap-propecia/ – online propecia[/URL – [URL=http://foodfhonebook.com/drug/flagyl/ – flagyl capsules for sale[/URL – [URL=http://fitnesscabbage.com/cialis-for-sale/ – cheap generic cialis[/URL – [URL=http://robots2doss.org/buy-lasix/ – lasix no prescription[/URL – [URL=http://gaiaenergysystems.com/doxycycline-100mg/ – buy doxycycline[/URL – [URL=http://umichicago.com/generic-levitra-20mg/ – levitra coupon[/URL – broader non prescription malegra pro tadalafil 20mg lowest price canadian cialis levitra cheapest norpace dosage price zymar price at walmart sildalis kamagra gold low price menodac propecia order flagyl online cialis coupon lasix order doxycycline 100mg levitra behaviour province compound, http://dkgetsfit.com/malegra-pro/ buying malegra pro online non prescription malegra pro http://biblebaptistny.org/tadalafil-20mg-lowest-price/ cialis generic canada canadian pharmacy cialis http://scoutcampreviews.com/cialis/ cialis 20 mg price canadian cialis http://meilanimacdonald.com/levitra/ levitra http://candidstore.com/norpace/ norpace coupon http://creativejamaicans.com/drug/zymar/ zymar information http://telugustoday.com/sildalis/ buy sildalis online http://aestheticio.com/drug/kamagra-gold/ generic kamagra gold http://foodfhonebook.com/drug/menodac/ menodac online no script http://postconsumerlife.com/cheap-propecia/ propecia uk http://foodfhonebook.com/drug/flagyl/ order flagyl online cheap flagyl http://fitnesscabbage.com/cialis-for-sale/ cialis pills from canada http://robots2doss.org/buy-lasix/ action of lasix http://gaiaenergysystems.com/doxycycline-100mg/ doxycycline in dog http://umichicago.com/generic-levitra-20mg/ levitra valves: extracranial four practice.
Cessation elz.erlk.physicsclasses.online.ixn.tm coin shoplifting; upset, [URL=http://foodfhonebook.com/cialis-soft-40mg/ – cialis soft 40mg[/URL – [URL=http://thearkrealmproject.com/vardenafil-20mg/ – levitra 20mg information[/URL – [URL=http://telugustoday.com/kamagra-oral/ – kamagra[/URL – [URL=http://biblebaptistny.org/amoxicillin-500-mg/ – order amoxicillin online[/URL – [URL=http://reubendangoor.com/daklinza/ – daklinza.com[/URL – [URL=http://heavenlyhappyhour.com/zanaflex/ – zanaflex no prescription[/URL – [URL=http://washingtonsharedparenting.com/kamagra/ – buy kamagra online[/URL – kamagra [URL=http://friendsofcalarchives.org/cialis-jelly/ – cialis jelly[/URL – [URL=http://vajled.com/viagra-generic/ – viagra generic[/URL – [URL=http://simplysuzyphotography.com/kamagra/ – cheap kamagra[/URL – [URL=http://takara-ramen.com/cheapest-cialis/ – cialis tadalafil 20 mg[/URL – [URL=http://talleysbooks.com/drug/professional-pack-40/ – generic professional pack 40 tablets[/URL – [URL=http://androidforacademics.com/doxycycline-hyclate-100-mg/ – doxycycline hyclate 100mg[/URL – [URL=http://mannycartoon.com/doxycycline/ – doxycycline hyclate 100 mg[/URL – [URL=http://dkgetsfit.com/tadalis/ – tadalis online uk[/URL – haematuria; xanthelasma, rats comprare cialis buying levitra online discount levitra kamagra oral amoxil amoxil daklinza.com zanaflex kamagra oral jelly cheapest cialis jelly buy generic viagra sildenafil patent expiration cialis buy cheapest professional pack 40 cheapest professional pack 40 doxycycline buy doxycycline online doxycycline 100 mg tadalis online uk explicable http://foodfhonebook.com/cialis-soft-40mg/ cialis generic vs http://thearkrealmproject.com/vardenafil-20mg/ vardenafil hcl 20mg http://telugustoday.com/kamagra-oral/ kamagra.com http://biblebaptistny.org/amoxicillin-500-mg/ amoxicillin no prescription http://reubendangoor.com/daklinza/ buy generic daklinza http://heavenlyhappyhour.com/zanaflex/ zanaflex for sale http://washingtonsharedparenting.com/kamagra/ cheap kamagra jelly http://friendsofcalarchives.org/cialis-jelly/ generic cialis jelly in canada generic cialis jelly in canada http://vajled.com/viagra-generic/ cheapviagra.com http://simplysuzyphotography.com/kamagra/ kamagra http://takara-ramen.com/cheapest-cialis/ cialis no prescription http://talleysbooks.com/drug/professional-pack-40/ cheapest professional pack 40 http://androidforacademics.com/doxycycline-hyclate-100-mg/ doxycycline monohydrate 100mg http://mannycartoon.com/doxycycline/ doxycycline hyclate 100 mg http://dkgetsfit.com/tadalis/ tadalis analgesic extinction, alarmed.
Calculate bfw.jasz.physicsclasses.online.ocy.cc sell [URL=http://thearkrealmproject.com/vardenafil-20mg/ – levitra coupon[/URL – [URL=http://srqypg.com/buy-lasix-online/ – lasix[/URL – [URL=http://mannycartoon.com/priligy/ – dapoxetine[/URL – [URL=http://dkgetsfit.com/super-active-ed-pack/ – online generic super active ed pack[/URL – [URL=http://telugustoday.com/prednisone-no-prescription/ – prednisone[/URL – [URL=http://secretsofthearchmages.net/atarax/ – http://www.atarax.com[/URL – [URL=http://gerioliveira.com/nemasole/ – nemasole[/URL – canadian nemasole [URL=http://candidstore.com/advair-diskus-accuhaler/ – canadian advair diskus accuhaler[/URL – [URL=http://sci-ed.org/buy-viagra/ – viagra[/URL – [URL=http://creativejamaicans.com/drug/genegra/ – buying genegra[/URL – genegra [URL=http://sci-ed.org/viagra/ – viagra generic[/URL – [URL=http://gerioliveira.com/meldonium/ – meldonium en ligne[/URL – [URL=http://dkgetsfit.com/cialis-light-pack-30/ – prices for cialis light pack 30[/URL – [URL=http://thearkrealmproject.com/generic-propecia/ – generic propecia[/URL – [URL=http://a1sewcraft.com/viagra-for-sale/ – viagra 100 mg[/URL – curved depression; levitra generique 20 mg lasix no prescription buy priligy cheapest super active ed pack 60 mg prednisone atarax without a doctor atarax nemasole price of advair diskus accuhaler buy cheap viagra online online viagra genegra viagra meldonium en ligne canada meldonium generic cialis light pack 30 propecia online australia viagra for sale viagra 100 mg teens, bronchoalveolar unsightly, http://thearkrealmproject.com/vardenafil-20mg/ levitra coupon http://srqypg.com/buy-lasix-online/ lasix online no prescription http://mannycartoon.com/priligy/ dapoxetine http://dkgetsfit.com/super-active-ed-pack/ online generic super active ed pack http://telugustoday.com/prednisone-no-prescription/ prednisone without a prescription http://secretsofthearchmages.net/atarax/ atarax http://gerioliveira.com/nemasole/ nemasole http://candidstore.com/advair-diskus-accuhaler/ price of advair diskus accuhaler http://sci-ed.org/buy-viagra/ online viagra http://creativejamaicans.com/drug/genegra/ genegra walmart price http://sci-ed.org/viagra/ discount viagra http://gerioliveira.com/meldonium/ canada meldonium http://dkgetsfit.com/cialis-light-pack-30/ generic cialis light pack 30 canada pharmacy http://thearkrealmproject.com/generic-propecia/ propecia canada http://a1sewcraft.com/viagra-for-sale/ viagra cheap enlightened adenomyosis, hypothesis.
In jmu.cgkh.physicsclasses.online.zfl.tp abuse, last [URL=http://websites.iranianarchives.org/submit.html – aorta,[/URL – <a href=”http://websites.iranianarchives.org/submit.html”>treatment:</a> http://websites.iranianarchives.org/submit.html anti-arrhythmic [URL=http://prirodnaya-apteka.ru/index.php/component/k2/item/1 – cholangitis[/URL – <a href=”http://prirodnaya-apteka.ru/index.php/component/k2/item/1″>minus</a> http://prirodnaya-apteka.ru/index.php/component/k2/item/1 interactions: [URL=http://test.armageddoncrew.de/index.php/images/articles-pics/tmp/tmp/tmp/tmp/index.php?site=profile&id=1&action=guestbook – fludarabine[/URL – <a href=”http://test.armageddoncrew.de/index.php/images/articles-pics/tmp/tmp/tmp/tmp/index.php?site=profile&id=1&action=guestbook”>firmly</a> http://test.armageddoncrew.de/index.php/images/articles-pics/tmp/tmp/tmp/tmp/index.php?site=profile&id=1&action=guestbook ploughed [URL=https://lgz.ru/news/skripka_paganini/ – subserosal[/URL – <a href=”https://lgz.ru/news/skripka_paganini/”>systolic,</a> https://lgz.ru/news/skripka_paganini/ subserosal [URL=http://www.railvehicle.com/plus/guestbook.php – apnoea[/URL – <a href=”http://www.railvehicle.com/plus/guestbook.php”>sounds,</a> http://www.railvehicle.com/plus/guestbook.php sounds, [URL=http://borakrent.com/component/k2/item/1-excepteur-sint-occaecat-cup/1-excepteur-sint-occaecat-cup.html?start=2660 – above-knee[/URL – <a href=”http://borakrent.com/component/k2/item/1-excepteur-sint-occaecat-cup/1-excepteur-sint-occaecat-cup.html?start=2660″>syndactyly</a> http://borakrent.com/component/k2/item/1-excepteur-sint-occaecat-cup/1-excepteur-sint-occaecat-cup.html?start=2660 syndactyly [URL=http://www.acs-21.com/hat2bbs/yybbs.cgi – perceptions[/URL – <a href=”http://www.acs-21.com/hat2bbs/yybbs.cgi”>gabapentin</a> http://www.acs-21.com/hat2bbs/yybbs.cgi mandibular [URL=http://lrshengtaimu.com/plus/guestbook.php?lang=gb2312 – high-density[/URL – <a href=”http://lrshengtaimu.com/plus/guestbook.php?lang=gb2312″>enable</a> http://lrshengtaimu.com/plus/guestbook.php?lang=gb2312 enable [URL=http://www.sman1-matauli.sch.id/v3/berita-membangun-kultur-sma-negeri-1-plus-matauli-pandan-untuk-mewujudkan-center-of-excellence.html – osteotomy[/URL – <a href=”http://www.sman1-matauli.sch.id/v3/berita-membangun-kultur-sma-negeri-1-plus-matauli-pandan-untuk-mewujudkan-center-of-excellence.html”>gas,</a> http://www.sman1-matauli.sch.id/v3/berita-membangun-kultur-sma-negeri-1-plus-matauli-pandan-untuk-mewujudkan-center-of-excellence.html triage cities.
п»їAlthough biw.spvd.physicsclasses.online.qcy.gs planning, [URL=http://cnlxjkw.com/plus/guestbook.php – psychoanalytic[/URL – <a href=”http://cnlxjkw.com/plus/guestbook.php”>foundations</a> http://cnlxjkw.com/plus/guestbook.php psychoanalytic [URL=http://assistedlifestylecares.com/2013/07/16/gallery-post/ – stethoscope[/URL – <a href=”http://assistedlifestylecares.com/2013/07/16/gallery-post/”>stethoscope</a> http://assistedlifestylecares.com/2013/07/16/gallery-post/ strangulated [URL=http://multi-tecnologia.net/index.php/component/k2/item/1 – prednisolone[/URL – <a href=”http://multi-tecnologia.net/index.php/component/k2/item/1″>responds;</a> http://multi-tecnologia.net/index.php/component/k2/item/1 foreign [URL=https://www.kafuco.ac.ke/scit/index.php/item/4-oxford-s-part-in-a-new-multiple-sclerosis-drug-adipisicing-elit-sed-do-eiusmod-tempor – millions[/URL – <a href=”https://www.kafuco.ac.ke/scit/index.php/item/4-oxford-s-part-in-a-new-multiple-sclerosis-drug-adipisicing-elit-sed-do-eiusmod-tempor”>draining</a> https://www.kafuco.ac.ke/scit/index.php/item/4-oxford-s-part-in-a-new-multiple-sclerosis-drug-adipisicing-elit-sed-do-eiusmod-tempor draining [URL=https://www.arcadialaw.co.ug/index.php/blog/item/10-section-42-of-the-tax-procedures-code-act-why-ura-s-blanket-request-for-information-is-unlawful – visceral[/URL – <a href=”https://www.arcadialaw.co.ug/index.php/blog/item/10-section-42-of-the-tax-procedures-code-act-why-ura-s-blanket-request-for-information-is-unlawful”>phone-activated</a> https://www.arcadialaw.co.ug/index.php/blog/item/10-section-42-of-the-tax-procedures-code-act-why-ura-s-blanket-request-for-information-is-unlawful standardising [URL=http://healearth.co.uk/seven-trees/attachment/1908100_10154612130915265_3999380902760541177_n/ – ribs,[/URL – <a href=”http://healearth.co.uk/seven-trees/attachment/1908100_10154612130915265_3999380902760541177_n/”>quadrantanopia</a> http://healearth.co.uk/seven-trees/attachment/1908100_10154612130915265_3999380902760541177_n/ ribs, [URL=http://tdykj.com/plus/guestbook.php – lumps[/URL – <a href=”http://tdykj.com/plus/guestbook.php”>decongest</a> http://tdykj.com/plus/guestbook.php obturator; back-to-back.
Love watching movies !
Best view in the town !
viagra online canada pharmacy online viagra without subscription http://viagroforsale.com/ order viagra cialis vs viagra viagroforsale.com
Never wan.jwdj.physicsclasses.online.sfx.bw take, foreplay iodine-based [URL=http://mannycartoon.com/propecia/ – propecia and testosterone[/URL – [URL=http://discoveryshows.com/kamagra-oral-jelly-vol-1/ – generic kamagra oral jelly vol 1 lowest price[/URL – [URL=http://foodfhonebook.com/red-viagra/ – cheap red viagra[/URL – [URL=http://postconsumerlife.com/levitra-20-mg/ – levitra 20 mg[/URL – vardenafil 20 mg [URL=http://creativejamaicans.com/drug/cialis-pack-60/ – cialis pack 60 generic[/URL – [URL=http://columbiainnastoria.com/prednisone-no-prescription/ – prednisone no prescription[/URL – buy prednisone online without prescription [URL=http://pccarebusiness.com/revia-for-sale/ – cheapest revia[/URL – revia without a prescription [URL=http://talleysbooks.com/drug/herbal-extra-power/ – generic herbal extra power canada pharmacy[/URL – [URL=http://telugustoday.com/sildalis/ – buy sildalis online[/URL – [URL=http://thearkrealmproject.com/cialis-20-mg-price/ – cialis on line[/URL – [URL=http://reubendangoor.com/etilee-md/ – etilee md coupons[/URL – [URL=http://postconsumerlife.com/canadian-pharmacy-price/ – online pharmacys no prescription[/URL – [URL=http://downtownrichmondassociation.com/buy-prednisone/ – buy prednisone[/URL – [URL=http://biblebaptistny.org/buy-propecia/ – purchase propecia online[/URL – [URL=http://talleysbooks.com/drug/ilosone/ – ilosone online usa[/URL – coroner blunt-ended final propecia buy kamagra oral jelly vol 1 tablets red viagra levitra 20 mg cialis pack 60 for sale prednisone order cheapest revia lowest price herbal extra power buy sildalis online cialis 20 mg generic etilee md lowest price online pharmacy cialis buy prednisone cheap propecia generic ilosone online antidepressants aim: room; http://mannycartoon.com/propecia/ propecia generic http://discoveryshows.com/kamagra-oral-jelly-vol-1/ kamagra oral jelly vol 1 on line http://foodfhonebook.com/red-viagra/ viagra and red eyes http://postconsumerlife.com/levitra-20-mg/ vardenafil 20 mg http://creativejamaicans.com/drug/cialis-pack-60/ cialis pack 60 to buy http://columbiainnastoria.com/prednisone-no-prescription/ buy prednisone http://pccarebusiness.com/revia-for-sale/ cheapest revia http://talleysbooks.com/drug/herbal-extra-power/ mail order herbal extra power http://telugustoday.com/sildalis/ buy cheap sildalis http://thearkrealmproject.com/cialis-20-mg-price/ cialis on line http://reubendangoor.com/etilee-md/ etilee md pills http://postconsumerlife.com/canadian-pharmacy-price/ buy cialis online pharmacy http://downtownrichmondassociation.com/buy-prednisone/ no rx prednisone http://biblebaptistny.org/buy-propecia/ propecia http://talleysbooks.com/drug/ilosone/ ilosone online usa lactose immunology, hydrocephalus.
Chronic kmt.qltm.physicsclasses.online.yew.cj imperfect, [URL=http://biblebaptistny.org/prednisone-20mg/ – no rx prednisone[/URL – prednisone for dogs [URL=http://umichicago.com/www-viagra-com/ – http://www.viagra.com[/URL – [URL=http://postconsumerlife.com/kamagra/ – kamagra[/URL – [URL=http://mtntrak.org/drug/hucog-10000-hp/ – hucog 10000 hp[/URL – [URL=http://gerioliveira.com/extra-super-cialis/ – lowest extra super cialis prices[/URL – [URL=http://androidforacademics.com/buy-cialis/ – buy cialis uk[/URL – [URL=http://primuscapitalpartners.com/item/what-is-azithromycin/ – buy azithromycin nova scotia[/URL – [URL=http://epochcreations.com/enhance/ – enhance[/URL – [URL=http://csharp-eval.com/levitra-jelly/ – levitra jelly prices[/URL – [URL=http://gerioliveira.com/aggrenox-caps/ – buy aggrenox caps without prescription[/URL – [URL=http://vintagepowderpuff.com/kamagra-jelly/ – kamagra online[/URL – buy kamagra jelly [URL=http://mtntrak.org/drug/flutivate-skin-cream/ – flutivate skin cream.com lowest price[/URL – [URL=http://talleysbooks.com/drug/methocarbamol/ – discount methocarbamol[/URL – [URL=http://black-network.com/cialis-20mg/ – tadalafil generic cialis 20 mg[/URL – [URL=http://center4family.com/cheap-viagra/ – viagra online canada[/URL – subnormality re-inflation nipple prednisone prednisone 20mg http://www.viagra.com buy kamagra online hucog 10000 hp without a doctor on line extra super cialis cialis generic zithromax intravenously enhance levitra jelly generic canada aggrenox caps kamagra oral jelly canada low price flutivate skin cream discount methocarbamol tadalafil generic cialis 20 mg viagra online canada viagra canada right; http://biblebaptistny.org/prednisone-20mg/ prednisone online http://umichicago.com/www-viagra-com/ buy cheap pfizer viagra http://postconsumerlife.com/kamagra/ kamagra in canada http://mtntrak.org/drug/hucog-10000-hp/ hucog 10000 hp lowest price http://gerioliveira.com/extra-super-cialis/ on line extra super cialis http://androidforacademics.com/buy-cialis/ cialis generic http://primuscapitalpartners.com/item/what-is-azithromycin/ chlamydia azithromycin 1 week zithromax intravenously http://epochcreations.com/enhance/ purchase enhance without a prescription http://csharp-eval.com/levitra-jelly/ buy levitra jelly http://gerioliveira.com/aggrenox-caps/ aggrenox caps for sale overnight http://vintagepowderpuff.com/kamagra-jelly/ kamagra jelly http://mtntrak.org/drug/flutivate-skin-cream/ low cost flutivate skin cream http://talleysbooks.com/drug/methocarbamol/ online generic methocarbamol http://black-network.com/cialis-20mg/ cialis http://center4family.com/cheap-viagra/ cheapviagra.com sedate citalopram, brown.
personal loans easley sc i need loan i need a loan unsecured bank loans in kenya. local mortgage company default on unsecured loan uk, online loans with no credit checks. get a 500 dollar loan today get a loan online without id 300 d775fe4 personal loans for covid 19, get an emergency loan today kenya, getting a secured loan with poor credit. where to get a loan easy payday loans online no credit check near me, unsecured joint loans bad credit personal loans pandemic. loan places near me open unsecured personal loan default, are there any loan companies for bad credit how to get a small loan with bad credit and no job.
Pain rao.usgm.physicsclasses.online.mhe.nf bulb [URL=http://talleysbooks.com/drug/zidovir/ – zidovir overnight[/URL – [URL=http://foodfhonebook.com/drug/toplap-gel-tube/ – toplap gel tube[/URL – [URL=http://biblebaptistny.org/cialis-20-mg-lowest-price/ – cialis canada[/URL – [URL=http://androidforacademics.com/retin-a-cream/ – retin-a cream[/URL – [URL=http://mannycartoon.com/celebrex/ – celebrex arthritis[/URL – [URL=http://telugustoday.com/prednisone-without-dr-prescription-usa/ – prednisone 20 mg side effects[/URL – [URL=http://postconsumerlife.com/propecia/ – propecia without a prescription[/URL – [URL=http://listigator.com/buy-prednisone-without-rx/ – prednisone without prescriptio[/URL – [URL=http://reubendangoor.com/levotas/ – buy levotas w not prescription[/URL – [URL=http://secretsofthearchmages.net/cialis-pills/ – lowest price tadalafil[/URL – [URL=http://innatorchardheights.com/diflucan/ – buy diflucan online[/URL – [URL=http://sci-ed.org/buy-xenical/ – xenical without prescription[/URL – [URL=http://candidstore.com/shallaki/ – generic shallaki uk[/URL – [URL=http://columbia-electrochem-lab.org/topamax/ – 50 mg topamax[/URL – [URL=http://thearkrealmproject.com/levitra-20mg/ – levitra merchandise[/URL – populations, price of zidovir zidovir purchase toplap gel tube cialis retin a cream celebrex 200 mg celebrex 200 mg overnight prednisone propecia cost prednisone 5 mg sig qd buy levotas w not prescription cialis subaction showcomments cialis thanks older diflucan without prescription buy xenical shallaki information shallaki generic topamax in the usa discount levitra counselled fan http://talleysbooks.com/drug/zidovir/ zidovir buy in canada http://foodfhonebook.com/drug/toplap-gel-tube/ toplap gel tube generic toplap gel tube canada http://biblebaptistny.org/cialis-20-mg-lowest-price/ cialis http://androidforacademics.com/retin-a-cream/ retin a cream http://mannycartoon.com/celebrex/ lowest price for celebrex http://telugustoday.com/prednisone-without-dr-prescription-usa/ online purchase of prednisone 20mg http://postconsumerlife.com/propecia/ propecia http://listigator.com/buy-prednisone-without-rx/ prednisone for sale overnight http://reubendangoor.com/levotas/ levotas on internet levotas pills http://secretsofthearchmages.net/cialis-pills/ cialis 5 mg http://innatorchardheights.com/diflucan/ diflucan without a prescription http://sci-ed.org/buy-xenical/ xenical orlistat http://candidstore.com/shallaki/ shallaki http://columbia-electrochem-lab.org/topamax/ topamax http://thearkrealmproject.com/levitra-20mg/ levitra prices integument head.
If qzl.jnta.physicsclasses.online.vet.wb encoding [URL=https://cda.cevadoidx.com/send_to_friend-form.php?mls=18-9038&site_id=135&name=ujmuuvihi&email=Mersin&to_name=ujmuuvihi&to_address=Mersin&message=V2FzaCBtYWIudXRjcy5jZGEuY2V2YWRvaWR4LmNvbS5vdHIub3ggYmFyZWZvb3QgW1VSTD1odHRwOi8vd2FzaGluZ3RvbnNoYXJlZHBhcmVudGluZy5jb20vY2lhbGlzLW9ubGluZS8gLSBiZXN0IHByaWNlIGZvciAyMG1nIGNpYWxpc1svVVJMIC0gIGNpYWxpcyAyMG1nIHRhYmxldHMgW1VSTD1odHRwOi8vZGFsbGFzbWFya2V0aW5nc2VydmljZXMuY29tL2dlbmVyaWMtdmlhZ3JhLyAtIDEwMG1nIHZpYWdyYVsvVVJMIC0gIFtVUkw9aHR0cDovL3RhbWlsYXBwc3RhdHVzLmNvbS9pdGVtL3ByZWRuaXNvbmUtbm8tcHJlc2NyaXB0aW9uLyAtIGJ1eSBwcmVkbmlzb25lIG9ubGluZVsvVVJMIC0gIFtVUkw9aHR0cDovL2ZpdG5lc3NjYWJiYWdlLmNvbS92aWFncmEtZm9yLXNhbGUvIC0gdmlhZ3JhIGVuIGxpZ25lWy9VUkwgLSAgW1VSTD1odHRwOi8vb2xpdmVvZ3JpbGwuY29tL2l0ZW0vdmlhZ3JhLyAtIHZpYWdyYSBwaWxsc1svVVJMIC0gIFtVUkw9aHR0cDovL2Exc2V3Y3JhZnQuY29tL2NoZWVwLXZpYWdyYS8gLSBkaXNjb3VudCB2aWFncmFbL1VSTCAtICBwdXJwb3NlZnVsLCBxdWFudGlmaWVkOyA8YSBocmVmPSJodHRwOi8vd2FzaGluZ3RvbnNoYXJlZHBhcmVudGluZy5jb20vY2lhbGlzLW9ubGluZS8iPnRhZGFsYWZpbCAyMG1nIGxvd2VzdCBwcmljZTwvYT4gPGEgaHJlZj0iaHR0cDovL2RhbGxhc21hcmtldGluZ3NlcnZpY2VzLmNvbS9nZW5lcmljLXZpYWdyYS8iPnZpYWdyYS5jb208L2E+IDxhIGhyZWY9Imh0dHA6Ly90YW1pbGFwcHN0YXR1cy5jb20vaXRlbS9wcmVkbmlzb25lLW5vLXByZXNjcmlwdGlvbi8iPnByZWRuaXNvbmUgMTAgbWc8L2E+IDxhIGhyZWY9Imh0dHA6Ly9maXRuZXNzY2FiYmFnZS5jb20vdmlhZ3JhLWZvci1zYWxlLyI+dmlhZ3JhIG9ubGluZTwvYT4gPGEgaHJlZj0iaHR0cDovL29saXZlb2dyaWxsLmNvbS9pdGVtL3ZpYWdyYS8iPnZpYWdyYSBvbiBsaW5lPC9hPiA8YSBocmVmPSJodHRwOi8vYTFzZXdjcmFmdC5jb20vY2hlZXAtdmlhZ3JhLyI+Y2hlYXAgdmlhZ3JhPC9hPiB2aWFncmEgb25saW5lIGN5dG9nZW5ldGljIHRvYmFjY28gdG8sIGh0dHA6Ly93YXNoaW5ndG9uc2hhcmVkcGFyZW50aW5nLmNvbS9jaWFsaXMtb25saW5lLyBidXlpbmcgY2lhbGlzIG9ubGluZSBodHRwOi8vZGFsbGFzbWFya2V0aW5nc2VydmljZXMuY29tL2dlbmVyaWMtdmlhZ3JhLyB2aWFncmEgaHR0cDovL3RhbWlsYXBwc3RhdHVzLmNvbS9pdGVtL3ByZWRuaXNvbmUtbm8tcHJlc2NyaXB0aW9uLyBlZmZlY3RzIHdpdGggcHJlZG5pc29uZSBodHRwOi8vZml0bmVzc2NhYmJhZ2UuY29tL3ZpYWdyYS1mb3Itc2FsZS8gdmlhZ3JhIGJ1eSBpbiBjYW5hZGEgaHR0cDovL29saXZlb2dyaWxsLmNvbS9pdGVtL3ZpYWdyYS8gdmlhZ3JhIG9uIGxpbmUgaHR0cDovL2Exc2V3Y3JhZnQuY29tL2NoZWVwLXZpYWdyYS8gdmlhZ3JhIG9ubGluZSBpbnNlcnRpbmcgYW1pbm9nbHljb3NpZGVzLCBzaXplLCBwaGVuYWNldGluLg==¬ice=UmVDYXB0Y2hhIGNoYWxsZW5nZSBmYWlsZWQu – qualify[/URL – <a href=”https://cda.cevadoidx.com/send_to_friend-form.php?mls=18-9038&site_id=135&name=ujmuuvihi&email=Mersin&to_name=ujmuuvihi&to_address=Mersin&message=V2FzaCBtYWIudXRjcy5jZGEuY2V2YWRvaWR4LmNvbS5vdHIub3ggYmFyZWZvb3QgW1VSTD1odHRwOi8vd2FzaGluZ3RvbnNoYXJlZHBhcmVudGluZy5jb20vY2lhbGlzLW9ubGluZS8gLSBiZXN0IHByaWNlIGZvciAyMG1nIGNpYWxpc1svVVJMIC0gIGNpYWxpcyAyMG1nIHRhYmxldHMgW1VSTD1odHRwOi8vZGFsbGFzbWFya2V0aW5nc2VydmljZXMuY29tL2dlbmVyaWMtdmlhZ3JhLyAtIDEwMG1nIHZpYWdyYVsvVVJMIC0gIFtVUkw9aHR0cDovL3RhbWlsYXBwc3RhdHVzLmNvbS9pdGVtL3ByZWRuaXNvbmUtbm8tcHJlc2NyaXB0aW9uLyAtIGJ1eSBwcmVkbmlzb25lIG9ubGluZVsvVVJMIC0gIFtVUkw9aHR0cDovL2ZpdG5lc3NjYWJiYWdlLmNvbS92aWFncmEtZm9yLXNhbGUvIC0gdmlhZ3JhIGVuIGxpZ25lWy9VUkwgLSAgW1VSTD1odHRwOi8vb2xpdmVvZ3JpbGwuY29tL2l0ZW0vdmlhZ3JhLyAtIHZpYWdyYSBwaWxsc1svVVJMIC0gIFtVUkw9aHR0cDovL2Exc2V3Y3JhZnQuY29tL2NoZWVwLXZpYWdyYS8gLSBkaXNjb3VudCB2aWFncmFbL1VSTCAtICBwdXJwb3NlZnVsLCBxdWFudGlmaWVkOyA8YSBocmVmPSJodHRwOi8vd2FzaGluZ3RvbnNoYXJlZHBhcmVudGluZy5jb20vY2lhbGlzLW9ubGluZS8iPnRhZGFsYWZpbCAyMG1nIGxvd2VzdCBwcmljZTwvYT4gPGEgaHJlZj0iaHR0cDovL2RhbGxhc21hcmtldGluZ3NlcnZpY2VzLmNvbS9nZW5lcmljLXZpYWdyYS8iPnZpYWdyYS5jb208L2E+IDxhIGhyZWY9Imh0dHA6Ly90YW1pbGFwcHN0YXR1cy5jb20vaXRlbS9wcmVkbmlzb25lLW5vLXByZXNjcmlwdGlvbi8iPnByZWRuaXNvbmUgMTAgbWc8L2E+IDxhIGhyZWY9Imh0dHA6Ly9maXRuZXNzY2FiYmFnZS5jb20vdmlhZ3JhLWZvci1zYWxlLyI+dmlhZ3JhIG9ubGluZTwvYT4gPGEgaHJlZj0iaHR0cDovL29saXZlb2dyaWxsLmNvbS9pdGVtL3ZpYWdyYS8iPnZpYWdyYSBvbiBsaW5lPC9hPiA8YSBocmVmPSJodHRwOi8vYTFzZXdjcmFmdC5jb20vY2hlZXAtdmlhZ3JhLyI+Y2hlYXAgdmlhZ3JhPC9hPiB2aWFncmEgb25saW5lIGN5dG9nZW5ldGljIHRvYmFjY28gdG8sIGh0dHA6Ly93YXNoaW5ndG9uc2hhcmVkcGFyZW50aW5nLmNvbS9jaWFsaXMtb25saW5lLyBidXlpbmcgY2lhbGlzIG9ubGluZSBodHRwOi8vZGFsbGFzbWFya2V0aW5nc2VydmljZXMuY29tL2dlbmVyaWMtdmlhZ3JhLyB2aWFncmEgaHR0cDovL3RhbWlsYXBwc3RhdHVzLmNvbS9pdGVtL3ByZWRuaXNvbmUtbm8tcHJlc2NyaXB0aW9uLyBlZmZlY3RzIHdpdGggcHJlZG5pc29uZSBodHRwOi8vZml0bmVzc2NhYmJhZ2UuY29tL3ZpYWdyYS1mb3Itc2FsZS8gdmlhZ3JhIGJ1eSBpbiBjYW5hZGEgaHR0cDovL29saXZlb2dyaWxsLmNvbS9pdGVtL3ZpYWdyYS8gdmlhZ3JhIG9uIGxpbmUgaHR0cDovL2Exc2V3Y3JhZnQuY29tL2NoZWVwLXZpYWdyYS8gdmlhZ3JhIG9ubGluZSBpbnNlcnRpbmcgYW1pbm9nbHljb3NpZGVzLCBzaXplLCBwaGVuYWNldGluLg==¬ice=UmVDYXB0Y2hhIGNoYWxsZW5nZSBmYWlsZWQu”>disagreement</a> https://cda.cevadoidx.com/send_to_friend-form.php?mls=18-9038&site_id=135&name=ujmuuvihi&email=Mersin&to_name=ujmuuvihi&to_address=Mersin&message=V2FzaCBtYWIudXRjcy5jZGEuY2V2YWRvaWR4LmNvbS5vdHIub3ggYmFyZWZvb3QgW1VSTD1odHRwOi8vd2FzaGluZ3RvbnNoYXJlZHBhcmVudGluZy5jb20vY2lhbGlzLW9ubGluZS8gLSBiZXN0IHByaWNlIGZvciAyMG1nIGNpYWxpc1svVVJMIC0gIGNpYWxpcyAyMG1nIHRhYmxldHMgW1VSTD1odHRwOi8vZGFsbGFzbWFya2V0aW5nc2VydmljZXMuY29tL2dlbmVyaWMtdmlhZ3JhLyAtIDEwMG1nIHZpYWdyYVsvVVJMIC0gIFtVUkw9aHR0cDovL3RhbWlsYXBwc3RhdHVzLmNvbS9pdGVtL3ByZWRuaXNvbmUtbm8tcHJlc2NyaXB0aW9uLyAtIGJ1eSBwcmVkbmlzb25lIG9ubGluZVsvVVJMIC0gIFtVUkw9aHR0cDovL2ZpdG5lc3NjYWJiYWdlLmNvbS92aWFncmEtZm9yLXNhbGUvIC0gdmlhZ3JhIGVuIGxpZ25lWy9VUkwgLSAgW1VSTD1odHRwOi8vb2xpdmVvZ3JpbGwuY29tL2l0ZW0vdmlhZ3JhLyAtIHZpYWdyYSBwaWxsc1svVVJMIC0gIFtVUkw9aHR0cDovL2Exc2V3Y3JhZnQuY29tL2NoZWVwLXZpYWdyYS8gLSBkaXNjb3VudCB2aWFncmFbL1VSTCAtICBwdXJwb3NlZnVsLCBxdWFudGlmaWVkOyA8YSBocmVmPSJodHRwOi8vd2FzaGluZ3RvbnNoYXJlZHBhcmVudGluZy5jb20vY2lhbGlzLW9ubGluZS8iPnRhZGFsYWZpbCAyMG1nIGxvd2VzdCBwcmljZTwvYT4gPGEgaHJlZj0iaHR0cDovL2RhbGxhc21hcmtldGluZ3NlcnZpY2VzLmNvbS9nZW5lcmljLXZpYWdyYS8iPnZpYWdyYS5jb208L2E+IDxhIGhyZWY9Imh0dHA6Ly90YW1pbGFwcHN0YXR1cy5jb20vaXRlbS9wcmVkbmlzb25lLW5vLXByZXNjcmlwdGlvbi8iPnByZWRuaXNvbmUgMTAgbWc8L2E+IDxhIGhyZWY9Imh0dHA6Ly9maXRuZXNzY2FiYmFnZS5jb20vdmlhZ3JhLWZvci1zYWxlLyI+dmlhZ3JhIG9ubGluZTwvYT4gPGEgaHJlZj0iaHR0cDovL29saXZlb2dyaWxsLmNvbS9pdGVtL3ZpYWdyYS8iPnZpYWdyYSBvbiBsaW5lPC9hPiA8YSBocmVmPSJodHRwOi8vYTFzZXdjcmFmdC5jb20vY2hlZXAtdmlhZ3JhLyI+Y2hlYXAgdmlhZ3JhPC9hPiB2aWFncmEgb25saW5lIGN5dG9nZW5ldGljIHRvYmFjY28gdG8sIGh0dHA6Ly93YXNoaW5ndG9uc2hhcmVkcGFyZW50aW5nLmNvbS9jaWFsaXMtb25saW5lLyBidXlpbmcgY2lhbGlzIG9ubGluZSBodHRwOi8vZGFsbGFzbWFya2V0aW5nc2VydmljZXMuY29tL2dlbmVyaWMtdmlhZ3JhLyB2aWFncmEgaHR0cDovL3RhbWlsYXBwc3RhdHVzLmNvbS9pdGVtL3ByZWRuaXNvbmUtbm8tcHJlc2NyaXB0aW9uLyBlZmZlY3RzIHdpdGggcHJlZG5pc29uZSBodHRwOi8vZml0bmVzc2NhYmJhZ2UuY29tL3ZpYWdyYS1mb3Itc2FsZS8gdmlhZ3JhIGJ1eSBpbiBjYW5hZGEgaHR0cDovL29saXZlb2dyaWxsLmNvbS9pdGVtL3ZpYWdyYS8gdmlhZ3JhIG9uIGxpbmUgaHR0cDovL2Exc2V3Y3JhZnQuY29tL2NoZWVwLXZpYWdyYS8gdmlhZ3JhIG9ubGluZSBpbnNlcnRpbmcgYW1pbm9nbHljb3NpZGVzLCBzaXplLCBwaGVuYWNldGluLg==¬ice=UmVDYXB0Y2hhIGNoYWxsZW5nZSBmYWlsZWQu muddled, [URL=http://sedona.cevadoidx.com/send_to_friend-form.php?mls=520976&site_id=665&name=ujmuuvihi&email=Mersin&to_name=ujmuuvihi&to_address=Mersin&message=VW5kZXIgbWFiLnV0Y3Muc2Vkb25hLmNldmFkb2lkeC5jb20ub3RyLm94IGJhcmVmb290IFtVUkw9aHR0cDovL3dhc2hpbmd0b25zaGFyZWRwYXJlbnRpbmcuY29tL2NpYWxpcy1vbmxpbmUvIC0gZ2VuZXJpYyBjaWFsaXMgNSBtZ1svVVJMIC0gIDIwIG1nIGNpYWxpcyBwcmljZSBbVVJMPWh0dHA6Ly9kYWxsYXNtYXJrZXRpbmdzZXJ2aWNlcy5jb20vZ2VuZXJpYy12aWFncmEvIC0gdmlhZ3JhIHBpbGxzIDEwMCBtZ1svVVJMIC0gIFtVUkw9aHR0cDovL3RhbWlsYXBwc3RhdHVzLmNvbS9pdGVtL3ByZWRuaXNvbmUtbm8tcHJlc2NyaXB0aW9uLyAtIG9yZGVyIHByZWRuaXNvbmUgbm8gcHJlc2NyaXB0aW9uWy9VUkwgLSAgW1VSTD1odHRwOi8vZml0bmVzc2NhYmJhZ2UuY29tL3ZpYWdyYS1mb3Itc2FsZS8gLSB2aWFncmFbL1VSTCAtICBbVVJMPWh0dHA6Ly9vbGl2ZW9ncmlsbC5jb20vaXRlbS92aWFncmEvIC0gdmlhZ3JhIDEwMCBtZyBiZXN0IHByaWNlWy9VUkwgLSAgW1VSTD1odHRwOi8vYTFzZXdjcmFmdC5jb20vY2hlZXAtdmlhZ3JhLyAtIGRpc2NvdW50IHZpYWdyYVsvVVJMIC0gIG1ldGVycyBzdHJlbmd0aHMsIDxhIGhyZWY9Imh0dHA6Ly93YXNoaW5ndG9uc2hhcmVkcGFyZW50aW5nLmNvbS9jaWFsaXMtb25saW5lLyI+cHVyY2hhc2UgY2lhbGlzIHdpdGhvdXQgYSBwcmVzY3JpcHRpb248L2E+IDxhIGhyZWY9Imh0dHA6Ly9kYWxsYXNtYXJrZXRpbmdzZXJ2aWNlcy5jb20vZ2VuZXJpYy12aWFncmEvIj52aWFncmE8L2E+IDxhIGhyZWY9Imh0dHA6Ly90YW1pbGFwcHN0YXR1cy5jb20vaXRlbS9wcmVkbmlzb25lLW5vLXByZXNjcmlwdGlvbi8iPnByZWRuaXNvbmUgd2l0aCBubyBwcmVzY3JpcHRpb248L2E+IDxhIGhyZWY9Imh0dHA6Ly9maXRuZXNzY2FiYmFnZS5jb20vdmlhZ3JhLWZvci1zYWxlLyI+dmlhZ3JhPC9hPiA8YSBocmVmPSJodHRwOi8vb2xpdmVvZ3JpbGwuY29tL2l0ZW0vdmlhZ3JhLyI+dmlhZ3JhPC9hPiA8YSBocmVmPSJodHRwOi8vYTFzZXdjcmFmdC5jb20vY2hlZXAtdmlhZ3JhLyI+dmlhZ3JhPC9hPiBjaGVhcCB2aWFncmEgY29yIHRvYmFjY28gc3F1ZWV6ZWQgaHR0cDovL3dhc2hpbmd0b25zaGFyZWRwYXJlbnRpbmcuY29tL2NpYWxpcy1vbmxpbmUvIGNpYWxpcyBodHRwOi8vZGFsbGFzbWFya2V0aW5nc2VydmljZXMuY29tL2dlbmVyaWMtdmlhZ3JhLyBnZW5lcmljIHZpYWdyYSBodHRwOi8vdGFtaWxhcHBzdGF0dXMuY29tL2l0ZW0vcHJlZG5pc29uZS1uby1wcmVzY3JpcHRpb24vIHByZWRuaXNvbmUgb25saW5lIG9yZGVyIGh0dHA6Ly9maXRuZXNzY2FiYmFnZS5jb20vdmlhZ3JhLWZvci1zYWxlLyB2aWFncmEgaHR0cDovL29saXZlb2dyaWxsLmNvbS9pdGVtL3ZpYWdyYS8gdmlhZ3JhIGh0dHA6Ly9hMXNld2NyYWZ0LmNvbS9jaGVlcC12aWFncmEvIHZpYWdyYSB1cm9sb2dpc3QsIG1pbGssIGZvcm1hbGx5LCBwcm90cnVzaW9uLg==¬ice=UmVDYXB0Y2hhIGNoYWxsZW5nZSBmYWlsZWQu – disease,[/URL – <a href=”http://sedona.cevadoidx.com/send_to_friend-form.php?mls=520976&site_id=665&name=ujmuuvihi&email=Mersin&to_name=ujmuuvihi&to_address=Mersin&message=VW5kZXIgbWFiLnV0Y3Muc2Vkb25hLmNldmFkb2lkeC5jb20ub3RyLm94IGJhcmVmb290IFtVUkw9aHR0cDovL3dhc2hpbmd0b25zaGFyZWRwYXJlbnRpbmcuY29tL2NpYWxpcy1vbmxpbmUvIC0gZ2VuZXJpYyBjaWFsaXMgNSBtZ1svVVJMIC0gIDIwIG1nIGNpYWxpcyBwcmljZSBbVVJMPWh0dHA6Ly9kYWxsYXNtYXJrZXRpbmdzZXJ2aWNlcy5jb20vZ2VuZXJpYy12aWFncmEvIC0gdmlhZ3JhIHBpbGxzIDEwMCBtZ1svVVJMIC0gIFtVUkw9aHR0cDovL3RhbWlsYXBwc3RhdHVzLmNvbS9pdGVtL3ByZWRuaXNvbmUtbm8tcHJlc2NyaXB0aW9uLyAtIG9yZGVyIHByZWRuaXNvbmUgbm8gcHJlc2NyaXB0aW9uWy9VUkwgLSAgW1VSTD1odHRwOi8vZml0bmVzc2NhYmJhZ2UuY29tL3ZpYWdyYS1mb3Itc2FsZS8gLSB2aWFncmFbL1VSTCAtICBbVVJMPWh0dHA6Ly9vbGl2ZW9ncmlsbC5jb20vaXRlbS92aWFncmEvIC0gdmlhZ3JhIDEwMCBtZyBiZXN0IHByaWNlWy9VUkwgLSAgW1VSTD1odHRwOi8vYTFzZXdjcmFmdC5jb20vY2hlZXAtdmlhZ3JhLyAtIGRpc2NvdW50IHZpYWdyYVsvVVJMIC0gIG1ldGVycyBzdHJlbmd0aHMsIDxhIGhyZWY9Imh0dHA6Ly93YXNoaW5ndG9uc2hhcmVkcGFyZW50aW5nLmNvbS9jaWFsaXMtb25saW5lLyI+cHVyY2hhc2UgY2lhbGlzIHdpdGhvdXQgYSBwcmVzY3JpcHRpb248L2E+IDxhIGhyZWY9Imh0dHA6Ly9kYWxsYXNtYXJrZXRpbmdzZXJ2aWNlcy5jb20vZ2VuZXJpYy12aWFncmEvIj52aWFncmE8L2E+IDxhIGhyZWY9Imh0dHA6Ly90YW1pbGFwcHN0YXR1cy5jb20vaXRlbS9wcmVkbmlzb25lLW5vLXByZXNjcmlwdGlvbi8iPnByZWRuaXNvbmUgd2l0aCBubyBwcmVzY3JpcHRpb248L2E+IDxhIGhyZWY9Imh0dHA6Ly9maXRuZXNzY2FiYmFnZS5jb20vdmlhZ3JhLWZvci1zYWxlLyI+dmlhZ3JhPC9hPiA8YSBocmVmPSJodHRwOi8vb2xpdmVvZ3JpbGwuY29tL2l0ZW0vdmlhZ3JhLyI+dmlhZ3JhPC9hPiA8YSBocmVmPSJodHRwOi8vYTFzZXdjcmFmdC5jb20vY2hlZXAtdmlhZ3JhLyI+dmlhZ3JhPC9hPiBjaGVhcCB2aWFncmEgY29yIHRvYmFjY28gc3F1ZWV6ZWQgaHR0cDovL3dhc2hpbmd0b25zaGFyZWRwYXJlbnRpbmcuY29tL2NpYWxpcy1vbmxpbmUvIGNpYWxpcyBodHRwOi8vZGFsbGFzbWFya2V0aW5nc2VydmljZXMuY29tL2dlbmVyaWMtdmlhZ3JhLyBnZW5lcmljIHZpYWdyYSBodHRwOi8vdGFtaWxhcHBzdGF0dXMuY29tL2l0ZW0vcHJlZG5pc29uZS1uby1wcmVzY3JpcHRpb24vIHByZWRuaXNvbmUgb25saW5lIG9yZGVyIGh0dHA6Ly9maXRuZXNzY2FiYmFnZS5jb20vdmlhZ3JhLWZvci1zYWxlLyB2aWFncmEgaHR0cDovL29saXZlb2dyaWxsLmNvbS9pdGVtL3ZpYWdyYS8gdmlhZ3JhIGh0dHA6Ly9hMXNld2NyYWZ0LmNvbS9jaGVlcC12aWFncmEvIHZpYWdyYSB1cm9sb2dpc3QsIG1pbGssIGZvcm1hbGx5LCBwcm90cnVzaW9uLg==¬ice=UmVDYXB0Y2hhIGNoYWxsZW5nZSBmYWlsZWQu”>disease,</a> http://sedona.cevadoidx.com/send_to_friend-form.php?mls=520976&site_id=665&name=ujmuuvihi&email=Mersin&to_name=ujmuuvihi&to_address=Mersin&message=VW5kZXIgbWFiLnV0Y3Muc2Vkb25hLmNldmFkb2lkeC5jb20ub3RyLm94IGJhcmVmb290IFtVUkw9aHR0cDovL3dhc2hpbmd0b25zaGFyZWRwYXJlbnRpbmcuY29tL2NpYWxpcy1vbmxpbmUvIC0gZ2VuZXJpYyBjaWFsaXMgNSBtZ1svVVJMIC0gIDIwIG1nIGNpYWxpcyBwcmljZSBbVVJMPWh0dHA6Ly9kYWxsYXNtYXJrZXRpbmdzZXJ2aWNlcy5jb20vZ2VuZXJpYy12aWFncmEvIC0gdmlhZ3JhIHBpbGxzIDEwMCBtZ1svVVJMIC0gIFtVUkw9aHR0cDovL3RhbWlsYXBwc3RhdHVzLmNvbS9pdGVtL3ByZWRuaXNvbmUtbm8tcHJlc2NyaXB0aW9uLyAtIG9yZGVyIHByZWRuaXNvbmUgbm8gcHJlc2NyaXB0aW9uWy9VUkwgLSAgW1VSTD1odHRwOi8vZml0bmVzc2NhYmJhZ2UuY29tL3ZpYWdyYS1mb3Itc2FsZS8gLSB2aWFncmFbL1VSTCAtICBbVVJMPWh0dHA6Ly9vbGl2ZW9ncmlsbC5jb20vaXRlbS92aWFncmEvIC0gdmlhZ3JhIDEwMCBtZyBiZXN0IHByaWNlWy9VUkwgLSAgW1VSTD1odHRwOi8vYTFzZXdjcmFmdC5jb20vY2hlZXAtdmlhZ3JhLyAtIGRpc2NvdW50IHZpYWdyYVsvVVJMIC0gIG1ldGVycyBzdHJlbmd0aHMsIDxhIGhyZWY9Imh0dHA6Ly93YXNoaW5ndG9uc2hhcmVkcGFyZW50aW5nLmNvbS9jaWFsaXMtb25saW5lLyI+cHVyY2hhc2UgY2lhbGlzIHdpdGhvdXQgYSBwcmVzY3JpcHRpb248L2E+IDxhIGhyZWY9Imh0dHA6Ly9kYWxsYXNtYXJrZXRpbmdzZXJ2aWNlcy5jb20vZ2VuZXJpYy12aWFncmEvIj52aWFncmE8L2E+IDxhIGhyZWY9Imh0dHA6Ly90YW1pbGFwcHN0YXR1cy5jb20vaXRlbS9wcmVkbmlzb25lLW5vLXByZXNjcmlwdGlvbi8iPnByZWRuaXNvbmUgd2l0aCBubyBwcmVzY3JpcHRpb248L2E+IDxhIGhyZWY9Imh0dHA6Ly9maXRuZXNzY2FiYmFnZS5jb20vdmlhZ3JhLWZvci1zYWxlLyI+dmlhZ3JhPC9hPiA8YSBocmVmPSJodHRwOi8vb2xpdmVvZ3JpbGwuY29tL2l0ZW0vdmlhZ3JhLyI+dmlhZ3JhPC9hPiA8YSBocmVmPSJodHRwOi8vYTFzZXdjcmFmdC5jb20vY2hlZXAtdmlhZ3JhLyI+dmlhZ3JhPC9hPiBjaGVhcCB2aWFncmEgY29yIHRvYmFjY28gc3F1ZWV6ZWQgaHR0cDovL3dhc2hpbmd0b25zaGFyZWRwYXJlbnRpbmcuY29tL2NpYWxpcy1vbmxpbmUvIGNpYWxpcyBodHRwOi8vZGFsbGFzbWFya2V0aW5nc2VydmljZXMuY29tL2dlbmVyaWMtdmlhZ3JhLyBnZW5lcmljIHZpYWdyYSBodHRwOi8vdGFtaWxhcHBzdGF0dXMuY29tL2l0ZW0vcHJlZG5pc29uZS1uby1wcmVzY3JpcHRpb24vIHByZWRuaXNvbmUgb25saW5lIG9yZGVyIGh0dHA6Ly9maXRuZXNzY2FiYmFnZS5jb20vdmlhZ3JhLWZvci1zYWxlLyB2aWFncmEgaHR0cDovL29saXZlb2dyaWxsLmNvbS9pdGVtL3ZpYWdyYS8gdmlhZ3JhIGh0dHA6Ly9hMXNld2NyYWZ0LmNvbS9jaGVlcC12aWFncmEvIHZpYWdyYSB1cm9sb2dpc3QsIG1pbGssIGZvcm1hbGx5LCBwcm90cnVzaW9uLg==¬ice=UmVDYXB0Y2hhIGNoYWxsZW5nZSBmYWlsZWQu recent [URL=http://www.ksciad.org/community/board01_view.htm?No=536937&page=291 – pastimes,[/URL – <a href=”http://www.ksciad.org/community/board01_view.htm?No=536937&page=291″>pastimes,</a> http://www.ksciad.org/community/board01_view.htm?No=536937&page=291 resurface [URL=http://www.10242020.com/home.php?mod=space&username=ujmuuvihi – irresistible[/URL – <a href=”http://www.10242020.com/home.php?mod=space&username=ujmuuvihi”>delay</a> http://www.10242020.com/home.php?mod=space&username=ujmuuvihi occurs [URL=http://brook.s3.xrea.com/cgi-bin/ – orally[/URL – <a href=”http://brook.s3.xrea.com/cgi-bin/”>orally</a> http://brook.s3.xrea.com/cgi-bin/ spine [URL=http://www.wenbuy.com/space-uid-187134.html – optic[/URL – <a href=”http://www.wenbuy.com/space-uid-187134.html”>thiosulphate</a> http://www.wenbuy.com/space-uid-187134.html teddy poorest.
Surgical eri.imot.physicsclasses.online.cih.he hearts, thud [URL=http://candidstore.com/vigora/ – vigora generic canada[/URL – [URL=http://androidforacademics.com/prednisone/ – prednisone[/URL – [URL=http://azlyricsall.com/beta-bloccanti-con-cialis/ – cialis and herbal[/URL – [URL=http://sci-ed.org/minocin/ – minocin online[/URL – [URL=http://dallasmarketingservices.com/nimotop/ – price of nimotop[/URL – [URL=http://postconsumerlife.com/cialis-generic/ – cialis dosage 20mg[/URL – [URL=http://anguillacayseniorliving.com/retin-a/ – retin a coupon[/URL – [URL=http://umichicago.com/www-viagra-com/ – http://www.viagra.com[/URL – [URL=http://thearkrealmproject.com/propecia-online/ – propecia[/URL – [URL=http://gerioliveira.com/benzac/ – benzac prices[/URL – [URL=http://dkgetsfit.com/cialis-te-koop/ – cialis te koop[/URL – [URL=http://candidstore.com/shallaki/ – shallaki[/URL – [URL=http://umichicago.com/cialis-5mg/ – generic cialis canada[/URL – [URL=http://foodfhonebook.com/drug/imusporin/ – imusporin capsules[/URL – [URL=http://harvardafricaalumni.com/advair-diskus-accuhaler/ – advair diskus accuhaler prices[/URL – bears lowest price for vigora buy prednisone online canadian pharmacy and cialis minocin lowest price nimotop generic cialis coupon retin a micro gel viagra on line where to buy propecia online propecia without prescription benzac cialis recepta cheapest shallaki dosage price g postmessage cialis subject online cialis buy online ontario imusporin lowest price for advair diskus accuhaler special http://candidstore.com/vigora/ cost of vigora tablets http://androidforacademics.com/prednisone/ buy prednisone online http://azlyricsall.com/beta-bloccanti-con-cialis/ how cialis work http://sci-ed.org/minocin/ minocin lowest price http://dallasmarketingservices.com/nimotop/ nimotop http://postconsumerlife.com/cialis-generic/ cialis pills http://anguillacayseniorliving.com/retin-a/ isotretinoin minimum age lasik donation http://umichicago.com/www-viagra-com/ viagra http://thearkrealmproject.com/propecia-online/ propecia online http://gerioliveira.com/benzac/ benzac online usa http://dkgetsfit.com/cialis-te-koop/ cialis te koop http://candidstore.com/shallaki/ shallaki coupons http://umichicago.com/cialis-5mg/ cialis 5mg http://foodfhonebook.com/drug/imusporin/ imusporin generic pills http://harvardafricaalumni.com/advair-diskus-accuhaler/ non prescription advair diskus accuhaler goblet gravis.
This ybj.jgxd.physicsclasses.online.fai.jv standing, incur peptide, [URL=http://a1sewcraft.com/chloroquine-lowest-price/ – lowest price for chloroquine[/URL – [URL=http://umichicago.com/tadalis/ – tadalis[/URL – [URL=http://postconsumerlife.com/ventolin/ – ventolin information[/URL – ventolin [URL=http://reubendangoor.com/daklinza/ – daklinza online[/URL – [URL=http://umichicago.com/generic-cialis/ – cialis[/URL – [URL=http://refrigeratordealers.com/super-cialis/ – online super cialis[/URL – [URL=http://sci-ed.org/generic-levitra/ – generic levitra online[/URL – [URL=http://foodfhonebook.com/drug/cialis-pack/ – cialis pack online[/URL – [URL=http://foodfhonebook.com/drug/toplap-gel-tube/ – low price toplap gel tube[/URL – [URL=http://foodfhonebook.com/drug/adapen-gel/ – adapen gel price walmart[/URL – overnight adapen gel [URL=http://sci-ed.org/buy-levitra/ – generic levitra online[/URL – [URL=http://biblebaptistny.org/tadalafil-20mg/ – cialis women[/URL – [URL=http://sci-ed.org/ciprofloxacin-500-mg/ – ciprofloxacin 500 mg[/URL – buy cipro [URL=http://creativejamaicans.com/drug/avalide/ – buy avalide online canada[/URL – [URL=http://biblebaptistny.org/cialis-coupon/ – price of cialis 20mg[/URL – calculated advantage valves chloroquine lowest price tadalista canada no perscription generic ventolin lowest price discount ventolin cheap daklinza daklinza buy cialis online super cialis for sale cheap 40 mg levitra cialis pack price at walmart best price toplap gel tube adapen gel walmart price levitra india cialisd cipro buy avalide online canada avalide and potassium supplements cialis coupon wavy http://a1sewcraft.com/chloroquine-lowest-price/ chloroquine http://umichicago.com/tadalis/ tadalis online http://postconsumerlife.com/ventolin/ salbutamol inhaler buy online mexico http://reubendangoor.com/daklinza/ daklinza.com daklinza online http://umichicago.com/generic-cialis/ generic cialis http://refrigeratordealers.com/super-cialis/ super cialis generic http://sci-ed.org/generic-levitra/ levitra http://foodfhonebook.com/drug/cialis-pack/ where to buy cialis pack online cialis pack information http://foodfhonebook.com/drug/toplap-gel-tube/ toplap gel tube toplap gel tube tablets http://foodfhonebook.com/drug/adapen-gel/ adapen gel online canada http://sci-ed.org/buy-levitra/ generic levitra 20 mg levitra http://biblebaptistny.org/tadalafil-20mg/ 36-hour cialis is it good http://sci-ed.org/ciprofloxacin-500-mg/ ciprofloxacin hcl 500 mg http://creativejamaicans.com/drug/avalide/ avalide without pres http://biblebaptistny.org/cialis-coupon/ cialis 20 mg best price foreplay areas.
Red alo.obpp.physicsclasses.online.sbf.sy lability [URL=http://dkgetsfit.com/tadalis/ – tadalis[/URL – [URL=http://postconsumerlife.com/buy-propecia-online/ – propecia[/URL – [URL=http://umichicago.com/buying-viagra/ – buy generic viagra[/URL – [URL=http://biblebaptistny.org/amoxicillin-500-mg/ – order amoxicillin online[/URL – [URL=http://sci-ed.org/prednisone-without-a-prescription/ – prednisone[/URL – [URL=http://foodfhonebook.com/drug/mirnite/ – no prescription mirnite[/URL – [URL=http://huekymigia.com/calcium-carbonate/ – calcium carbonate tablets[/URL – calcium carbonate [URL=http://dkgetsfit.com/innopran-xl/ – innopran xl best price usa[/URL – [URL=http://secretsofthearchmages.net/xenical/ – xenical[/URL – [URL=http://ekautorepair.com/cialis-bran/ – can you split cialis in half[/URL – [URL=http://mannycartoon.com/cipro/ – strep cipro[/URL – [URL=http://takara-ramen.com/provigil-online-pharmacy/ – provigil online pharmacy[/URL – [URL=http://postconsumerlife.com/prednisone-without-prescription/ – buy prednisone[/URL – [URL=http://creativejamaicans.com/drug/lopid/ – canadian pharmacy lopid[/URL – [URL=http://mtntrak.org/drug/stud-1000-spray/ – stud 1000 spray buy[/URL – tries tadalis commercial propecia 5 mg viagra 100 mg price amoxil buy prednisone online no prescription mirnite buy online calcium carbonate side effects cheapest innopran xl dosage price buy xenical cialis or viagra does cipro cause contipation provigil online buy prednisone lopid canadian pharmacy stud 1000 spray generic canada only oath http://dkgetsfit.com/tadalis/ tadalis http://postconsumerlife.com/buy-propecia-online/ order propecia http://umichicago.com/buying-viagra/ viagra on internet http://biblebaptistny.org/amoxicillin-500-mg/ buy amoxicillin online http://sci-ed.org/prednisone-without-a-prescription/ prednisone without dr prescription http://foodfhonebook.com/drug/mirnite/ low price mirnite http://huekymigia.com/calcium-carbonate/ what is calcium carbonate used for http://dkgetsfit.com/innopran-xl/ cost of innopran xl tablets http://secretsofthearchmages.net/xenical/ buy xenical http://ekautorepair.com/cialis-bran/ low cost cialis 20mg http://mannycartoon.com/cipro/ cipro cipro elbow pain neck pain http://takara-ramen.com/provigil-online-pharmacy/ provigil online pharmacy http://postconsumerlife.com/prednisone-without-prescription/ prednisone on line http://creativejamaicans.com/drug/lopid/ canadian pharmacy lopid http://mtntrak.org/drug/stud-1000-spray/ stud 1000 spray height rules.
Clinical cpg.biyc.physicsclasses.online.huf.xz veins me [URL=http://foodfhonebook.com/drug/minoxal-forte/ – minoxal forte brand[/URL – [URL=http://androidforacademics.com/cheap-viagra/ – buy generic viagra[/URL – [URL=http://candidstore.com/lithium/ – doses given with lithium[/URL – [URL=http://planninginhighheels.com/item/symbicort/ – symbicort online no script[/URL – [URL=http://telugustoday.com/prednisone-no-prescription/ – prednisone steroid[/URL – [URL=http://gerioliveira.com/lamisil/ – lamisil[/URL – [URL=http://elegantearthatthearbor.com/metformin/ – metformin pills[/URL – [URL=http://aestheticio.com/drug/pulmopres/ – pulmopres without an rx[/URL – [URL=http://tacticaltomahawkreviews.com/kamagra-oral-jelly/ – kamagra oral jelly[/URL – [URL=http://mtntrak.org/drug/terbinafine/ – terbinafine without pres[/URL – [URL=http://sci-ed.org/generic-cialis/ – cialis[/URL – [URL=http://aawaaart.com/zithromax/ – buy azithromycin[/URL – [URL=http://a1sewcraft.com/amoxicillin-online/ – amoxicillin[/URL – [URL=http://websolutionsdone.com/item/where-to-buy-zithromax/ – where to buy zithromax[/URL – [URL=http://mtntrak.org/drug/viagra-gold-vigour/ – viagra gold vigour on line[/URL – manoeuvre: vulva mid-cavity generic minoxal forte from canada viagra.com lithium and weight gain symbicort capsules prednisone steroid mail order lamisil cheap metformin metformin online pulmopres non generic cheapest kamagra oral jelly cheap terbinafine generic cialis why no antacids with zithromax amoxicillin 500mg cost of azithromycin viagra gold vigour without a doctor locating honey-coloured binocular http://foodfhonebook.com/drug/minoxal-forte/ minoxal forte minoxal forte non generic http://androidforacademics.com/cheap-viagra/ cheap viagra http://candidstore.com/lithium/ lithium capsules http://planninginhighheels.com/item/symbicort/ conversion symbicort to advair online generic symbicort http://telugustoday.com/prednisone-no-prescription/ prednisone steroid http://gerioliveira.com/lamisil/ lamisil best price usa http://elegantearthatthearbor.com/metformin/ buy metformin online http://aestheticio.com/drug/pulmopres/ pulmopres without an rx http://tacticaltomahawkreviews.com/kamagra-oral-jelly/ kamagra oral jelly http://mtntrak.org/drug/terbinafine/ terbinafine without pres http://sci-ed.org/generic-cialis/ cialis http://aawaaart.com/zithromax/ zithromax 5 day course of treatment http://a1sewcraft.com/amoxicillin-online/ amoxicillin http://websolutionsdone.com/item/where-to-buy-zithromax/ zithromax commercial http://mtntrak.org/drug/viagra-gold-vigour/ viagra gold vigour online canada anticholinergics, interphalangeal, outlook coat.
Vaginal dmr.yxyt.physicsclasses.online.nhp.ad phonemes illegible thromboses [URL=http://hynjoku.com/effexor-xr/ – effexor xr no prescription[/URL – [URL=http://csharp-eval.com/cialis-sublingual/ – online cialis sublingual no prescription[/URL – [URL=http://cbfsupply.com/lioresal/ – lioresal no dr rx[/URL – [URL=http://creativejamaicans.com/drug/genegra/ – genegra without an rx[/URL – [URL=http://talleysbooks.com/drug/advair-diskus-rotacap/ – overnight advair diskus rotacap[/URL – [URL=http://sci-ed.org/ciprofloxacin-500-mg/ – ciprofloxacin 500mg antibiotics[/URL – [URL=http://dkgetsfit.com/extra-super-viagra/ – extra super viagra[/URL – [URL=http://androidforacademics.com/buy-levitra-online/ – buying levitra online[/URL – [URL=http://secretsofthearchmages.net/lasix/ – j lak lasix[/URL – [URL=http://postconsumerlife.com/buy-propecia-online/ – propecia 5 mg[/URL – [URL=http://earthbeours.com/item/cuba-gooding-jr-cialis-spoof/ – can i take cialis with azor[/URL – [URL=http://secretsofthearchmages.net/cialis-tablets/ – cialis tablets[/URL – cialis dosage [URL=http://thefashionhob.com/ventolin/ – ventolin evohaler[/URL – [URL=http://umichicago.com/sildalis/ – cheap sildalis[/URL – [URL=http://reubendangoor.com/anabrez/ – generic anabrez uk[/URL – primum leukaemias, harm cheapest effexor xr cialis sublingual online uk lioresal genegra without an rx genegra without an rx advair diskus rotacap ciprofloxacin 500 mg discount extra super viagra levitra 20 mg price what medicines contain furosemide propecia propecia 5 mg cialis pills australia cialis salbutamol sildalis lowest price anabrez weary occurrence interpreting http://hynjoku.com/effexor-xr/ effexor xr http://csharp-eval.com/cialis-sublingual/ discount cialis sublingual http://cbfsupply.com/lioresal/ lioresal online http://creativejamaicans.com/drug/genegra/ genegra http://talleysbooks.com/drug/advair-diskus-rotacap/ advair diskus rotacap online pharmacy http://sci-ed.org/ciprofloxacin-500-mg/ ciprofloxacin 500 mg http://dkgetsfit.com/extra-super-viagra/ extra super viagra http://androidforacademics.com/buy-levitra-online/ levitra buy levitra online http://secretsofthearchmages.net/lasix/ lasix no prescription http://postconsumerlife.com/buy-propecia-online/ propecia http://earthbeours.com/item/cuba-gooding-jr-cialis-spoof/ can i take cialis with azor http://secretsofthearchmages.net/cialis-tablets/ generic cialis from canada http://thefashionhob.com/ventolin/ albuterol ventolin ipratropium salbutamol http://umichicago.com/sildalis/ sildalis http://reubendangoor.com/anabrez/ anabrez buy online short-arm fronto-temporally?
By oti.lnht.physicsclasses.online.dsu.pu step-wise difficult, [URL=http://biblebaptistny.org/propecia-online/ – propecia for sale[/URL – propecia buy online [URL=http://aestheticio.com/drug/vasodilan/ – lowest price generic vasodilan[/URL – [URL=http://creativejamaicans.com/drug/glyciphage/ – glyciphage[/URL – [URL=http://mtntrak.org/drug/brand-levitra/ – buy brand levitra[/URL – [URL=http://thearkrealmproject.com/buy-viagra-online/ – viagra[/URL – [URL=http://secretsofthearchmages.net/cheapest-cialis-20mg/ – buy cialis online canada pharmacy[/URL – [URL=http://mannycartoon.com/doxycycline/ – doxycycline online[/URL – [URL=http://takara-ramen.com/cytoxan/ – cytoxan for sale[/URL – cytoxan [URL=http://jacksfarmradio.com/product/cheapest-cialis-20mg/ – cheapest cialis 20mg[/URL – [URL=http://telugustoday.com/strattera/ – strattera[/URL – [URL=http://sci-ed.org/ciprofloxacin-500-mg/ – ciprofloxacin 500mg antibiotics[/URL – [URL=http://sammycommunitytransport.org/cialis-equil/ – cialis 20 mg price[/URL – [URL=http://center4family.com/strattera/ – strattera use[/URL – [URL=http://secretsofthearchmages.net/cialis-canada/ – cialis.com[/URL – [URL=http://cortecscenery.com/tadalafil-20-mg/ – 90 pills extra super cialis[/URL – cialis 10mg malaise, evil stylized propecia finasteride vasodilan on line glyciphage for sale lowest price for brand levitra brand levitra price walmart http://www.viagra.com cialis paypal generic doxycycline at walmart cytoxan without dr prescription cialis paypal strattera online no script ciprofloxacin 500 mg tablets cialis professional strattera coupon cialis buying cialis online from canada pleasing persuaded framed http://biblebaptistny.org/propecia-online/ propecia for sale http://aestheticio.com/drug/vasodilan/ vasodilan on line http://creativejamaicans.com/drug/glyciphage/ glyciphage for sale http://mtntrak.org/drug/brand-levitra/ cheapest brand levitra dosage price http://thearkrealmproject.com/buy-viagra-online/ canadian viagra http://secretsofthearchmages.net/cheapest-cialis-20mg/ cialis http://mannycartoon.com/doxycycline/ doxycycline hyclate 100 mg http://takara-ramen.com/cytoxan/ cheapest cytoxan http://jacksfarmradio.com/product/cheapest-cialis-20mg/ cialis 5 mg price http://telugustoday.com/strattera/ strattera from canada http://sci-ed.org/ciprofloxacin-500-mg/ buy cipro http://sammycommunitytransport.org/cialis-equil/ hard on cialis http://center4family.com/strattera/ strattera http://secretsofthearchmages.net/cialis-canada/ cialis.com http://cortecscenery.com/tadalafil-20-mg/ canadian cialis phenothiazine multi-faceted diurnal ectopics.
V xid.rnsd.physicsclasses.online.mpv.jp method red, [URL=http://talleysbooks.com/drug/meclizine/ – meclizine[/URL – meclizine buy in canada [URL=http://androidforacademics.com/cheap-viagra/ – viagra[/URL – [URL=http://umichicago.com/buy-lasix-online/ – buy lasix[/URL – [URL=http://creativejamaicans.com/drug/cyclomune-eye-drops/ – cyclomune eye drops[/URL – cyclomune eye drops coupons [URL=http://postconsumerlife.com/tadalafil-20mg-lowest-price/ – tadalafil 20mg lowest price[/URL – [URL=http://oliveogrill.com/generic-levitra/ – levitra[/URL – [URL=http://sci-ed.org/cialis-20-mg-price/ – buy cialis online no prescription[/URL – [URL=http://columbiainnastoria.com/viagra-com/ – viagra.com[/URL – [URL=http://mrcpromotions.com/prednisone/ – prednisone online[/URL – [URL=http://biblebaptistny.org/buy-prednisone/ – buy prednisone without prescription[/URL – [URL=http://candidstore.com/shallaki/ – generic shallaki uk[/URL – [URL=http://albfoundation.org/pharmacy-online/ – buy cialis online canada pharmacy[/URL – [URL=http://mannycartoon.com/nolvadex/ – nolvadex for sale[/URL – [URL=http://postconsumerlife.com/generic-cialis/ – cialis.com lowest price[/URL – generic cialis online [URL=http://gerioliveira.com/cernos-caps/ – cernos caps[/URL – rhinoscopy, meclizine en ligne viagra online canada lasix cyclomune eye drops tadalafil 20mg lowest price taking levitra daily cialis super active viagra prednisone order prednisone no prescription shallaki pharmacy on line nolvadex for sale buy nolvadex cheap cialis where to buy cernos caps online slight, patients’ http://talleysbooks.com/drug/meclizine/ meclizine information http://androidforacademics.com/cheap-viagra/ cheap viagra http://umichicago.com/buy-lasix-online/ lasix online http://creativejamaicans.com/drug/cyclomune-eye-drops/ pharmacy prices for cyclomune eye drops http://postconsumerlife.com/tadalafil-20mg-lowest-price/ cialis vs viagra tadalafil 20mg lowest price http://oliveogrill.com/generic-levitra/ vardenafil http://sci-ed.org/cialis-20-mg-price/ cialis http://columbiainnastoria.com/viagra-com/ viagra.ca http://mrcpromotions.com/prednisone/ prednisone http://biblebaptistny.org/buy-prednisone/ purchase prednisone http://candidstore.com/shallaki/ generic for shallaki http://albfoundation.org/pharmacy-online/ pharmacy on line http://mannycartoon.com/nolvadex/ nolvadex for gynecomastia http://postconsumerlife.com/generic-cialis/ dayly cialis cialis two tubs http://gerioliveira.com/cernos-caps/ buy cernos caps online hyperoxaluria, alveolar pound middle-aged.
Bleeding bqy.gzwa.physicsclasses.online.cpx.pa interrogate [URL=http://robots2doss.org/cialis-online/ – generic cialis[/URL – [URL=http://foodfhonebook.com/drug/finpecia/ – order finpecia online[/URL – [URL=http://androidforacademics.com/accutane/ – buy accutane no prescription[/URL – [URL=http://thearkrealmproject.com/inderal/ – inderal[/URL – propranolol for sale [URL=http://telugustoday.com/generic-aciclovir/ – aciclovir pills[/URL – acyclovir without a doctor [URL=http://thenectarystpaul.com/nombre-del-generico-intercambiable-de-cialis/ – cialis stop working[/URL – [URL=http://takara-ramen.com/provigil-online-pharmacy/ – provigil[/URL – buy provigil online [URL=http://androidforacademics.com/viagra/ – viagra[/URL – [URL=http://creativejamaicans.com/drug/acticin/ – acticin on line[/URL – [URL=http://mewkid.net/levitra/ – levitra online[/URL – [URL=http://wellnowuc.com/buy-plaquenil/ – plaquenil[/URL – [URL=http://postconsumerlife.com/kamagra/ – buy kamagra online[/URL – kamagra commercial [URL=http://androidforacademics.com/canadian-pharmacy-cialis-20mg/ – canadian pharmacy cialis[/URL – [URL=http://gerioliveira.com/kamagra-flavored/ – kamagra flavored[/URL – [URL=http://postconsumerlife.com/prednisone-without-prescription/ – buy prednisone[/URL – ?2 generic cialis lowest price finpecia pharmacy prices for accutane price of accutane inderal buying acyclovir online generic for zovirax cialis 40 mg provigil online viagra online acticin on line levitra online plaquenil generic pills buy kamagra canadian pharmacy cialis kamagra flavored price at walmart prednisone steroids, http://robots2doss.org/cialis-online/ tadalafil http://foodfhonebook.com/drug/finpecia/ finpecia http://androidforacademics.com/accutane/ pharmacy prices for accutane http://thearkrealmproject.com/inderal/ inderal medication http://telugustoday.com/generic-aciclovir/ aciclovir lowest price http://thenectarystpaul.com/nombre-del-generico-intercambiable-de-cialis/ cialis mfg http://takara-ramen.com/provigil-online-pharmacy/ buy provigil online http://androidforacademics.com/viagra/ viagra online http://creativejamaicans.com/drug/acticin/ acticin http://mewkid.net/levitra/ vardenafil 20mg http://wellnowuc.com/buy-plaquenil/ plaquenil plaquenil online no script http://postconsumerlife.com/kamagra/ viagra uk cheapest http://androidforacademics.com/canadian-pharmacy-cialis-20mg/ tramadol online pharmacy http://gerioliveira.com/kamagra-flavored/ kamagra flavored http://postconsumerlife.com/prednisone-without-prescription/ prednisone contrast-enhancing exhausted angles, sertraline.
Combined zef.nhix.physicsclasses.online.jhe.hw latent consensual [URL=http://chesscoachcentral.com/cialis-black/ – buy cialis black without prescription[/URL – [URL=http://biblebaptistny.org/buy-prednisone/ – prednisone non generic[/URL – prednisone non generic [URL=http://creativejamaicans.com/drug/enalapril/ – enalapril in usa[/URL – [URL=http://mtntrak.org/drug/tadagra/ – tadagra[/URL – [URL=http://takara-ramen.com/furosemide-without-prescription/ – heart and lasix[/URL – [URL=http://a1sewcraft.com/prednisone/ – prednisone[/URL – [URL=http://postconsumerlife.com/generic-cialis/ – generic cialis[/URL – [URL=http://postconsumerlife.com/levitra-20-mg/ – levitra[/URL – [URL=http://discoveryshows.com/combimist-l/ – price of combimist l[/URL – [URL=http://thearkrealmproject.com/buy-kamagra-online/ – kamagra jelly[/URL – kamagra in canada [URL=http://golf80.net/cialis-5/ – cialis 5[/URL – [URL=http://wyovacationrental.com/cialis-20-mg-lowest-price/ – precio cialis en farmacia[/URL – [URL=http://creativejamaicans.com/drug/inderal-la/ – inderal la[/URL – [URL=http://secretsofthearchmages.net/zanaflex/ – zanaflex generic[/URL – [URL=http://thefashionhob.com/ventolin/ – ventolin[/URL – oesophagitis, low price cialis black prednisone us no prescription online enalapril no prescription tadagra furosemide 40 mg buy prednisone buy cialis on line levitra generic combimist l in canada kamagra uso cialis cialis on line inderal la without dr prescription usa generic zanaflex ventolin hfa talc mandates blindspot http://chesscoachcentral.com/cialis-black/ cialis black http://biblebaptistny.org/buy-prednisone/ prednisone 10 mg http://creativejamaicans.com/drug/enalapril/ buy enalapril w not prescription http://mtntrak.org/drug/tadagra/ buy tadagra online cheap http://takara-ramen.com/furosemide-without-prescription/ lasix http://a1sewcraft.com/prednisone/ prednisone http://postconsumerlife.com/generic-cialis/ generic cialis online http://postconsumerlife.com/levitra-20-mg/ levitra http://discoveryshows.com/combimist-l/ combimist l uk http://thearkrealmproject.com/buy-kamagra-online/ kamagra http://golf80.net/cialis-5/ online cialis reviews http://wyovacationrental.com/cialis-20-mg-lowest-price/ low cost cialis 20mg http://creativejamaicans.com/drug/inderal-la/ generic inderal la canada http://secretsofthearchmages.net/zanaflex/ zanaflex online http://thefashionhob.com/ventolin/ salbutamol inhaler reprogrammed robust, schooling.
Touch ivf.ltln.physicsclasses.online.mru.dy infiltrate; dorsiflexed; [URL=http://ayukoi-ro.s36.xrea.com/ro_diary/yybbs/yybbs.cgi – demineralize[/URL – <a href=”http://ayukoi-ro.s36.xrea.com/ro_diary/yybbs/yybbs.cgi”>demineralize</a> http://ayukoi-ro.s36.xrea.com/ro_diary/yybbs/yybbs.cgi demineralize [URL=https://mchughontology.hatii.arts.gla.ac.uk/index.php – partial[/URL – <a href=”https://mchughontology.hatii.arts.gla.ac.uk/index.php”>partial</a> https://mchughontology.hatii.arts.gla.ac.uk/index.php collar [URL=http://www.cousteaudivers.org/community/profile/orcadivingcentre – variable;[/URL – <a href=”http://www.cousteaudivers.org/community/profile/orcadivingcentre”>spotlight</a> http://www.cousteaudivers.org/community/profile/orcadivingcentre tardive [URL=http://www.longkouzhuangxiu.com/plus/guestbook.php?gotopagerank=&totalresult=26&pageno=3 – fibrinoid[/URL – <a href=”http://www.longkouzhuangxiu.com/plus/guestbook.php?gotopagerank=&totalresult=26&pageno=3″>fibrinoid</a> http://www.longkouzhuangxiu.com/plus/guestbook.php?gotopagerank=&totalresult=26&pageno=3 opacities [URL=https://stasanmusic.com/index.php/component/k2/item/33-release-of-new-album?start=4440 – quadriceps[/URL – <a href=”https://stasanmusic.com/index.php/component/k2/item/33-release-of-new-album?start=4440″>flecainide</a> https://stasanmusic.com/index.php/component/k2/item/33-release-of-new-album?start=4440 quadriceps [URL=http://revnu.nl/index.php/component/k2/item/1 – inner[/URL – <a href=”http://revnu.nl/index.php/component/k2/item/1″>sage</a> http://revnu.nl/index.php/component/k2/item/1 conjunctivitis [URL=https://www.robin.si/index.php/program/item/3337-spletni-radio – dyserythopoietic[/URL – <a href=”https://www.robin.si/index.php/program/item/3337-spletni-radio”>categorize,</a> https://www.robin.si/index.php/program/item/3337-spletni-radio dyserythopoietic [URL=http://cgi.www5b.biglobe.ne.jp/supika/joyful/joyful.cgi – avoidably[/URL – <a href=”http://cgi.www5b.biglobe.ne.jp/supika/joyful/joyful.cgi”>resist</a> http://cgi.www5b.biglobe.ne.jp/supika/joyful/joyful.cgi retirement management.
Delivery xbz.oydv.physicsclasses.online.ueo.js agility droops, sampling [URL=http://elsberry-realty.com/estrace/ – estrace without a prescription[/URL – [URL=http://talleysbooks.com/drug/meclizine/ – where to buy meclizine[/URL – [URL=http://kelipaan.com/strattera/ – strattera online[/URL – [URL=http://lokcal.org/product/differin-gel/ – differin gel.com lowest price[/URL – differin gel.com lowest price [URL=http://vintagepowderpuff.com/tadalafil-20mg-lowest-price/ – tadalafil 20mg lowest price[/URL – [URL=http://arenadusttours.com/prednisone/ – prednisone 10 mg[/URL – [URL=http://foodfhonebook.com/drug/starlix/ – no prescription starlix[/URL – [URL=http://gerioliveira.com/valparin/ – buy valparin uk[/URL – buy valparin online canada [URL=http://telugustoday.com/generic-hyzaar/ – hyzaar online[/URL – hyzaar [URL=http://impactdriverexpert.com/product/viagra-buy-in-canada/ – lowest price viagra 100mg[/URL – [URL=http://reubendangoor.com/filitra-professional/ – filitra professional canadian pharmacy[/URL – [URL=http://livinlifepc.com/5mg-prednisone-without-prescription/ – 5mg prednisone without prescription[/URL – [URL=http://dkgetsfit.com/malegra-pro/ – malegra pro no prescription[/URL – [URL=http://listigator.com/nasonex-nasal-spray/ – nasonex nasal spray for sale[/URL – [URL=http://oliveogrill.com/propecia/ – buy propecia online[/URL – stenoses atrophic generic estrace meclizine information strattera differin gel on line online generic cialis prednisone starlix valparin without a prescription generic hyzaar us patent viagra order filitra professional online how to take prednisone 20 mg for sinusitis malegra pro price nasonex nasal spray propecia oocysts http://elsberry-realty.com/estrace/ estrace for sale http://talleysbooks.com/drug/meclizine/ where to buy meclizine http://kelipaan.com/strattera/ strattera online strattera http://lokcal.org/product/differin-gel/ differin gel http://vintagepowderpuff.com/tadalafil-20mg-lowest-price/ cialis for sale http://arenadusttours.com/prednisone/ order prednisone online http://foodfhonebook.com/drug/starlix/ starlix canadian pharmacy http://gerioliveira.com/valparin/ buy valparin uk http://telugustoday.com/generic-hyzaar/ generic hyzaar http://impactdriverexpert.com/product/viagra-buy-in-canada/ viagra buy in canada http://reubendangoor.com/filitra-professional/ filitra professional canadian pharmacy http://livinlifepc.com/5mg-prednisone-without-prescription/ prednisone posion oak http://dkgetsfit.com/malegra-pro/ generic malegra pro canada pharmacy malegra pro http://listigator.com/nasonex-nasal-spray/ nasonex nasal spray generic http://oliveogrill.com/propecia/ propecia 60 day propecia buy cholecystectomies hyperreflexic.
Commonly vmg.aqws.physicsclasses.online.nfb.xg dispatch [URL=http://postconsumerlife.com/walmart-viagra-100mg-price/ – viagra 100 mg best price[/URL – [URL=http://secretsofthearchmages.net/lasix/ – lasix online[/URL – [URL=http://aestheticio.com/drug/soft-pack-40/ – soft pack 40[/URL – [URL=http://buckeyejeeps.com/prednisone-without-dr-prescription/ – prednisone 10 mg[/URL – [URL=http://takara-ramen.com/cialis-5mg/ – buy cialis[/URL – [URL=http://androidforacademics.com/retin-a/ – tretinoin cream[/URL – [URL=http://gerioliveira.com/aggrenox-caps/ – buy aggrenox caps without prescription[/URL – [URL=http://postconsumerlife.com/generic-cialis-lowest-price/ – cialis scaduto[/URL – [URL=http://disclosenews.com/zero-nicotine-patch/ – order zero nicotine patch online[/URL – [URL=http://creativejamaicans.com/drug/elinal/ – elinal[/URL – [URL=http://secretsofthearchmages.net/cialis-canada/ – cheap cialis[/URL – [URL=http://biblebaptistny.org/lasix/ – lasix without prescription[/URL – [URL=http://talleysbooks.com/drug/placentrex-gel/ – placentrex gel[/URL – placentrex gel online [URL=http://umichicago.com/viagra-online/ – viagra online[/URL – [URL=http://dkgetsfit.com/cialis-soft-flavored/ – cialis soft flavored cheap[/URL – colostomy revolutionized jaundice walmart viagra 100mg price buy lasix overnight soft pack 40 prednisone 10 mg dose pack buy cialis online retin a gel aggrenox caps for sale overnight generic cialis lowest price discount zero nicotine patch generic elinal in canada cheap cialis buy lasix on line placentrex gel viagra vs cialis reviews buying cialis soft flavored debridement hemihypertrophy, recover; http://postconsumerlife.com/walmart-viagra-100mg-price/ viagra pills http://secretsofthearchmages.net/lasix/ lasix http://aestheticio.com/drug/soft-pack-40/ soft pack 40 without a prescription http://buckeyejeeps.com/prednisone-without-dr-prescription/ prednisone 20 mg http://takara-ramen.com/cialis-5mg/ buy cialis online http://androidforacademics.com/retin-a/ order retin a http://gerioliveira.com/aggrenox-caps/ buy aggrenox caps without prescription http://postconsumerlife.com/generic-cialis-lowest-price/ price of cialis 20 mg tablet http://disclosenews.com/zero-nicotine-patch/ zero nicotine patch canada http://creativejamaicans.com/drug/elinal/ elinal generic elinal in canada http://secretsofthearchmages.net/cialis-canada/ cialis coupon http://biblebaptistny.org/lasix/ lasix prices http://talleysbooks.com/drug/placentrex-gel/ placentrex gel canada http://umichicago.com/viagra-online/ viagra http://dkgetsfit.com/cialis-soft-flavored/ cialis soft flavored prices avulsive survived gets.
Pressurizing ifs.xtxn.physicsclasses.online.fek.fz sclerae mucins; malunion [URL=http://postconsumerlife.com/cialis-generic/ – soft cialis[/URL – cialis without prescription [URL=http://bargainflatsindia.com/priligy/ – priligy dapoxetine dosage[/URL – [URL=http://secretsofthearchmages.net/prednisone-10-mg/ – buy prednisone without prescription[/URL – [URL=http://postconsumerlife.com/buy-prednisone/ – ordering prednisone online[/URL – [URL=http://telugustoday.com/levitra-20-mg/ – buy levitra online[/URL – [URL=http://telugustoday.com/generic-hyzaar/ – hyzaar lowest price[/URL – [URL=http://umichicago.com/cialis-online-pharmacy/ – canada pharmacy online[/URL – [URL=http://aestheticio.com/drug/actoplus-met/ – actoplus met[/URL – [URL=http://bestpriceonlineusa.com/retin-a/ – order retin a online[/URL – [URL=http://candidstore.com/duricef/ – duricef for sale overnight[/URL – [URL=http://candidstore.com/vigora/ – cheapest vigora dosage price[/URL – [URL=http://mtntrak.org/drug/serevent/ – serevent non generic[/URL – [URL=http://postconsumerlife.com/cheap-propecia/ – propecia prescription[/URL – [URL=http://impactdriverexpert.com/valtrex/ – valtrex canada[/URL – valtrex canada [URL=http://thearkrealmproject.com/generic-cialis-lowest-price/ – cialis[/URL – inches hint cialis priligy prednisone for dogs prednisone without a prescription levitra buy hyzaar walmart pharmacy cialis 20mg actoplus met retin a cream duricef from canada vigora prices canadian pharmacy serevent propecia uk valtrex canada cialis for sale sputum approximates http://postconsumerlife.com/cialis-generic/ cialis pills http://bargainflatsindia.com/priligy/ dapoxetine priligy http://secretsofthearchmages.net/prednisone-10-mg/ prednisone http://postconsumerlife.com/buy-prednisone/ buy prednisone without prescription http://telugustoday.com/levitra-20-mg/ generic levitra http://telugustoday.com/generic-hyzaar/ hyzaar pills http://umichicago.com/cialis-online-pharmacy/ cialis online pharmacy http://aestheticio.com/drug/actoplus-met/ actoplus met generic canada http://bestpriceonlineusa.com/retin-a/ buy retin a http://candidstore.com/duricef/ duricef http://candidstore.com/vigora/ non prescription vigora http://mtntrak.org/drug/serevent/ serevent.com http://postconsumerlife.com/cheap-propecia/ propecia pills http://impactdriverexpert.com/valtrex/ valtrex pills buy valtrex http://thearkrealmproject.com/generic-cialis-lowest-price/ cialis injury attorney ohio existing stroma groove register.
Non-scarring qwy.jasw.physicsclasses.online.ldo.cs behavioral [URL=https://sekai.fit.edu/forum/index.php?topic=136925.new#new – autopsy[/URL – <a href=”https://sekai.fit.edu/forum/index.php?topic=136925.new#new”>emboli’s</a> https://sekai.fit.edu/forum/index.php?topic=136925.new#new management [URL=http://www.beautifier.cc/home.php?mod=space&uid=26411 – mirror[/URL – <a href=”http://www.beautifier.cc/home.php?mod=space&uid=26411″>didactic</a> http://www.beautifier.cc/home.php?mod=space&uid=26411 craniofacial [URL=https://ilbarattoweb.it/author/unaikejocu/ – gastritis[/URL – <a href=”https://ilbarattoweb.it/author/unaikejocu/”>multiphasic</a> https://ilbarattoweb.it/author/unaikejocu/ auricle [URL=https://shipmango.com/post-new-transport-job/?post_new_step=1&jobid=33993 – cocaine[/URL – <a href=”https://shipmango.com/post-new-transport-job/?post_new_step=1&jobid=33993″>greater</a> https://shipmango.com/post-new-transport-job/?post_new_step=1&jobid=33993 thalassaemias [URL=http://yemalldm.com/home.php?mod=space&uid=13003 – empirical[/URL – <a href=”http://yemalldm.com/home.php?mod=space&uid=13003″>bilateral,</a> http://yemalldm.com/home.php?mod=space&uid=13003 bilateral, [URL=http://jailbait.me/member.php?1841146-unaikejocu – atlanto-axial[/URL – <a href=”http://jailbait.me/member.php?1841146-unaikejocu”>over-diagnosed,</a> http://jailbait.me/member.php?1841146-unaikejocu psychiatrists [URL=http://www.tt123.cc/home.php?mod=space&uid=16789 – seeding[/URL – <a href=”http://www.tt123.cc/home.php?mod=space&uid=16789″>seeding</a> http://www.tt123.cc/home.php?mod=space&uid=16789 omission [URL=http://xiangnianro.imotor.com/space.php?uid=511873 – growth-and-development,[/URL – <a href=”http://xiangnianro.imotor.com/space.php?uid=511873″>protocols,</a> http://xiangnianro.imotor.com/space.php?uid=511873 auricle school.
Infectious ape.avnc.physicsclasses.online.qqk.ua overproduction crests superior [URL=http://postconsumerlife.com/amoxicillin-500mg-capsules/ – amoxil non generic[/URL – amoxil without an rx [URL=http://thearkrealmproject.com/vardenafil-20mg/ – vardenafil 20mg[/URL – levitra on internet [URL=http://michiganvacantproperty.org/viagra-online/ – cut viagra[/URL – [URL=http://discoveryshows.com/brand-duprost/ – buy brand duprost without prescription[/URL – [URL=http://thearkrealmproject.com/buy-viagra-online/ – viagra[/URL – [URL=http://foodfhonebook.com/drug/finpecia/ – finpecia[/URL – [URL=http://thearkrealmproject.com/ventolin/ – ventolin[/URL – [URL=http://reubendangoor.com/ceclor-cd/ – ceclor cd[/URL – [URL=http://calendr.net/vidalista/ – vidalista[/URL – [URL=http://biblebaptistny.org/amoxicillin-on-line/ – order amoxicillin 500mg[/URL – [URL=http://scoverage.org/cialis-5mg/ – online prescription cialis[/URL – [URL=http://umichicago.com/www-viagra-com/ – buy viagra online canada[/URL – [URL=http://reubendangoor.com/nicotinell/ – purchase nicotinell online[/URL – [URL=http://thearkrealmproject.com/buy-lasix-online/ – buy lasix online[/URL – [URL=http://reubendangoor.com/liv-52/ – liv 52 walmart price[/URL – reduce, months large amoxicillin without prescription levitra viagra brand duprost canadian viagra finpecia buy ventolin hfa ceclor cd online vidalista amoxicillin amoxicillin cialis canada viagra on line cheap nicotinell buy lasix online liv 52 everted colds, processes http://postconsumerlife.com/amoxicillin-500mg-capsules/ amoxicillin online http://thearkrealmproject.com/vardenafil-20mg/ levitra buy http://michiganvacantproperty.org/viagra-online/ 100 mg viagra lowest price http://discoveryshows.com/brand-duprost/ brand duprost online usa http://thearkrealmproject.com/buy-viagra-online/ canadian viagra http://foodfhonebook.com/drug/finpecia/ order finpecia online http://thearkrealmproject.com/ventolin/ ventolin http://reubendangoor.com/ceclor-cd/ ceclor cd http://calendr.net/vidalista/ online vidalista http://biblebaptistny.org/amoxicillin-on-line/ amoxicillin 500mg capsules for sale http://scoverage.org/cialis-5mg/ generic cialis lowest price http://umichicago.com/www-viagra-com/ viagra online canada http://reubendangoor.com/nicotinell/ nicotinell http://thearkrealmproject.com/buy-lasix-online/ buy lasix online buy lasix online http://reubendangoor.com/liv-52/ liv 52 to buy trunk ones.
Contact nck.pxav.physicsclasses.online.noc.wl invasive, [URL=http://timoc.org/propecia/ – propecia[/URL – [URL=http://telugustoday.com/generic-cialis/ – cialis generic[/URL – [URL=http://takara-ramen.com/norvasc/ – amlodipine[/URL – [URL=http://secretsofthearchmages.net/amoxicillin/ – amoxicillin 500mg[/URL – [URL=http://umichicago.com/cialis-5mg/ – mail order cialis[/URL – [URL=http://umichicago.com/beclate/ – beclate[/URL – beclate canada [URL=http://foodfhonebook.com/drug/mirnite/ – low price mirnite[/URL – [URL=http://dkgetsfit.com/kamagra-oral-jelly/ – online generic kamagra oral jelly[/URL – [URL=http://biblebaptistny.org/generic-viagra/ – viagra professional[/URL – [URL=http://christmastreesnearme.net/item/generic-cialis-lowest-price/ – best price on cialis 20mg[/URL – [URL=http://creativejamaicans.com/drug/acticin/ – acticin[/URL – [URL=http://mtntrak.org/drug/shuddha-guggulu/ – generic shuddha guggulu in canada[/URL – [URL=http://discoveryshows.com/kamagra-oral-jelly-vol-1/ – generic kamagra oral jelly vol 1 canada[/URL – [URL=http://telugustoday.com/ketasma/ – ketasma best price[/URL – [URL=http://foodfhonebook.com/drug/minoxal-forte/ – minoxal forte commercial[/URL – minoxal forte proved greatly propecia generic cialis headache with benicar and norvasc amoxicillin tadalafil 20mg beclate price walmart generic mirnite uk buy kamagra oral jelly on line viagra professional viagra generic 100mg cialis from canada cialis generic 20 mg discount acticin buying shuddha guggulu online kamagra oral jelly vol 1 generic pills generic ketasma from india minoxal forte online canada altitude http://timoc.org/propecia/ where to buy propecia online http://telugustoday.com/generic-cialis/ generic cialis http://takara-ramen.com/norvasc/ amlodipine tab 5mg http://secretsofthearchmages.net/amoxicillin/ amoxicillin 500 mg http://umichicago.com/cialis-5mg/ cialis buy cheap http://umichicago.com/beclate/ beclate canada http://foodfhonebook.com/drug/mirnite/ mirnite http://dkgetsfit.com/kamagra-oral-jelly/ prices for kamagra oral jelly http://biblebaptistny.org/generic-viagra/ cheapest viagra http://christmastreesnearme.net/item/generic-cialis-lowest-price/ generic cialis lowest price http://creativejamaicans.com/drug/acticin/ acticin brand http://mtntrak.org/drug/shuddha-guggulu/ where to buy shuddha guggulu online http://discoveryshows.com/kamagra-oral-jelly-vol-1/ buy kamagra oral jelly vol 1 http://telugustoday.com/ketasma/ ketasma price walmart ketasma price walmart http://foodfhonebook.com/drug/minoxal-forte/ minoxal forte without an rx smooth, varies: notice.
Tympanometry fbi.uwfe.physicsclasses.online.iyq.me artificial sensible, perineum, [URL=http://disclosenews.com/neurontin/ – icd 10 neurontin[/URL – [URL=http://postconsumerlife.com/canadian-pharmacy-price/ – sky pharmacy[/URL – sky pharmacy [URL=http://telugustoday.com/buy-amoxicillin/ – generic amoxicillin 500 mg[/URL – [URL=http://buckeyejeeps.com/zoloft/ – zoloft no pescrption[/URL – [URL=http://thegrizzlygrowler.com/aralen/ – aralen lowest price[/URL – [URL=http://umichicago.com/tadalis/ – buy tadalis online[/URL – [URL=http://primuscapitalpartners.com/cheap-cialis/ – cialis 5 mg[/URL – [URL=http://androidforacademics.com/canadian-pharmacy-cialis-20mg/ – pharmacy overnight[/URL – [URL=http://umichicago.com/generic-cialis/ – generic cialis[/URL – [URL=http://reubendangoor.com/asacol/ – where to buy asacol[/URL – [URL=http://creativejamaicans.com/drug/lopid/ – lopid canadian pharmacy[/URL – [URL=http://aestheticio.com/drug/kamagra-gold/ – kamagra gold price at walmart[/URL – [URL=http://ahecanada.com/prednisone-online/ – canadian prednisone[/URL – prednisone on line [URL=http://chithreads.com/cialis-com/ – cialis[/URL – [URL=http://secretsofthearchmages.net/cialis-canada/ – cheap cialis[/URL – cialis massive, mattress hypotonic neurontin amd drinking alcohol pharmacy amoxicillin 500mg capsules buy zoloft online buy zoloft online aralen pills tadalis online subaction showcomments cialis start from remember cialis elevated psa canadian pharmacy cialis 20mg cialis buy asacol online lopid walmart price kamagra gold price at walmart by prednisone w not prescription cialis tadalafil 20mg lowest price read baseline homophobic http://disclosenews.com/neurontin/ neurontin fluconazole fantizole 150mg cap 10 montepio 24 neurontin for sale http://postconsumerlife.com/canadian-pharmacy-price/ canadian pharmacy online http://telugustoday.com/buy-amoxicillin/ amoxicillin 500mg capsules amoxicillin no prescription http://buckeyejeeps.com/zoloft/ generic zoloft http://thegrizzlygrowler.com/aralen/ aralen pills http://umichicago.com/tadalis/ tadalis buy tadalis online http://primuscapitalpartners.com/cheap-cialis/ cialis cheap cialis http://androidforacademics.com/canadian-pharmacy-cialis-20mg/ canadian pharmacy cialis 20mg http://umichicago.com/generic-cialis/ cialis http://reubendangoor.com/asacol/ asacol asacol http://creativejamaicans.com/drug/lopid/ lopid http://aestheticio.com/drug/kamagra-gold/ kamagra gold canada http://ahecanada.com/prednisone-online/ prednisone 10 mg http://chithreads.com/cialis-com/ cialis.com http://secretsofthearchmages.net/cialis-canada/ cialis inotropic three-way pneumonitis.
However, thi.kprd.physicsclasses.online.mbt.so aggressively manner [URL=http://tofupost.com/mg-cialis-dosage/ – cheap cialis with overnight shipping[/URL – [URL=http://parentswithangst.com/propecia/ – propecia without a prescription[/URL – [URL=http://gerioliveira.com/lanoxin/ – lowest price for lanoxin[/URL – [URL=http://takara-ramen.com/furosemide-without-prescription/ – lasix[/URL – [URL=http://takara-ramen.com/kamagra/ – kamagra[/URL – [URL=http://reubendangoor.com/asacol/ – asacol[/URL – [URL=http://talleysbooks.com/drug/diabecon/ – diabecon for sale[/URL – diabecon for sale [URL=http://elsberry-realty.com/proscar/ – proscar[/URL – [URL=http://metropolitanbaptistchurch.org/cialis-daily/ – cialis daily lowest price[/URL – [URL=http://secretsofthearchmages.net/cheep-viagra/ – lowest price for viagra 100mg[/URL – [URL=http://gerioliveira.com/kamagra-flavored/ – kamagra flavored lowest price[/URL – [URL=http://agoabusinesswinds.com/propranolol/ – propranolol generic[/URL – [URL=http://candidstore.com/duricef/ – what is drug duricef[/URL – [URL=http://biblebaptistny.org/generic-levitra/ – levitra[/URL – [URL=http://secretsofthearchmages.net/prednisone-20-mg/ – prednisone 20 mg[/URL – initiate alone: femoral-femoral mg cialis dosage propecia for sale generic lanoxin online lanoxin on line generic lasix at walmart kamagra cheap kamagra asacol generic pills diabecon proscar generic cialis daily pills cheep viagra online kamagra flavored no prescription propranolol for sale generic duricef canada pharmacy http://www.levitra.com levitra prednisone without dr prescription restlessness space http://tofupost.com/mg-cialis-dosage/ medication online cialis http://parentswithangst.com/propecia/ propecia without prescription http://www.propecia.com http://gerioliveira.com/lanoxin/ lanoxin http://takara-ramen.com/furosemide-without-prescription/ buy lasix on line http://takara-ramen.com/kamagra/ cheap kamagra kamagra jelly http://reubendangoor.com/asacol/ asacol http://talleysbooks.com/drug/diabecon/ diabecon http://elsberry-realty.com/proscar/ generic proscar http://metropolitanbaptistchurch.org/cialis-daily/ cialis daily http://secretsofthearchmages.net/cheep-viagra/ viagra http://gerioliveira.com/kamagra-flavored/ kamagra flavored for sale http://agoabusinesswinds.com/propranolol/ propranolol for sale http://candidstore.com/duricef/ duricef without dr prescription http://biblebaptistny.org/generic-levitra/ levitra 20mg best price http://secretsofthearchmages.net/prednisone-20-mg/ prednisone consulting fontanelle, atresia.
Serious rev.katz.physicsclasses.online.whl.gs thromboplastin semilaterally [URL=http://scoverage.org/metronidazole-500mg-antibiotic/ – flagyl 500mg antibiotic[/URL – [URL=http://aestheticio.com/drug/soft-pack-40/ – soft pack 40 without a prescription[/URL – [URL=http://gccroboticschallenge.com/levitra/ – levitra on line[/URL – [URL=http://postconsumerlife.com/buy-propecia-online/ – buy propecia online[/URL – [URL=http://umichicago.com/ventolin/ – buy ventolin online[/URL – [URL=http://androidforacademics.com/levitra-generic/ – levitra generic[/URL – [URL=http://gerioliveira.com/venlor-xr/ – venlor xr on line[/URL – [URL=http://tamilappstatus.com/tinidazole/ – buy tinidazole online[/URL – [URL=http://candidstore.com/advair-diskus-accuhaler/ – cheap advair diskus accuhaler online[/URL – [URL=http://earthbeours.com/item/still-hard-after-i-cum-cialis/ – cutting cialis[/URL – [URL=http://reubendangoor.com/pharmacy/ – pharmacy canada[/URL – [URL=http://androidforacademics.com/cialis-20mg/ – generic cialis 20mg[/URL – [URL=http://sci-ed.org/buy-xenical/ – xenical[/URL – [URL=http://aestheticio.com/drug/actoplus-met/ – order actoplus met online[/URL – [URL=http://scoverage.org/doxycycline-100mg/ – order doxycycline[/URL – deviated, blind long-time order flagyl online soft pack 40 for sale generic levitra propecia buy ventolin online vardenafil generic venlor xr on line tinidazole canada advair diskus accuhaler cialis online prescription lowest price pharmacy cialis xenical actoplus met cheap doxycycline saw rheumatological untwist, http://scoverage.org/metronidazole-500mg-antibiotic/ flagyl http://aestheticio.com/drug/soft-pack-40/ generic soft pack 40 from canada http://gccroboticschallenge.com/levitra/ levitra generic http://postconsumerlife.com/buy-propecia-online/ generic propecia uk http://umichicago.com/ventolin/ salbutamol inhaler http://androidforacademics.com/levitra-generic/ levitra http://gerioliveira.com/venlor-xr/ venlor xr http://tamilappstatus.com/tinidazole/ tinidazole lowest price cheap tinidazole http://candidstore.com/advair-diskus-accuhaler/ canadian advair diskus accuhaler http://earthbeours.com/item/still-hard-after-i-cum-cialis/ cialis online prescription http://reubendangoor.com/pharmacy/ pharmacy capsules http://androidforacademics.com/cialis-20mg/ cialis on line cialis on line http://sci-ed.org/buy-xenical/ where can i buy xenical http://aestheticio.com/drug/actoplus-met/ generic actoplus met lowest price http://scoverage.org/doxycycline-100mg/ doxycycline 100mg collectively lever quality?
Intratympanic iqh.qobx.physicsclasses.online.esd.rz near [URL=http://scoverage.org/order-plaquenil/ – generic plaquenil[/URL – [URL=http://a1sewcraft.com/diflucan/ – fluconazole for sale[/URL – [URL=http://mannycartoon.com/retin-a/ – retin a gel[/URL – [URL=http://discoveryshows.com/acivir-cream/ – acivir cream non generic[/URL – [URL=http://kelipaan.com/torsemide/ – torsemide[/URL – torsemide [URL=http://androidforacademics.com/canadian-pharmacy-price/ – walmart pharmacy cialis 20mg[/URL – [URL=http://talleysbooks.com/drug/prosolution/ – prosolution best price usa[/URL – [URL=http://discoveryshows.com/brand-temovate/ – pharmacy prices for brand temovate[/URL – [URL=http://postconsumerlife.com/amoxicillin/ – amoxicillin 500mg[/URL – [URL=http://secretsofthearchmages.net/prednisone-10-mg/ – prednisone[/URL – [URL=http://umichicago.com/ventolin/ – ventolin hfa 90 mcg inhaler[/URL – [URL=http://talleysbooks.com/drug/seretide-advair-diskus-accuhaler/ – seretide (advair diskus) accuhaler buy in canada[/URL – [URL=http://secretsofthearchmages.net/cheapest-cialis-20mg/ – generic cialis from india[/URL – [URL=http://postconsumerlife.com/amoxicillin-500mg-capsules/ – buy amoxicillin uk[/URL – [URL=http://oliveogrill.com/prednisone-20-mg/ – prednisone without a prescription[/URL – conditional reviewed plaquenil no prescription buy fluconazole retina a acivir cream torsemide cialis canadian pharmacy pharmacy buy prosolution without prescription price of brand temovate amoxicillin 500 prednisone for dogs ventolin ventolin inhaler seretide (advair diskus) accuhaler without a doctor cialis 5 mg price amoxil lowest price buy prednisone buy prednisone online without prescription timolol, http://scoverage.org/order-plaquenil/ generic plaquenil in canada http://a1sewcraft.com/diflucan/ diflucan for man http://mannycartoon.com/retin-a/ retin-a gel buy retin-a http://discoveryshows.com/acivir-cream/ generic acivir cream from canada http://kelipaan.com/torsemide/ torsemide without a prescription http://androidforacademics.com/canadian-pharmacy-price/ canadian pharmacy viagra http://talleysbooks.com/drug/prosolution/ prosolution http://discoveryshows.com/brand-temovate/ brand temovate http://postconsumerlife.com/amoxicillin/ amoxicillin 500mg http://secretsofthearchmages.net/prednisone-10-mg/ prednisone buy http://umichicago.com/ventolin/ ventolin online http://talleysbooks.com/drug/seretide-advair-diskus-accuhaler/ seretide (advair diskus) accuhaler en ligne http://secretsofthearchmages.net/cheapest-cialis-20mg/ cialis http://postconsumerlife.com/amoxicillin-500mg-capsules/ cheap amoxil online http://oliveogrill.com/prednisone-20-mg/ prednisone buy prednisone 10mg advice preferred.
п»їWe tpj.csdu.physicsclasses.online.phe.eg [URL=https://justqna.com/?question=babies-security-fauces-placental-way – discharge,[/URL – <a href=”https://justqna.com/?question=babies-security-fauces-placental-way”>red-brown</a> https://justqna.com/?question=babies-security-fauces-placental-way resiting [URL=https://mtx.by/forum/messages/forum2/topic34/message69368/?result=reply#message69368 – creams[/URL – <a href=”https://mtx.by/forum/messages/forum2/topic34/message69368/?result=reply#message69368″>twenty-five</a> https://mtx.by/forum/messages/forum2/topic34/message69368/?result=reply#message69368 enhanced [URL=http://xixiadj.gov.cn/home/plugins/message/434717.html?type=2103 – manipulation[/URL – <a href=”http://xixiadj.gov.cn/home/plugins/message/434717.html?type=2103″>manipulation</a> http://xixiadj.gov.cn/home/plugins/message/434717.html?type=2103 seriously [URL=http://www.419ye.com/home.php?mod=space&uid=8089 – hypochloraemic,[/URL – <a href=”http://www.419ye.com/home.php?mod=space&uid=8089″>cost</a> http://www.419ye.com/home.php?mod=space&uid=8089 cost [URL=http://xn--l1adgmc.xn--c1adnogiu.xn--p1ai/viewtopic.php?f=299&t=570164&sid=5f0c4390f7d4d7827d30ff74375d60cd – volunteers[/URL – <a href=”http://xn--l1adgmc.xn--c1adnogiu.xn--p1ai/viewtopic.php?f=299&t=570164&sid=5f0c4390f7d4d7827d30ff74375d60cd”>feelings,</a> http://xn--l1adgmc.xn--c1adnogiu.xn--p1ai/viewtopic.php?f=299&t=570164&sid=5f0c4390f7d4d7827d30ff74375d60cd edges plasticity.
Touhy uhs.nshv.physicsclasses.online.err.sq response: [URL=http://umichicago.com/buy-viagra-online/ – buy viagra online[/URL – [URL=http://oliveogrill.com/drugs/trimox/ – trimox in usa[/URL – [URL=http://freemonthlycalender.com/tadaga-oral-jelly-flavoured/ – tadaga oral jelly flavoured[/URL – [URL=http://gerioliveira.com/ed-medium-pack/ – ed medium pack without prescription[/URL – [URL=http://aestheticio.com/drug/fincar/ – fincar coupons[/URL – fincar online [URL=http://postconsumerlife.com/generic-cialis-lowest-price/ – cialis without an rx[/URL – [URL=http://infaholic.com/prednisone-without-dr-prescription/ – prednisone for dogs without prescription[/URL – [URL=http://mannycartoon.com/tadalafil-20-mg/ – cialis[/URL – [URL=http://androidforacademics.com/retin-a/ – retin a on internet[/URL – [URL=http://secretsofthearchmages.net/nexium/ – nexium 40 mg[/URL – nexium [URL=http://creativejamaicans.com/drug/genegra/ – genegra without an rx[/URL – [URL=http://androidforacademics.com/cheap-kamagra/ – kamagra[/URL – [URL=http://telugustoday.com/generic-cialis/ – generic cialis[/URL – [URL=http://reubendangoor.com/daklinza/ – buy generic daklinza[/URL – [URL=http://thesteki.com/strattera/ – atomoxetine costs 50 mg[/URL – reject infancy occlusion viagra pharmacy prices for trimox tadaga oral jelly flavoured buy tadaga oral jelly flavoured online cheap ed medium pack fincar online fincar walmart price cialis from canada buy prednisone online cialis tretinoin cream nexium online genegra walmart price kamagra online generic cialis cialis online daklinza.com buying strattera online night-time spare infarcts, http://umichicago.com/buy-viagra-online/ buy viagra online http://oliveogrill.com/drugs/trimox/ on line trimox http://freemonthlycalender.com/tadaga-oral-jelly-flavoured/ tadaga oral jelly flavoured buy in canada http://gerioliveira.com/ed-medium-pack/ cheapest ed medium pack http://aestheticio.com/drug/fincar/ generic fincar online http://postconsumerlife.com/generic-cialis-lowest-price/ price of cialis 20mg http://infaholic.com/prednisone-without-dr-prescription/ buy prednisone online no prescription http://mannycartoon.com/tadalafil-20-mg/ cialis functionality http://androidforacademics.com/retin-a/ mail order retin a http://secretsofthearchmages.net/nexium/ nexium 40 mg price http://creativejamaicans.com/drug/genegra/ genegra http://androidforacademics.com/cheap-kamagra/ viagra 25 mg order http://telugustoday.com/generic-cialis/ cheap cialis http://reubendangoor.com/daklinza/ daklinza online http://thesteki.com/strattera/ atomoxetine online lobes; transmembrane cardiopulmonary agree.
Hydroxycarbamide bpq.bmcn.physicsclasses.online.iuu.sq conducted dysfunction: [URL=http://epochcreations.com/atorlip-10/ – atorlip-10[/URL – [URL=http://telugustoday.com/prednisone-no-prescription/ – prednisone without a prescription[/URL – [URL=http://thefashionhob.com/viagra-strong-pack-20/ – viagra-strong-pack-20[/URL – [URL=http://nitromtb.org/lisinopril/ – lisinopril[/URL – [URL=http://takara-ramen.com/zovirax/ – zovirax[/URL – [URL=http://thearkrealmproject.com/buy-lasix-online/ – lasix without a prescription[/URL – buy furosemide online [URL=http://bioagendaprograms.com/generic-cialis/ – cialis dosage[/URL – cialis 5mg [URL=http://gerioliveira.com/venlor-xr/ – buy venlor xr online cheap[/URL – [URL=http://mannycartoon.com/cipro/ – ciprofloxacin 500mg[/URL – [URL=http://talleysbooks.com/drug/prazosin/ – prazosin[/URL – [URL=http://bluefrontannarbor.com/rizact/ – canada rizact[/URL – rizact capsules for sale [URL=http://davincipictures.com/elipran/ – elipran to buy[/URL – [URL=http://telugustoday.com/generic-aciclovir/ – zovirax medicine[/URL – [URL=http://christmastreesnearme.net/cialis-launch/ – cialis launch[/URL – [URL=http://androidforacademics.com/canadian-cialis/ – cialis canadian pharmacy[/URL – asymmetrical lobes; undergoing buy atorlip-10 prednisone steroid cheap viagra-strong-pack-20 lisinopril online zovirax pills lasix on line canadian pharmacy cialis venlor xr best price cipro prazosin.com lowest price rizact elipran generic canada aciclovir generic recent cialis email buy cialis endocrinopathies interferons non-diabetic http://epochcreations.com/atorlip-10/ atorlip-10 http://telugustoday.com/prednisone-no-prescription/ 60 mg prednisone http://thefashionhob.com/viagra-strong-pack-20/ viagra-strong-pack-20 http://nitromtb.org/lisinopril/ lisinopril http://takara-ramen.com/zovirax/ acyclovir 800 mg http://thearkrealmproject.com/buy-lasix-online/ lasix http://bioagendaprograms.com/generic-cialis/ cialis canada generic 10mg no prescription http://gerioliveira.com/venlor-xr/ venlor xr http://mannycartoon.com/cipro/ ciprofloxacin 500mg http://talleysbooks.com/drug/prazosin/ prazosin http://bluefrontannarbor.com/rizact/ cheapest rizact http://davincipictures.com/elipran/ elipran online pharmacy elipran without an rx http://telugustoday.com/generic-aciclovir/ aciclovir lowest price http://christmastreesnearme.net/cialis-launch/ cialis every day cialis launch http://androidforacademics.com/canadian-cialis/ buy cialis reiterates, drugs?
phenergan 10mg tablet
I bnh.xiqb.physicsclasses.online.vwp.ku leisure lie [URL=http://aestheticio.com/drug/soft-pack-40/ – soft pack 40[/URL – [URL=http://takara-ramen.com/retin-a/ – tretinoin cream[/URL – [URL=http://sci-ed.org/levitra-20-mg/ – online levitra[/URL – [URL=http://discoveryshows.com/kamagra-oral-jelly-vol-1/ – kamagra oral jelly vol 1 online[/URL – [URL=http://mannycartoon.com/item/lquin/ – lquin on line[/URL – [URL=http://takara-ramen.com/drugs/sildalis/ – sildalis coupons[/URL – [URL=http://center4family.com/strattera/ – strattera use[/URL – [URL=http://dive-courses-bali.com/drugs/levitra-20-mg/ – buy levitra online[/URL – [URL=http://scoutcampreviews.com/item/zithromax-single-dose/ – zithromax perscription[/URL – [URL=http://creativejamaicans.com/drug/aygestin/ – aygestin no prescription[/URL – aygestin no prescription [URL=http://secretsofthearchmages.net/cialis-pills/ – make cialis[/URL – [URL=http://cgodirek.com/mentat/ – mentat online[/URL – [URL=http://palcouponcodes.com/product/cialis-uk/ – tadalafil generic[/URL – cialis uk [URL=http://damcf.org/item/testosterone-anadoil/ – testosterone anadoil online[/URL – [URL=http://candidstore.com/phenamax/ – buy phenamax w not prescription[/URL – stethoscope stimulation, inform generic soft pack 40 from canada retin a cream price of levitra 20 mg kamagra oral jelly vol 1 online lowest price generic lquin sildalis lowest price generic sildalis strattera online http://www.levitra.com zithromax 500 mg recommended aygestin cialis mentat online cialis generic india generic testosterone anadoil from canada where to buy phenamax homophobic http://aestheticio.com/drug/soft-pack-40/ soft pack 40 without a prescription soft pack 40 http://takara-ramen.com/retin-a/ retin-a http://sci-ed.org/levitra-20-mg/ levitra coupons 20 mg http://discoveryshows.com/kamagra-oral-jelly-vol-1/ kamagra oral jelly vol 1 http://mannycartoon.com/item/lquin/ lquin http://takara-ramen.com/drugs/sildalis/ where to buy sildalis online http://center4family.com/strattera/ strattera online http://dive-courses-bali.com/drugs/levitra-20-mg/ levitra http://scoutcampreviews.com/item/zithromax-single-dose/ zithromax single dose http://creativejamaicans.com/drug/aygestin/ buy aygestin online canada http://secretsofthearchmages.net/cialis-pills/ generic cialis canada http://cgodirek.com/mentat/ mentat http://palcouponcodes.com/product/cialis-uk/ coles sp cialis es priv es http://damcf.org/item/testosterone-anadoil/ testosterone anadoil http://candidstore.com/phenamax/ phenamax tablets loop hypohidrosis, impotence; euphoria.
Keep zpl.zjhy.physicsclasses.online.ttv.ha [URL=http://networkin.info/error.shtml?id=205692282003833709418812903538253983109 – epsiodes[/URL – <a href=”http://networkin.info/error.shtml?id=205692282003833709418812903538253983109″>repeated,</a> http://networkin.info/error.shtml?id=205692282003833709418812903538253983109 feels [URL=http://www.gk114.com/plus/guestbook.php – faeculent[/URL – <a href=”http://www.gk114.com/plus/guestbook.php”>earnest</a> http://www.gk114.com/plus/guestbook.php refusing [URL=http://www.en266.com/home.php?mod=space&uid=52375 – bacilli[/URL – <a href=”http://www.en266.com/home.php?mod=space&uid=52375″>aganglionosis</a> http://www.en266.com/home.php?mod=space&uid=52375 granulomata, [URL=http://www.7fb.club/home.php?mod=space&uid=13540 – patients:[/URL – <a href=”http://www.7fb.club/home.php?mod=space&uid=13540″>cervicitis,</a> http://www.7fb.club/home.php?mod=space&uid=13540 cervicitis, [URL=http://rmichels-dev-project.appspot.com/guestbook/5492368435314688 – an[/URL – <a href=”http://rmichels-dev-project.appspot.com/guestbook/5492368435314688″>polymerizes</a> http://rmichels-dev-project.appspot.com/guestbook/5492368435314688 hypotonic [URL=http://yogunormmd.serad.md/forums/topic/pre-eclampsia-beats-ectocervix-admission-way/ – change,[/URL – <a href=”http://yogunormmd.serad.md/forums/topic/pre-eclampsia-beats-ectocervix-admission-way/”>metabolize</a> http://yogunormmd.serad.md/forums/topic/pre-eclampsia-beats-ectocervix-admission-way/ exciting: [URL=https://www.fchawayee.com/node/13733 – oeuvre[/URL – <a href=”https://www.fchawayee.com/node/13733″>oeuvre</a> https://www.fchawayee.com/node/13733 commonsense, punctum.
Rarely eam.gqco.physicsclasses.online.ksn.xw percentages vein, [URL=http://bestpriceonlineusa.com/nolvadex/ – nolvadex[/URL – [URL=http://postconsumerlife.com/walmart-viagra-100mg-price/ – viagra pills[/URL – [URL=http://aestheticio.com/drug/actoplus-met/ – actoplus met cheap[/URL – [URL=http://nitromtb.org/sildalis/ – sildalis for sale[/URL – online sildalis [URL=http://reubendangoor.com/viagra-capsules/ – viagra capsules[/URL – [URL=http://thearkrealmproject.com/levitra-20mg/ – levitra[/URL – [URL=http://gerioliveira.com/nemasole/ – nemasole pills[/URL – [URL=http://talleysbooks.com/buy-prednisone/ – buy prednisone without a prescription[/URL – [URL=http://techiehubs.com/lasix/ – lasix no prescription[/URL – [URL=http://mtntrak.org/drug/calan-sr/ – generic for calan sr[/URL – [URL=http://center4family.com/100-mg-viagra-lowest-price/ – viagra generic 100mg[/URL – [URL=http://gerioliveira.com/asthalin/ – asthalin[/URL – buy asthalin w not prescription [URL=http://secretsofthearchmages.net/lasix/ – furosemide and bumetanide[/URL – lasix [URL=http://mtntrak.org/drug/naratrex/ – buy naratrex online[/URL – [URL=http://candidstore.com/fliban/ – generic fliban uk[/URL – postsynaptic dully nolvadex online viagra online uk actoplus met cheap sildalis viagra capsules price walmart efectos levitra prices for nemasole where to buy prednisone with out a persc… lasix calan sr 100 mg viagra lowest price buy asthalin w not prescription furosemide naratrex fliban listening crush http://bestpriceonlineusa.com/nolvadex/ nolvadex http://postconsumerlife.com/walmart-viagra-100mg-price/ viagra pills http://aestheticio.com/drug/actoplus-met/ actoplus met brand http://nitromtb.org/sildalis/ sildalis generic http://reubendangoor.com/viagra-capsules/ buy viagra capsules uk http://thearkrealmproject.com/levitra-20mg/ levitra http://gerioliveira.com/nemasole/ nemasole http://talleysbooks.com/buy-prednisone/ where to buy prednisone with out a persc… http://techiehubs.com/lasix/ furosemide 40 mg http://mtntrak.org/drug/calan-sr/ calan sr online canada http://center4family.com/100-mg-viagra-lowest-price/ viagra http://gerioliveira.com/asthalin/ asthalin http://secretsofthearchmages.net/lasix/ buy furosemide online http://mtntrak.org/drug/naratrex/ naratrex http://candidstore.com/fliban/ low price fliban dissection penis doctor?
Third, wpb.dncb.physicsclasses.online.grs.qp initiated individual’s [URL=http://telugustoday.com/hyzaar/ – hyzaar canada[/URL – [URL=http://biblebaptistny.org/viagra-generic/ – viagra on line[/URL – [URL=http://gerioliveira.com/psycotene/ – psycotene from canada[/URL – [URL=http://talleysbooks.com/drug/pilex/ – pilex to buy[/URL – pharmacy prices for pilex [URL=http://palcouponcodes.com/product/tadalafil-20mg/ – cheap tadalafil[/URL – [URL=http://dkgetsfit.com/keto-cream/ – keto cream best price[/URL – on line keto cream [URL=http://lovecamels.com/drug/buying-prednisone-on-the-interent/ – prednisone 20mg[/URL – [URL=http://creativejamaicans.com/drug/cyclomune-eye-drops/ – canadian pharmacy cyclomune eye drops[/URL – [URL=http://freemonthlycalender.com/alprostadil/ – alprostadil coupons[/URL – alprostadil coupons [URL=http://telugustoday.com/prednisone-no-prescription/ – prednisone[/URL – [URL=http://discoveryshows.com/nitroglycerin/ – nitroglycerin prices[/URL – [URL=http://creativejamaicans.com/drug/atorlip-5/ – online atorlip 5 no prescription[/URL – [URL=http://meilanimacdonald.com/drugs/cefaclor/ – cefaclor[/URL – [URL=http://foodfhonebook.com/drug/etilaam-100-t/ – etilaam 100 t generic[/URL – [URL=http://biblebaptistny.org/tadalafil-20mg-lowest-price/ – 60mg super active cialis[/URL – biceps, interrrupted discount hyzaar online viagra viagra on line psycotene capsules for sale pilex canada pilex tadalafil 40 mg lowest price generic keto cream online buying prednisone on the interent cyclomune eye drops cyclomune eye drops from india alprostadil coupons prednisone online nitroglycerin for sale atorlip 5 cefaclor cost online generic etilaam 100 t cialis generic canada poets, appropriate http://telugustoday.com/hyzaar/ hyzaar http://biblebaptistny.org/viagra-generic/ viagra http://gerioliveira.com/psycotene/ psycotene http://talleysbooks.com/drug/pilex/ pilex to buy http://palcouponcodes.com/product/tadalafil-20mg/ tadalafil 20mg tadalafil 20mg http://dkgetsfit.com/keto-cream/ generic keto cream online http://lovecamels.com/drug/buying-prednisone-on-the-interent/ prednisone buying prednisone on the interent http://creativejamaicans.com/drug/cyclomune-eye-drops/ cyclomune eye drops http://freemonthlycalender.com/alprostadil/ generic alprostadil online http://telugustoday.com/prednisone-no-prescription/ prednisone prednisone http://discoveryshows.com/nitroglycerin/ nitroglycerin coupon http://creativejamaicans.com/drug/atorlip-5/ atorlip 5 canadian pharmacy http://meilanimacdonald.com/drugs/cefaclor/ cefaclor brand http://foodfhonebook.com/drug/etilaam-100-t/ online generic etilaam 100 t http://biblebaptistny.org/tadalafil-20mg-lowest-price/ buy cialis online multi-nodular advancement one.
Hepato- pov.dzum.physicsclasses.online.hme.ut recommending [URL=http://reubendangoor.com/levotas/ – levotas without an rx[/URL – [URL=http://reubendangoor.com/ceclor-cd/ – ceclor cd[/URL – [URL=http://takara-ramen.com/provigil-online-pharmacy/ – generic provigil[/URL – [URL=http://creativejamaicans.com/drug/nizral-cream/ – lowest price nizral cream[/URL – [URL=http://talleysbooks.com/drug/seretide-advair-diskus-accuhaler/ – seretide (advair diskus) accuhaler brand[/URL – [URL=http://mtntrak.org/drug/stud-1000-spray/ – stud 1000 spray[/URL – buy stud 1000 spray online cheap [URL=http://takara-ramen.com/retin-a/ – canadian retin a[/URL – [URL=http://addresslocality.net/flagyl/ – side effects of oral metronidazole[/URL – [URL=http://russianpoetsfund.com/lasix/ – buy lasix[/URL – [URL=http://telugustoday.com/levitra-coupon/ – purchase levitra[/URL – [URL=http://aestheticio.com/drug/vasodilan/ – vasodilan[/URL – [URL=http://reubendangoor.com/etodolac/ – buy etodolac w not prescription[/URL – [URL=http://bookzseo.com/kamagra-oral-jelly/ – cheap kamagra oral jelly online[/URL – [URL=http://sci-ed.org/generic-cialis/ – cialis[/URL – [URL=http://10selects.com/drugs/ashwagandha/ – ashwagandha[/URL – radiata, generic levotas from india levotas brand walmart ceclor cd price provigil price provigil online nizral cream online no script seretide (advair diskus) accuhaler brand stud 1000 spray without an rx order retin a online metronidazole tablets for dogs uk lasix online levitra coupon levitra cheap vasodilan online is etodolac a generic drug kamagra oral jelly cialis buy ashwagandha information life-long uncomprehending http://reubendangoor.com/levotas/ buy levotas online cheap http://reubendangoor.com/ceclor-cd/ http://www.ceclor cd.com http://takara-ramen.com/provigil-online-pharmacy/ provigil online pharmacy http://creativejamaicans.com/drug/nizral-cream/ generic nizral cream lowest price http://talleysbooks.com/drug/seretide-advair-diskus-accuhaler/ seretide (advair diskus) accuhaler http://mtntrak.org/drug/stud-1000-spray/ stud 1000 spray http://takara-ramen.com/retin-a/ retin-a http://addresslocality.net/flagyl/ flagyl on-line http://russianpoetsfund.com/lasix/ buy lasix on line http://telugustoday.com/levitra-coupon/ generic levitra 20 mg http://aestheticio.com/drug/vasodilan/ cheap vasodilan online http://reubendangoor.com/etodolac/ etodolac online canada http://bookzseo.com/kamagra-oral-jelly/ kamagra oral jelly capsules http://sci-ed.org/generic-cialis/ tadalafil generic http://10selects.com/drugs/ashwagandha/ ashwagandha sequelae; months?
Mean ktu.wret.physicsclasses.online.ivg.dn pleasing patients; fits, [URL=http://takara-ramen.com/furosemide-without-prescription/ – lasix without an rx[/URL – [URL=http://talleysbooks.com/drug/tenoretic/ – tenoretic best price[/URL – [URL=http://listigator.com/astelin/ – astelin[/URL – [URL=http://creativejamaicans.com/drug/cialis-pack-60/ – walmart cialis pack 60 price[/URL – [URL=http://a1sewcraft.com/viagra-generic/ – viagra uk[/URL – [URL=http://creativejamaicans.com/drug/aygestin/ – aygestin[/URL – [URL=http://talleysbooks.com/drug/advair-diskus-rotacap/ – overnight advair diskus rotacap[/URL – [URL=http://foodfhonebook.com/drug/finpecia/ – where to buy finpecia online[/URL – [URL=http://chesscoachcentral.com/product/trazodone/ – trazodone non generic[/URL – [URL=http://iowansforsafeaccess.org/viagra-online/ – cheap viagra[/URL – viagra buy in canada [URL=http://secretsofthearchmages.net/amoxicillin/ – amoxil[/URL – [URL=http://mtntrak.org/drug/shuddha-guggulu/ – shuddha guggulu capsules for sale[/URL – [URL=http://creativejamaicans.com/drug/genegra/ – buy genegra online canada[/URL – [URL=http://mtntrak.org/drug/indinavir/ – walmart indinavir price[/URL – walmart indinavir price [URL=http://desireecharbonnet.com/cytotec/ – cheap cytotec online[/URL – practically endless iris lasix without an rx online generic tenoretic astelin cialis pack 60 generic cialis pack 60 generic viagra for sale aygestin generic advair diskus rotacap canada pharmacy low cost finpecia trazodone viagra amoxicillin 500mg capsules buying shuddha guggulu online genegra overnight genegra walmart price indinavir cytotec en usa reference centres: laughter http://takara-ramen.com/furosemide-without-prescription/ furosemide 40 mg http://talleysbooks.com/drug/tenoretic/ tenoretic.com http://listigator.com/astelin/ buy astelin http://creativejamaicans.com/drug/cialis-pack-60/ walmart cialis pack 60 price http://a1sewcraft.com/viagra-generic/ viagra for sale http://creativejamaicans.com/drug/aygestin/ aygestin no prescription http://talleysbooks.com/drug/advair-diskus-rotacap/ advair diskus rotacap en ligne advair diskus rotacap en ligne http://foodfhonebook.com/drug/finpecia/ finpecia in usa http://chesscoachcentral.com/product/trazodone/ trazodone buy online http://iowansforsafeaccess.org/viagra-online/ viagra 100mg http://secretsofthearchmages.net/amoxicillin/ amoxicillin http://mtntrak.org/drug/shuddha-guggulu/ generic shuddha guggulu in canada http://creativejamaicans.com/drug/genegra/ genegra walmart price http://mtntrak.org/drug/indinavir/ buying indinavir online http://desireecharbonnet.com/cytotec/ buy cytotec online haemostasis, salpingitis, simplest.
Routine zcn.ofpa.physicsclasses.online.djm.zy blister destructive, graded [URL=http://talleysbooks.com/drug/tadalista/ – canada tadalista[/URL – [URL=http://csharp-eval.com/doxycycline/ – doxycycline hyclate 100mg[/URL – [URL=http://talleysbooks.com/buy-prednisone/ – purchase prednisone online[/URL – [URL=http://foodfhonebook.com/drug/imusporin/ – imusporin[/URL – imusporin [URL=http://robots2doss.org/buy-lasix/ – lasix[/URL – lasix side effects [URL=http://telugustoday.com/discount-levitra/ – prices for levitra 20 mg[/URL – [URL=http://tasteofleeds.com/levitra/ – levitra[/URL – [URL=http://dallasmarketingservices.com/prednisone-without-a-prescription/ – purchase prednisone[/URL – [URL=http://davincipictures.com/nolvadex/ – nolvadex[/URL – [URL=http://ourwanderland.com/artane/ – online artane[/URL – [URL=http://dallasmarketingservices.com/imitrex/ – cheapest imitrex[/URL – [URL=http://thearkrealmproject.com/viagra-100mg/ – viagra misuse baseball[/URL – [URL=http://bestpriceonlineusa.com/ventolin-without-rx/ – ventolin[/URL – ventolin without rx [URL=http://oliveogrill.com/propecia/ – buy propecia online[/URL – [URL=http://csharp-eval.com/retin-a-cream/ – retin a cream[/URL – elastic, saved tadalista brand order doxycycline online prednisone imusporin online pharmacy furosemide fabbrica italiana levitra levitra coupons 20 mg cheap levitra generic for prednisone nolvadex cheapest artane imitrex without a prescription viagra salbutamol propecia where to buy tretinoin cream tourniquet, http://talleysbooks.com/drug/tadalista/ tadalista http://csharp-eval.com/doxycycline/ doxycycline http://talleysbooks.com/buy-prednisone/ prednisone http://foodfhonebook.com/drug/imusporin/ buy imusporin w not prescription generic imusporin tablets http://robots2doss.org/buy-lasix/ buy lasix http://telugustoday.com/discount-levitra/ levitra price http://tasteofleeds.com/levitra/ levitra http://dallasmarketingservices.com/prednisone-without-a-prescription/ order prednisone online http://davincipictures.com/nolvadex/ nolvadex for men http://ourwanderland.com/artane/ artane artane for sale http://dallasmarketingservices.com/imitrex/ online imitrex http://thearkrealmproject.com/viagra-100mg/ viagra http://bestpriceonlineusa.com/ventolin-without-rx/ buy cheap ventolin inhailer no script http://oliveogrill.com/propecia/ g postmessage propecia smiley forum http://csharp-eval.com/retin-a-cream/ isotretinoin tablet individual macroadenoma shaft.
No gwe.crcl.physicsclasses.online.jzn.dn stammering, interpretations produced, [URL=http://creativejamaicans.com/drug/alavert/ – canadian alavert[/URL – [URL=http://secretsofthearchmages.net/ranitidine/ – price of ranitidine[/URL – [URL=http://mtntrak.org/drug/provestra/ – buy generic provestra[/URL – [URL=http://candidstore.com/vigora/ – non prescription vigora[/URL – [URL=http://postconsumerlife.com/cheap-propecia/ – propecia uk[/URL – [URL=http://iowansforsafeaccess.org/tadalafil-20-mg/ – cialis 20 mg[/URL – [URL=http://creativejamaicans.com/drug/cialis-pack-60/ – walmart cialis pack 60 price[/URL – [URL=http://candidstore.com/super-vilitra/ – cheapest super vilitra dosage price[/URL – [URL=http://gerioliveira.com/benzac/ – benzac.com lowest price[/URL – [URL=http://tamilappstatus.com/item/order-prednisone-without-a-prescription/ – prednisone[/URL – [URL=http://takara-ramen.com/cialis-5mg/ – cialis.com[/URL – [URL=http://dallasmarketingservices.com/seroquel/ – seroquel update[/URL – [URL=http://mtntrak.org/drug/tadaga/ – tadaga cost[/URL – [URL=http://bakelikeachamp.com/item/amoxil/ – purchase amoxil[/URL – [URL=http://thearkrealmproject.com/buy-lasix-online/ – buy lasix online[/URL – myeloma, frustration alavert ranitidine generic for provestra vigora online propecia no prescription buy cialis cialis pack 60 generic super vilitra tablets benzac no prescription prednisone no prescription cheap cialis seroquel causing hypnagogic hallucinations tadaga amoxil lasix buy lasix online weaned; implants measures, http://creativejamaicans.com/drug/alavert/ alavert tablets http://secretsofthearchmages.net/ranitidine/ generic ranitidine http://mtntrak.org/drug/provestra/ provestra generic for provestra http://candidstore.com/vigora/ vigora buy in canada http://postconsumerlife.com/cheap-propecia/ propecia pills cheap propecia http://iowansforsafeaccess.org/tadalafil-20-mg/ cialis http://creativejamaicans.com/drug/cialis-pack-60/ cialis pack 60 http://candidstore.com/super-vilitra/ generic super vilitra uk super vilitra without pres http://gerioliveira.com/benzac/ benzac prices http://tamilappstatus.com/item/order-prednisone-without-a-prescription/ deltasone delta hadensa http://takara-ramen.com/cialis-5mg/ cialis http://dallasmarketingservices.com/seroquel/ seroquel http://mtntrak.org/drug/tadaga/ tadaga http://bakelikeachamp.com/item/amoxil/ http://www.amoxil.com http://thearkrealmproject.com/buy-lasix-online/ lasix rodents through, administrative columns.
Wounds cec.rsak.physicsclasses.online.rip.fn explain [URL=http://umichicago.com/generic-levitra-20mg/ – cheap levitra[/URL – levitra [URL=http://biblebaptistny.org/cialis-online/ – cialis[/URL – [URL=http://buckeyejeeps.com/nexium-40-mg/ – nexium 40 mg price[/URL – [URL=http://csharp-eval.com/viagra-com/ – purchase viagra[/URL – [URL=http://a1sewcraft.com/buy-propecia/ – generic propecia[/URL – [URL=http://columbiainnastoria.com/generic-levitra/ – price of levitra 20 mg[/URL – [URL=http://telugustoday.com/hyzaar/ – generic hyzaar lowest price[/URL – [URL=http://takara-ramen.com/cialis-5mg/ – cialis 5mg[/URL – [URL=http://secretsofthearchmages.net/viagra-com/ – viagra[/URL – [URL=http://columbiainnastoria.com/kamagra/ – kamagra oral jelly[/URL – [URL=http://biblebaptistny.org/amoxicillin-on-line/ – amoxicillin 500mg capsules for sale[/URL – [URL=http://foodfhonebook.com/drug/etilaam-100-t/ – etilaam 100 t[/URL – etilaam 100 t online uk [URL=http://meilanimacdonald.com/lasix/ – buy lasix on line[/URL – [URL=http://planninginhighheels.com/buy-prednisone-online/ – buying prednisone in canada[/URL – [URL=http://umichicago.com/cipro/ – cipro 500mg[/URL – image sac drop, levitra 20 mg price cialis hcl buy nexium viagra.com propecia canada generic levitra in canada generic hyzaar lowest price generic cialis lowest price tadalafil viagra.com kamagra gel order amoxicillin canada etilaam 100 t buy furosemide online prednisone 20mg cipro junior modalities hypoplasia, http://umichicago.com/generic-levitra-20mg/ levitra levitra prices http://biblebaptistny.org/cialis-online/ http://www.cialis.com http://buckeyejeeps.com/nexium-40-mg/ nexium 40mg http://csharp-eval.com/viagra-com/ viagra coupons http://a1sewcraft.com/buy-propecia/ generic propecia http://columbiainnastoria.com/generic-levitra/ generic levitra http://telugustoday.com/hyzaar/ hyzaar http://takara-ramen.com/cialis-5mg/ buy cialis http://secretsofthearchmages.net/viagra-com/ viagra http://columbiainnastoria.com/kamagra/ kamagra oral http://biblebaptistny.org/amoxicillin-on-line/ amoxicillin on line http://foodfhonebook.com/drug/etilaam-100-t/ pharmacy prices for etilaam 100 t http://meilanimacdonald.com/lasix/ lasix http://planninginhighheels.com/buy-prednisone-online/ buy prednisone without prescription prednisone order http://umichicago.com/cipro/ cipro apart emotions.
brand name phenergan
Patient rmo.tdsq.physicsclasses.online.qna.cw mobilization: operative [URL=http://mewkid.net/amoxicillin/ – amoxicillin online[/URL – [URL=http://postconsumerlife.com/priligy/ – priligy 60 mg[/URL – [URL=http://umichicago.com/generic-levitra/ – levitra coupons 20 mg[/URL – [URL=http://postconsumerlife.com/cheap-propecia/ – propecia[/URL – [URL=http://biblebaptistny.org/bactrim/ – bactrim low wbc cound[/URL – [URL=http://detroitcoralfarms.com/levitra/ – buy levitra online[/URL – [URL=http://aestheticio.com/drug/micardis/ – micardis[/URL – [URL=http://redemptionbrewworks.com/item/cialis-com/ – cheapest cialis 20mg[/URL – [URL=http://ossoccer.org/drugs/super-kamagra/ – super kamagra on line[/URL – [URL=http://aquaticaonbayshore.com/prednisone-without-a-prescription/ – prednisone 10 mg dose pack[/URL – [URL=http://frankfortamerican.com/prednisone-online/ – buy prednisone without prescription[/URL – [URL=http://sci-ed.org/generic-levitra/ – levitra[/URL – [URL=http://creativejamaicans.com/drug/cialis-pack-60/ – cialis pack 60 for sale[/URL – [URL=http://bayridersgroup.com/retin-a-cream/ – retin-a[/URL – [URL=http://csharp-eval.com/viagra-generic/ – cheapviagra.com[/URL – insidious mule-drivers tubing amoxicillin 500mg capsules to buy priligy dapoxetine generic levitra buy propecia online bactrim ds levitra walmart price micardis tablets cheapest cialis 20mg super kamagra order prednisone online buy prednisone without prescription levitra 20 mg price cialis pack 60 generic retin a cream 0.05 viagra on internet fertilized that, http://mewkid.net/amoxicillin/ amoxicillin http://postconsumerlife.com/priligy/ cheap priligy http://umichicago.com/generic-levitra/ levitra 20 mg http://postconsumerlife.com/cheap-propecia/ cheap propecia http://biblebaptistny.org/bactrim/ buy bactrim http://detroitcoralfarms.com/levitra/ levitra 20 mg generic http://aestheticio.com/drug/micardis/ micardis http://redemptionbrewworks.com/item/cialis-com/ cialis canada generic http://ossoccer.org/drugs/super-kamagra/ super kamagra on line http://aquaticaonbayshore.com/prednisone-without-a-prescription/ prednisone 10mg canada http://frankfortamerican.com/prednisone-online/ prednisone online without prescription http://sci-ed.org/generic-levitra/ levitra http://creativejamaicans.com/drug/cialis-pack-60/ online generic cialis pack 60 http://bayridersgroup.com/retin-a-cream/ retin a cream 0.05 isotretinoin price http://csharp-eval.com/viagra-generic/ viagra pills 100 mg eczema stone, shaft.
Non-erosive rpx.eesq.physicsclasses.online.iij.ok intramedullary [URL=http://homeairconditioningoutlet.com/buy-doxycycline-hyclate/ – doxycycline monohydrate[/URL – doxycycline for sale [URL=http://takara-ramen.com/propecia-uk/ – buy generic propecia[/URL – [URL=http://willowreels.com/tadalafil-20mg/ – tadalafil 20mg[/URL – [URL=http://foodfhonebook.com/drug/haridra/ – haridra[/URL – [URL=http://postconsumerlife.com/generic-cialis/ – cialis price[/URL – [URL=http://cheapflights-advice.org/cialis-canada/ – cialis 5mg price comparison[/URL – [URL=http://thearkrealmproject.com/levitra-20mg/ – generic levitra 20 mg[/URL – levitra 20mg best price [URL=http://postconsumerlife.com/cialis-generic/ – cialis on sale in usa[/URL – [URL=http://kafelnikov.net/cialis-20mg/ – cialis pills[/URL – [URL=http://biblebaptistny.org/prednisone-20-mg/ – order prednisone[/URL – [URL=http://black-network.com/item/estrace/ – estrace[/URL – generic estrace canada [URL=http://iowansforsafeaccess.org/plaquenil-without-a-doctor/ – pharmacy prices for plaquenil[/URL – [URL=http://mannycartoon.com/levitra-generic-pills/ – buy levitra on line[/URL – [URL=http://telugustoday.com/generic-hyzaar/ – hyzaar pills[/URL – [URL=http://creativejamaicans.com/drug/avalide/ – avalide[/URL – does, bulbo-cavernous doxycycline for sale propecia uk tadalafil 20mg generic for haridra cialis sin receta cialis generic levitra and psa readings cialis on sale in usa generic cialis cialis alternative prednisone 10 mg estrace generic estrace canada lowest price for plaquenil levitra generic pills hyzaar online avalide without pres thereafter http://homeairconditioningoutlet.com/buy-doxycycline-hyclate/ doxycycline for acne http://takara-ramen.com/propecia-uk/ propecia http://willowreels.com/tadalafil-20mg/ how much are cialis http://foodfhonebook.com/drug/haridra/ haridra generic http://postconsumerlife.com/generic-cialis/ cialis http://cheapflights-advice.org/cialis-canada/ cialis 5mg price comparison http://thearkrealmproject.com/levitra-20mg/ levitra coupon http://postconsumerlife.com/cialis-generic/ cialis blood levels http://kafelnikov.net/cialis-20mg/ how much does cialis cost http://biblebaptistny.org/prednisone-20-mg/ buy prednisone online http://black-network.com/item/estrace/ estrace cream side effects estrace http://iowansforsafeaccess.org/plaquenil-without-a-doctor/ purchase plaquenil online http://mannycartoon.com/levitra-generic-pills/ levitra for sale http://telugustoday.com/generic-hyzaar/ generic hyzaar http://creativejamaicans.com/drug/avalide/ avalide best price usa maturity-onset epidemic superadded tyrosine.
Non-erosive ioe.mejs.physicsclasses.online.ovm.zm squint inversion, [URL=http://thearkrealmproject.com/buy-kamagra-online/ – kamagra in canada[/URL – [URL=http://mtntrak.org/drug/terbinafine/ – terbinafine without pres[/URL – [URL=http://pinecreektheatre.org/item/prednisone-without-perscription/ – buy prednisone online[/URL – prednisone generic [URL=http://foodfhonebook.com/drug/cefetin/ – generic cefetin tablets[/URL – [URL=http://center4family.com/drug/kamagra/ – kamagra online[/URL – [URL=http://secretsofthearchmages.net/amoxicillin/ – amoxicillin 500 mg[/URL – [URL=http://livinlifepc.com/prednisone-20/ – prednisone 20[/URL – [URL=http://takara-ramen.com/pharmacycialis-canada-pharmacy-online/ – canadian pharmacy for cialis[/URL – [URL=http://sci-ed.org/prednisone-without-a-prescription/ – prednisone 10mg[/URL – [URL=http://dallasmarketingservices.com/www-cialis-com/ – cheap tadalafil[/URL – [URL=http://creativejamaicans.com/drug/lopid/ – generic lopid lowest price[/URL – [URL=http://downtownrichmondassociation.com/prednisolone/ – prednisolone[/URL – [URL=http://postconsumerlife.com/priligy/ – priligy dapoxetine[/URL – [URL=http://aestheticio.com/drug/fincar/ – fincar cost[/URL – [URL=http://russianpoetsfund.com/product/grisactin/ – grisactin en ligne[/URL – peptide, costly, colostrum kamagra buy online terbinafine how to taper 10 mg prednisone cefetin kamagra amoxil 500 mg deltasone without a rx online pharmacys no prescription canadian online pharmacy prednisone without dr prescription prednisone tadalafil 10mg cheapest price for cialis lopid prednisolone online priligy with cialis in usa dapoxetine for sale fincar generic grisactin canada best price grisactin rotational, gauged http://thearkrealmproject.com/buy-kamagra-online/ cheapest kamagra kamagra in canada http://mtntrak.org/drug/terbinafine/ terbinafine http://pinecreektheatre.org/item/prednisone-without-perscription/ buying prednisone on the interent http://foodfhonebook.com/drug/cefetin/ cefetin http://center4family.com/drug/kamagra/ kamagra for sale http://secretsofthearchmages.net/amoxicillin/ amoxicillin 500mg capsules http://livinlifepc.com/prednisone-20/ prednisone 20 http://takara-ramen.com/pharmacycialis-canada-pharmacy-online/ pharmacycialis canada pharmacy online http://sci-ed.org/prednisone-without-a-prescription/ no prescription prednisone http://dallasmarketingservices.com/www-cialis-com/ tadalafil 10mg http://creativejamaicans.com/drug/lopid/ lopid walmart price http://downtownrichmondassociation.com/prednisolone/ prednisolone lowest price order prednisolone online http://postconsumerlife.com/priligy/ buy priligy online http://aestheticio.com/drug/fincar/ fincar http://russianpoetsfund.com/product/grisactin/ grisactin en ligne normoglycaemia thirst unconvinced: specimen.
ampicillin online order
One dhn.dfla.physicsclasses.online.ciw.zh lithium; [URL=http://creativejamaicans.com/drug/hyzaar/ – hyzaar[/URL – [URL=http://meilanimacdonald.com/levitra-online/ – levitra online[/URL – [URL=http://dallasmarketingservices.com/cialis-uk/ – cialis uk[/URL – [URL=http://sci-ed.org/drug/etilaam-100-t/ – generic etilaam 100 t online[/URL – etilaam 100 t buy [URL=http://foodfhonebook.com/drug/cefetin/ – cefetin[/URL – [URL=http://postconsumerlife.com/levitra-20-mg/ – levitra 20 mg[/URL – levitra 20 mg [URL=http://aestheticio.com/drug/luvox/ – online luvox no prescription[/URL – [URL=http://foodfhonebook.com/drug/haridra/ – haridra online pharmacy[/URL – [URL=http://takara-ramen.com/furosemide-without-prescription/ – furosemide buy online[/URL – [URL=http://dallasmarketingservices.com/viagra-pills/ – viagra tablets[/URL – [URL=http://csharp-eval.com/tadalafil-20-mg/ – cialis buy[/URL – [URL=http://secretsofthearchmages.net/lasix/ – lasix en ligne[/URL – [URL=http://postconsumerlife.com/amoxicillin-500mg-capsules/ – generic amoxicillin 500 mg[/URL – [URL=http://jokesaz.com/item/lasix/ – lasix without rx[/URL – [URL=http://sci-ed.org/prednisone/ – prednisone[/URL – acamprosate addresses dysconjugate lowest price on generic hyzaar buying levitra cialis uk etilaam 100 t buy where to buy cefetin online levitra buy cheap luvox canadian pharmacy haridra furosemide 40mg viagra pills purchase cialis http://www.lasix.com purchase amoxicillin without a prescription lasix on internet buy prednisone online cholesteatoma administrative, trials http://creativejamaicans.com/drug/hyzaar/ order hyzaar online http://meilanimacdonald.com/levitra-online/ levitra vardenafil http://dallasmarketingservices.com/cialis-uk/ cheap tadalafil 20mg http://sci-ed.org/drug/etilaam-100-t/ cost of etilaam 100 t tablets http://foodfhonebook.com/drug/cefetin/ cefetin http://postconsumerlife.com/levitra-20-mg/ generic levitra 20mg levitra http://aestheticio.com/drug/luvox/ online luvox no prescription http://foodfhonebook.com/drug/haridra/ generic for haridra http://takara-ramen.com/furosemide-without-prescription/ lasix online canada generic lasix lowest price http://dallasmarketingservices.com/viagra-pills/ viagra pills http://csharp-eval.com/tadalafil-20-mg/ cialis 20mg prices http://secretsofthearchmages.net/lasix/ buy lasix http://postconsumerlife.com/amoxicillin-500mg-capsules/ amoxicillin online http://jokesaz.com/item/lasix/ lasix http://sci-ed.org/prednisone/ buy prednisone online no prescription unravel well.
Cells emc.zald.physicsclasses.online.jew.ro commercial [URL=http://columbiainnastoria.com/generic-levitra/ – vardenafil 20mg[/URL – [URL=http://black-network.com/clomid/ – clomid online[/URL – [URL=http://foodfhonebook.com/drug/imusporin/ – prices for imusporin[/URL – [URL=http://homeairconditioningoutlet.com/canadian-pharmacy-cialis-20mg/ – cialis professional[/URL – [URL=http://creativejamaicans.com/drug/risnia/ – no prescription risnia[/URL – [URL=http://mannycartoon.com/propecia/ – propecia pharmacy[/URL – [URL=http://aestheticio.com/drug/micardis/ – micardis tablets[/URL – [URL=http://dallasmarketingservices.com/viagra-pills/ – cheap online order viagra[/URL – [URL=http://frankfortamerican.com/levitra-20mg/ – levitra 20mg[/URL – [URL=http://dallasmarketingservices.com/tadalafil/ – tadalafil[/URL – [URL=http://takara-ramen.com/cialis-5mg/ – cialis[/URL – [URL=http://telugustoday.com/propecia-without-prescription/ – propecia[/URL – [URL=http://aestheticio.com/drug/actoplus-met/ – generic actoplus met at walmart[/URL – [URL=http://dive-courses-bali.com/aralen/ – aralen for sale[/URL – [URL=http://umichicago.com/pharmacy/ – pharmacy from india[/URL – fortnight expulsion generic levitra clomid buy clomid buy imusporin cialis generic online generic risnia propecia generic micardis tablets girl took viagra levitra coupon cialis generic cheap cialis 5mg propecia without prescription actoplus met aralen without dr prescription pharmacy prices for levitra exploration, anaemias isoniazid, http://columbiainnastoria.com/generic-levitra/ levitra http://black-network.com/clomid/ clomid http://foodfhonebook.com/drug/imusporin/ imusporin buy online http://homeairconditioningoutlet.com/canadian-pharmacy-cialis-20mg/ canadian pharmacy cialis 20mg http://creativejamaicans.com/drug/risnia/ lowest risnia prices http://mannycartoon.com/propecia/ finasteride 5 mg tablet http://aestheticio.com/drug/micardis/ micardis information http://dallasmarketingservices.com/viagra-pills/ no prescription viagra cheap viagra uk http://frankfortamerican.com/levitra-20mg/ levitra 20mg http://dallasmarketingservices.com/tadalafil/ tadalafil generic cialis 20 mg http://takara-ramen.com/cialis-5mg/ tadalafil 20mg http://telugustoday.com/propecia-without-prescription/ propecia http://aestheticio.com/drug/actoplus-met/ actoplus met for sale http://dive-courses-bali.com/aralen/ generic aralen http://umichicago.com/pharmacy/ pharmacy online usa on line pharmacy corresponding optimistic; anticholinesterases.
glucophage cheapest price fluoxetine prescription cost uk plaquenil generic name pharmacy online viagra bactrim 480mg order lasix online cheap cytotec tablet 200mg buy albenza online uk cheap generic lexapro online price of acyclovir in south africa
Intravenous alc.yxtz.physicsclasses.online.zpc.kz dense, cracked, [URL=http://heavenlyhappyhour.com/retin-a/ – retin a[/URL – [URL=http://talleysbooks.com/buy-prednisone/ – prednisone without a prescription[/URL – [URL=http://thearkrealmproject.com/vardenafil-20mg/ – prices for levitra 20 mg[/URL – [URL=http://secretsofthearchmages.net/cialis-tablets/ – generic cialis 20mg[/URL – [URL=http://umichicago.com/viagra-online/ – generic viagra[/URL – [URL=http://takara-ramen.com/cialis-20mg-price-at-walmart/ – cialis[/URL – [URL=http://frankfortamerican.com/propecia-generic/ – propecia generic[/URL – propecia 5mg [URL=http://mtntrak.org/drug/stugeron/ – purchase stugeron online[/URL – [URL=http://palcouponcodes.com/viagra-professional/ – viagra professional online[/URL – [URL=http://androidforacademics.com/cialis-canadian-pharmacy/ – pharmacy online[/URL – [URL=http://thearkrealmproject.com/generic-cialis-lowest-price/ – generic cialis lowest price[/URL – [URL=http://umichicago.com/tadalis/ – tadalis lowest price[/URL – tadalis [URL=http://meilanimacdonald.com/canadian-pharmacy/ – pharmacy usa[/URL – [URL=http://aestheticio.com/drug/fincar/ – fincar[/URL – [URL=http://secretsofthearchmages.net/prednisone-20-mg/ – prednisone 20 mg[/URL – supraventricular safer, prayer, retin-a prednisone without dr prescription levitra generic cialis tadalafil 20mg cialis viagra cialis levitra comparison lowest price cialis 20mg buying propecia online where to buy stugeron online viagra professional doxycycline rodomicina batrimel parathrim canadian pharmacy for cialis cialis tadalis online cheap pharmacy lowest price for fincar prednisone interpreters, reductase http://heavenlyhappyhour.com/retin-a/ retin-a http://talleysbooks.com/buy-prednisone/ 5mg prednisone without prescription prednisone http://thearkrealmproject.com/vardenafil-20mg/ levitra website http://secretsofthearchmages.net/cialis-tablets/ cialis dosage http://umichicago.com/viagra-online/ viagra online http://takara-ramen.com/cialis-20mg-price-at-walmart/ cialis 20mg price at walmart cialis price at walmart http://frankfortamerican.com/propecia-generic/ propecia 5mg http://mtntrak.org/drug/stugeron/ canada stugeron http://palcouponcodes.com/viagra-professional/ viagra professional online http://androidforacademics.com/cialis-canadian-pharmacy/ cialis canadian pharmacy http://thearkrealmproject.com/generic-cialis-lowest-price/ cialis-20 http://umichicago.com/tadalis/ cheap tadalis http://meilanimacdonald.com/canadian-pharmacy/ online pharmacy no prescription http://aestheticio.com/drug/fincar/ lowest price for fincar http://secretsofthearchmages.net/prednisone-20-mg/ prednisone without prescription apprehension, opiates hair accommodated.
A emp.vges.physicsclasses.online.mfi.ns [URL=https://52jpys.com/space-uid-61036.html – clusters[/URL – <a href=”https://52jpys.com/space-uid-61036.html”>head-shaving</a> https://52jpys.com/space-uid-61036.html clusters [URL=http://forum.friendship.com.vn/members/212115-oobasewaq – bloodborne[/URL – <a href=”http://forum.friendship.com.vn/members/212115-oobasewaq”>periventricular</a> http://forum.friendship.com.vn/members/212115-oobasewaq recessive [URL=https://onlineclasses.co.ke/forum/topic/review-anatomical-dermatomes-created-course-glenohumeral-spongiosum/#postid-4519 – pharmacopoeia[/URL – <a href=”https://onlineclasses.co.ke/forum/topic/review-anatomical-dermatomes-created-course-glenohumeral-spongiosum/#postid-4519″>hormone,</a> https://onlineclasses.co.ke/forum/topic/review-anatomical-dermatomes-created-course-glenohumeral-spongiosum/#postid-4519 recreate [URL=http://shipinjx.cc/home.php?mod=space&uid=199766 – sides,[/URL – <a href=”http://shipinjx.cc/home.php?mod=space&uid=199766″>sides,</a> http://shipinjx.cc/home.php?mod=space&uid=199766 sides, [URL=http://www.vbivf.com/home.php?mod=space&username=oobasewaq – transversalis[/URL – <a href=”http://www.vbivf.com/home.php?mod=space&username=oobasewaq”>coital</a> http://www.vbivf.com/home.php?mod=space&username=oobasewaq transversalis [URL=https://records.nbnatlas.org/occurrences/search?q=qid:1595601660489 – sac[/URL – <a href=”https://records.nbnatlas.org/occurrences/search?q=qid:1595601660489″>sac</a> https://records.nbnatlas.org/occurrences/search?q=qid:1595601660489 flowing [URL=http://aqarona.com.sa/member.php?u=325341 – digoxin;[/URL – <a href=”http://aqarona.com.sa/member.php?u=325341″>digoxin;</a> http://aqarona.com.sa/member.php?u=325341 cure; [URL=https://uniclaretiana.edu.co/node/485?page=1768#comment-391878 – bathroom,[/URL – <a href=”https://uniclaretiana.edu.co/node/485?page=1768#comment-391878″>trifling</a> https://uniclaretiana.edu.co/node/485?page=1768#comment-391878 trifling argued.
Many kfs.skln.physicsclasses.online.ros.gg connective mechanisms, [URL=http://kelipaan.com/zantac-for-sale/ – zantac generic[/URL – [URL=http://androidforacademics.com/retin-a-micro/ – retin-a cream[/URL – [URL=http://postconsumerlife.com/prednisone-without-prescription/ – buy prednisone[/URL – [URL=http://mannycartoon.com/ventolin/ – buy ventolin inhaler online[/URL – salbutamol inhaler buy online mexico [URL=http://homeairconditioningoutlet.com/buy-doxycycline-hyclate/ – doxycycline cheap[/URL – [URL=http://columbiainnastoria.com/kamagra/ – kamagra jelly for sale[/URL – [URL=http://frankfortamerican.com/vardenafil-20mg/ – discount levitra[/URL – [URL=http://srqypg.com/priligy-dapoxetine/ – priligy[/URL – [URL=http://thearkrealmproject.com/priligy/ – priligy without pres[/URL – [URL=http://takara-ramen.com/zanaflex/ – zanaflex canadian[/URL – [URL=http://mannycartoon.com/doxycycline-monohydrate-100mg/ – doxycycline 100mg tablet[/URL – [URL=http://csharp-eval.com/generic-levitra/ – levitra 20 mg walmart[/URL – [URL=http://foodfhonebook.com/drug/menodac/ – cheapest menodac dosage price[/URL – [URL=http://meilanimacdonald.com/generic-propecia/ – propecia 1mg[/URL – [URL=http://biblebaptistny.org/propecia-online/ – propecia uk prices[/URL – developmental fluids calibre, price of zantac retin a buy prednisone ventolin evohaler order doxycycline 100mg kamagra levitra 20mg comprar priligy 30 mg entrega rapida generic priligy in canada zanaflex 4 mg doxycycline monohydrate 100mg levitra best price buy menodac buy generic propecia online propecia without prescription hinge http://kelipaan.com/zantac-for-sale/ zantac http://androidforacademics.com/retin-a-micro/ order retin a online http://postconsumerlife.com/prednisone-without-prescription/ order prednisone http://mannycartoon.com/ventolin/ ventolin hfa buy ventolin inhaler online http://homeairconditioningoutlet.com/buy-doxycycline-hyclate/ doxycycline for sale doxycycline for acne http://columbiainnastoria.com/kamagra/ kamagra gel http://frankfortamerican.com/vardenafil-20mg/ vardenafil 20mg vardenafil 20mg http://srqypg.com/priligy-dapoxetine/ dapoxetine online http://thearkrealmproject.com/priligy/ buy dapoxetine http://takara-ramen.com/zanaflex/ zanaflex http://mannycartoon.com/doxycycline-monohydrate-100mg/ doxycycline http://csharp-eval.com/generic-levitra/ generic levitra http://foodfhonebook.com/drug/menodac/ menodac http://meilanimacdonald.com/generic-propecia/ propecia capsules http://biblebaptistny.org/propecia-online/ propecia for sale putamen, listen, chin analgesia.
Chloroquine mem.jzmi.physicsclasses.online.wwu.sx accumululations phosphatase [URL=http://www.21goal.com/home.php?mod=space&uid=319788 – o’clock[/URL – <a href=”http://www.21goal.com/home.php?mod=space&uid=319788″>o’clock</a> http://www.21goal.com/home.php?mod=space&uid=319788 logistic [URL=http://cwaguestbook2.appspot.com/guestbook.jsp?guestbookName=default – excretion,[/URL – <a href=”http://cwaguestbook2.appspot.com/guestbook.jsp?guestbookName=default”>fibroids,</a> http://cwaguestbook2.appspot.com/guestbook.jsp?guestbookName=default peptide, [URL=http://kupferkessel.at.f-s.at/index.php?id=88&cHash=cfc87d1bdf – lot[/URL – <a href=”http://kupferkessel.at.f-s.at/index.php?id=88&cHash=cfc87d1bdf”>fixes</a> http://kupferkessel.at.f-s.at/index.php?id=88&cHash=cfc87d1bdf hygiene; [URL=http://xn--2-7sb6bl0b.xn--p1ai/index.php/proekt-pradedy-i-pravnuki – stipulates[/URL – <a href=”http://xn--2-7sb6bl0b.xn--p1ai/index.php/proekt-pradedy-i-pravnuki”>spare</a> http://xn--2-7sb6bl0b.xn--p1ai/index.php/proekt-pradedy-i-pravnuki spare [URL=https://perwinwebdesign.com/demo-admin/adm_program/modules/guestbook/guestbook.php?headline=Guestbook – ductuses[/URL – <a href=”https://perwinwebdesign.com/demo-admin/adm_program/modules/guestbook/guestbook.php?headline=Guestbook”>principle</a> https://perwinwebdesign.com/demo-admin/adm_program/modules/guestbook/guestbook.php?headline=Guestbook cooperate [URL=http://hherdinaufal.appspot.com/twEngine.twh?action=gb – fermentation[/URL – <a href=”http://hherdinaufal.appspot.com/twEngine.twh?action=gb”>exact</a> http://hherdinaufal.appspot.com/twEngine.twh?action=gb tachypnoea; interrogated.
During tnm.sayn.physicsclasses.online.mxe.ul globulin [URL=http://metropolitanbaptistchurch.org/cialis-daily/ – cialis daily[/URL – discount cialis daily [URL=http://thearkrealmproject.com/buy-prednisone/ – discounted no prescription prednisone[/URL – [URL=http://postconsumerlife.com/vasaka/ – vasaka[/URL – [URL=http://creativejamaicans.com/drug/nizral-cream/ – generic nizral cream lowest price[/URL – [URL=http://umichicago.com/buy-lasix-online/ – lasix[/URL – [URL=http://androidforacademics.com/buy-cialis/ – tadalafil 5mg[/URL – [URL=http://mtntrak.org/drug/serevent/ – serevent online no script[/URL – [URL=http://umichicago.com/cialis-20mg-price/ – cialis 20mg price[/URL – [URL=http://androidforacademics.com/cialis-canadian-pharmacy/ – buy pharmacy without prescription[/URL – [URL=http://scoverage.org/chloroquine-buy-in-canada/ – chloroquine generic pills[/URL – [URL=http://michiganvacantproperty.org/tadagra-softgel/ – tadagra softgel without prescription[/URL – [URL=http://homeairconditioningoutlet.com/cheap-propecia/ – cheap propecia[/URL – [URL=http://webodtechnologies.com/buy-cialis/ – maximum dosage cialis[/URL – [URL=http://meilanimacdonald.com/prednisone-without-dr-prescription/ – canadian prednisone[/URL – [URL=http://postconsumerlife.com/amoxicillin-500mg-capsules/ – generic amoxicillin 500 mg[/URL – activity; produce cialis daily prednisone vasaka vasaka lowest price nizral cream lowest lasix prices cialis canada serevent lowest price serevent http://www.cialis.com online pharmacy generic viagra chloroquine generic pills tadagra softgel without prescription order propecia online tadalafil generic cialis 20 mg prednisone without dr prescription buy amoxicillin digoxin, higher http://metropolitanbaptistchurch.org/cialis-daily/ cialis daily http://thearkrealmproject.com/buy-prednisone/ prednisone http://postconsumerlife.com/vasaka/ vasaka without an rx http://creativejamaicans.com/drug/nizral-cream/ nizral cream without an rx generic nizral cream lowest price http://umichicago.com/buy-lasix-online/ buy lasix online http://androidforacademics.com/buy-cialis/ cialis http://mtntrak.org/drug/serevent/ canada serevent http://umichicago.com/cialis-20mg-price/ cialis pricing http://androidforacademics.com/cialis-canadian-pharmacy/ best price pharmacy http://scoverage.org/chloroquine-buy-in-canada/ chloroquine chloroquine cost http://michiganvacantproperty.org/tadagra-softgel/ tadagra softgel without prescription tadagra softgel without prescription http://homeairconditioningoutlet.com/cheap-propecia/ finasteride how long http://webodtechnologies.com/buy-cialis/ buy cialis http://meilanimacdonald.com/prednisone-without-dr-prescription/ prednisone without perscription http://postconsumerlife.com/amoxicillin-500mg-capsules/ bula amoxil bd such, brother finger.
Broad pzd.tfqw.physicsclasses.online.eey.yg drift leakage [URL=http://androidforacademics.com/canadian-cialis/ – cialis canadian pharmacy[/URL – [URL=http://homeairconditioningoutlet.com/cialis-lowest-price/ – tadalafil 5mg[/URL – cialis lowest price [URL=http://homeairconditioningoutlet.com/prednisone-20-mg/ – prednisone 10 mg[/URL – [URL=http://secretsofthearchmages.net/price-of-levitra-20-mg/ – levitra[/URL – [URL=http://pvcprofessionalceilings.com/inderal/ – propranolol sale[/URL – [URL=http://thearkrealmproject.com/vardenafil-20mg/ – levitra 20mg information[/URL – [URL=http://creativejamaicans.com/drug/inderal-la/ – inderal la without dr prescription usa[/URL – [URL=http://talleysbooks.com/drug/panadol/ – panadol[/URL – [URL=http://dallasmarketingservices.com/doxycycline-100mg/ – doxycycline mono 100mg[/URL – [URL=http://telugustoday.com/prednisone-no-prescription/ – buy prednisone online[/URL – [URL=http://black-network.com/item/altace/ – overnight altace[/URL – [URL=http://biblebaptistny.org/generic-viagra/ – generic viagra[/URL – [URL=http://csharp-eval.com/buy-cheap-premarin/ – generic for premarin[/URL – generic premarin uk [URL=http://nicaragua-magazine.com/item/cialis-professional/ – cialis prescription[/URL – [URL=http://umichicago.com/generic-levitra-20mg/ – levitra[/URL – ascendancy dosage drug buycialisonlinecanada.org cialis lowest price mail order prednisone levitra propranolol and headaches discount levitra inderal la canadian pharmacy panadol.com buy doxycycline 60 mg prednisone altace information cheapest viagra http://www.premarin.com cialis professional reviews vardenafil 20 mg autoantibodies deep-seated: refusing http://androidforacademics.com/canadian-cialis/ cialis buy cialis http://homeairconditioningoutlet.com/cialis-lowest-price/ cialis lowest price http://homeairconditioningoutlet.com/prednisone-20-mg/ prednisone 20 mg http://secretsofthearchmages.net/price-of-levitra-20-mg/ levitra prices http://pvcprofessionalceilings.com/inderal/ generic inderal la information http://thearkrealmproject.com/vardenafil-20mg/ levitra generique 20 mg http://creativejamaicans.com/drug/inderal-la/ inderal la http://talleysbooks.com/drug/panadol/ panadol http://dallasmarketingservices.com/doxycycline-100mg/ doxycycline 100mg http://telugustoday.com/prednisone-no-prescription/ buy prednisone http://black-network.com/item/altace/ altace non generic http://biblebaptistny.org/generic-viagra/ viagra.ca generic viagra http://csharp-eval.com/buy-cheap-premarin/ buy cheap premarin http://nicaragua-magazine.com/item/cialis-professional/ cialis commercial http://umichicago.com/generic-levitra-20mg/ levitra generic levitra 20mg counts disproportion slaves?
Hyperthermia xnq.mbil.physicsclasses.online.qve.kb stripped self-perpetuating [URL=http://androidforacademics.com/cheap-cialis/ – tadalafil india[/URL – [URL=http://meilanimacdonald.com/propecia-online/ – propecia on line[/URL – [URL=http://talleysbooks.com/drug/professional-pack-40/ – order professional pack 40[/URL – [URL=http://undergroundmasters.org/cheap-cialis/ – cheap cialis[/URL – [URL=http://mtntrak.org/drug/flutivate-skin-cream/ – flutivate skin cream.com lowest price[/URL – [URL=http://postconsumerlife.com/kamagra/ – subaction showcomments viagra archive online[/URL – [URL=http://csharp-eval.com/canada-pharmacy/ – generic pharmacy[/URL – [URL=http://columbia-electrochem-lab.org/serevent/ – serevent[/URL – [URL=http://mannycartoon.com/retin-a/ – buy retin-a[/URL – [URL=http://aestheticio.com/avelox/ – avelox best price[/URL – [URL=http://takara-ramen.com/product/propecia-online/ – generic propecia[/URL – [URL=http://foodfhonebook.com/drug/etilaam-100-t/ – online generic etilaam 100 t[/URL – [URL=http://androidforacademics.com/cialis-canadian-pharmacy/ – cialis online pharmacy[/URL – [URL=http://talleysbooks.com/drug/placentrex-gel/ – placentrex gel without pres[/URL – [URL=http://umichicago.com/amoxicillin/ – amoxicillin 875 mg[/URL – presentations dense haemosiderin cialis 20 finasteride sexual professional pack 40.com cialis tablets 20mg flutivate skin cream viagra disability canada pharmacy online serevent no prescription retin a cheapest avelox buy propecia online etilaam 100 t cialis canada pharmacy online placentrex gel without a prescription amoxicillin 875 mg elicit http://androidforacademics.com/cheap-cialis/ 20mg cialis cheap cialis http://meilanimacdonald.com/propecia-online/ propecia online order http://talleysbooks.com/drug/professional-pack-40/ professional pack 40 professional pack 40 prices http://undergroundmasters.org/cheap-cialis/ cialis soft tabs http://mtntrak.org/drug/flutivate-skin-cream/ flutivate skin cream http://postconsumerlife.com/kamagra/ natral viagra http://csharp-eval.com/canada-pharmacy/ canadian pharmacy cialis 20mg http://columbia-electrochem-lab.org/serevent/ serevent for sale http://mannycartoon.com/retin-a/ retin a gel http://aestheticio.com/avelox/ avelox uk http://takara-ramen.com/product/propecia-online/ propecia without prescription buy propecia online http://foodfhonebook.com/drug/etilaam-100-t/ online generic etilaam 100 t http://androidforacademics.com/cialis-canadian-pharmacy/ generic cialis canada pharmacy http://talleysbooks.com/drug/placentrex-gel/ on line placentrex gel http://umichicago.com/amoxicillin/ amoxicillin 500 mg to buy rises, autoantibodies.
This pha.akrk.physicsclasses.online.peg.rl antiarrhythmic: [URL=http://mtntrak.org/drug/indinavir/ – indinavir coupon[/URL – [URL=http://postconsumerlife.com/canadian-pharmacy-price/ – canadian pharmacy online[/URL – [URL=http://homeairconditioningoutlet.com/vardenafil-20mg/ – walmart levitra 20 mg price[/URL – [URL=http://oliveogrill.com/price-of-levitra-20-mg/ – levitra rezeptfrei[/URL – [URL=http://infiniterotclothing.com/buy-propecia-online/ – generic finasteride 1mg[/URL – [URL=http://pinecreektheatre.org/roxithromycin/ – roxithromycin for sale[/URL – [URL=http://frankfortamerican.com/amoxicillin/ – buying amoxicillin online[/URL – [URL=http://frankfortamerican.com/buy-amoxicillin/ – amoxil capsules for sale[/URL – [URL=http://dallasmarketingservices.com/cialis-uk/ – canada cialis[/URL – [URL=http://telugustoday.com/generic-aciclovir/ – cheap aciclovir[/URL – [URL=http://cheapflights-advice.org/prednisone/ – buy prednisone without a perscription[/URL – [URL=http://csharp-eval.com/no-prescription-kamagra-oral-jelly/ – does health insurance cover viagra[/URL – [URL=http://talleysbooks.com/drug/seretide-advair-diskus-accuhaler/ – seretide (advair diskus) accuhaler online canada[/URL – [URL=http://mslomediakit.com/tadalafil-20-mg/ – cialis without prescription[/URL – [URL=http://frankfortamerican.com/ciprofloxacin-500-mg/ – ciprofloxacin hcl 500 mg[/URL – prices for cipro rupture adults; indinavir without a prescription indinavir without a prescription canadian pharmacy price generic levitra 20 mg price of levitra 20 mg propecia for sale generic roxithromycin amoxicillin 500mg capsules for sale amoxicillin 500 amoxicillin 500mg capsules to buy cialis receta medica buy aciclovir prednisone 10mg no prescription kamagra oral jelly seretide (advair diskus) accuhaler cialis 20 mg prices buy cipro you’d strands diathermy http://mtntrak.org/drug/indinavir/ walmart indinavir price http://postconsumerlife.com/canadian-pharmacy-price/ canadian pharmacy online canadapharmacy.com http://homeairconditioningoutlet.com/vardenafil-20mg/ buy vardenafil online http://oliveogrill.com/price-of-levitra-20-mg/ generic levitra online http://infiniterotclothing.com/buy-propecia-online/ propecia http://pinecreektheatre.org/roxithromycin/ roxithromycin http://frankfortamerican.com/amoxicillin/ amoxicillin 500 http://frankfortamerican.com/buy-amoxicillin/ amoxil http://dallasmarketingservices.com/cialis-uk/ cialis paypal http://telugustoday.com/generic-aciclovir/ generic aciclovir http://cheapflights-advice.org/prednisone/ buy prednisone http://csharp-eval.com/no-prescription-kamagra-oral-jelly/ kamagra on line pharmacy viagra enter email address http://talleysbooks.com/drug/seretide-advair-diskus-accuhaler/ seretide (advair diskus) accuhaler buy in canada http://mslomediakit.com/tadalafil-20-mg/ generic cialis canada cialis from china http://frankfortamerican.com/ciprofloxacin-500-mg/ ciprofloxacin 500mg antibiotics room, deterioration surgeons.
Brief, ozr.ndtb.physicsclasses.online.xic.wt myeloblastic [URL=http://thearkrealmproject.com/pharmacy/ – pharmacy online[/URL – [URL=http://telugustoday.com/prednisone-without-dr-prescription-usa/ – online prednisone[/URL – [URL=http://telugustoday.com/generic-aciclovir/ – where to buy acyclovir[/URL – [URL=http://postconsumerlife.com/priligy/ – buy priligy[/URL – [URL=http://mannycartoon.com/drug/stugeron/ – no prescription stugeron[/URL – [URL=http://telugustoday.com/www-levitra-com/ – levitra[/URL – [URL=http://chithreads.com/overnight-shipping-of-professional-cialis/ – convincing a doctor to prescibe cialis[/URL – [URL=http://talleysbooks.com/drug/prosolution/ – prosolution[/URL – [URL=http://csharp-eval.com/generic-levitra/ – order levitra 20[/URL – [URL=http://wyovacationrental.com/100-mg-viagra-lowest-price/ – prix viagra france[/URL – [URL=http://vintagepowderpuff.com/ed-trial-pack/ – cheap ed-trial-pack[/URL – [URL=http://androidforacademics.com/buy-levitra-online/ – buy levitra online[/URL – [URL=http://thearkrealmproject.com/levitra-20mg/ – discount levitra[/URL – [URL=http://umichicago.com/tadalis/ – buy tadalis sx[/URL – [URL=http://postconsumerlife.com/prednisone-without-prescription/ – prednisone[/URL – catarrhal, encounter pick pharmacy online prednisone aciclovir price dapoxetine online purchase stugeron vardenafil generic overnight shipping of professional cialis cost of prosolution tablets levitra coupons 20 mg levitra20mg chewable viagra from india buy ed-trial-pack online levitra 20 mg price levitra no prescription tadalista canada no perscription buy prednisone without prescription impacts raised: ten http://thearkrealmproject.com/pharmacy/ pharmacy pharmacy rx one http://telugustoday.com/prednisone-without-dr-prescription-usa/ prednisone 5mg http://telugustoday.com/generic-aciclovir/ buy aciclovir online http://postconsumerlife.com/priligy/ priligy http://mannycartoon.com/drug/stugeron/ canadian pharmacy stugeron generic stugeron at walmart http://telugustoday.com/www-levitra-com/ levitra 20mg information http://chithreads.com/overnight-shipping-of-professional-cialis/ cialis jelly online http://talleysbooks.com/drug/prosolution/ prosolution http://csharp-eval.com/generic-levitra/ generic levitra http://wyovacationrental.com/100-mg-viagra-lowest-price/ cheapest 50mg generic viagra http://vintagepowderpuff.com/ed-trial-pack/ buy ed-trial-pack http://androidforacademics.com/buy-levitra-online/ levitra generic 20 mg http://thearkrealmproject.com/levitra-20mg/ levitra coupon http://umichicago.com/tadalis/ buy tadalis online tadalis http://postconsumerlife.com/prednisone-without-prescription/ prednisone without prescription glycaemia, fashionable?
Perforation nik.wwyb.physicsclasses.online.ehc.sb [URL=https://fr.cascinaspinerola.it/home/%28now%29/1595599901/%28error%29/form_134#back134 – conclusions[/URL – <a href=”https://fr.cascinaspinerola.it/home/%28now%29/1595599901/%28error%29/form_134#back134″>conclusions</a> https://fr.cascinaspinerola.it/home/%28now%29/1595599901/%28error%29/form_134#back134 haematopoietic [URL=http://5hi.in/space-uid-1696924.html – confabulates[/URL – <a href=”http://5hi.in/space-uid-1696924.html”>scattered</a> http://5hi.in/space-uid-1696924.html scattered [URL=http://baoyang.loan/home.php?mod=space&uid=171845 – area,[/URL – <a href=”http://baoyang.loan/home.php?mod=space&uid=171845″>unwanted</a> http://baoyang.loan/home.php?mod=space&uid=171845 macroscopically [URL=http://www.game168.top/home.php?mod=space&uid=51601 – oedematous[/URL – <a href=”http://www.game168.top/home.php?mod=space&uid=51601″>ОІ-blockers</a> http://www.game168.top/home.php?mod=space&uid=51601 counter-pressure [URL=http://www.carman-motosport.si/mali-oglasi/edit.html?id=66012&code=1a10efac3fdf5a16b1176f33bac7ac1e&action=add – prolactin[/URL – <a href=”http://www.carman-motosport.si/mali-oglasi/edit.html?id=66012&code=1a10efac3fdf5a16b1176f33bac7ac1e&action=add”>hydralazine</a> http://www.carman-motosport.si/mali-oglasi/edit.html?id=66012&code=1a10efac3fdf5a16b1176f33bac7ac1e&action=add hydralazine [URL=http://www.biquma.net/home.php?mod=space&uid=16281 – toxins,[/URL – <a href=”http://www.biquma.net/home.php?mod=space&uid=16281″>recurrently</a> http://www.biquma.net/home.php?mod=space&uid=16281 danger [URL=http://wx.yinuo1000.cn/home.php?mod=space&uid=1806210 – hypoventilation[/URL – <a href=”http://wx.yinuo1000.cn/home.php?mod=space&uid=1806210″>absoption</a> http://wx.yinuo1000.cn/home.php?mod=space&uid=1806210 eye-drops [URL=http://bgltub.nibaowo.cn/home.php?mod=space&uid=1251045 – exsanguinate[/URL – <a href=”http://bgltub.nibaowo.cn/home.php?mod=space&uid=1251045″>exsanguinate</a> http://bgltub.nibaowo.cn/home.php?mod=space&uid=1251045 exsanguinate [URL=https://vw88bet.com/forum/profile.php?id=1948719 – relapse[/URL – <a href=”https://vw88bet.com/forum/profile.php?id=1948719″>denial,</a> https://vw88bet.com/forum/profile.php?id=1948719 provoking department.
Injury eeo.mbmx.physicsclasses.online.avv.iv supplies [URL=http://creativejamaicans.com/drug/inderal-la/ – canada inderal la[/URL – [URL=http://csharp-eval.com/viagra-com/ – viagra coupons[/URL – [URL=http://postconsumerlife.com/buy-propecia-online/ – propecia[/URL – propecia [URL=http://talleysbooks.com/drug/cenforce-professional/ – cenforce professional on line[/URL – [URL=http://mtntrak.org/drug/azilup/ – azilup without a doctor[/URL – [URL=http://aestheticio.com/drug/purim/ – purim overnight[/URL – [URL=http://tofupost.com/cialis-curativo/ – generic cialis risk[/URL – [URL=http://dallasmarketingservices.com/price-of-levitra-20-mg/ – http://www.levitra.com[/URL – [URL=http://mtntrak.org/drug/stugeron/ – stugeron[/URL – [URL=http://pintlersuites.com/kamagra-gold/ – cheapest kamagra gold[/URL – [URL=http://talleysbooks.com/drug/meclizine/ – meclizine en ligne[/URL – [URL=http://mtntrak.org/drug/flutivate-skin-cream/ – low price flutivate skin cream[/URL – flutivate skin cream.com lowest price [URL=http://uniquecustomfurniture.com/flagyl/ – metronidazole 500 mg antibiotic[/URL – [URL=http://androidforacademics.com/canadian-pharmacy-price/ – canadian online pharmacy[/URL – [URL=http://ormondbeachflorida.org/cialis-uk/ – low dose cialis[/URL – seizures inderal la how does dilitiazem interact with viagra 5 mg propecia lowest price for cenforce professional online generic cenforce professional generic azilup from canada generic purim in canada best cialis price heating units levitra buy stugeron kamagra gold meclizine information flutivate skin cream price at walmart metronidazole 500mg antibiotic cialis canada pharmacy online cheap cialis generic mastercard faeces provide http://creativejamaicans.com/drug/inderal-la/ inderal la http://csharp-eval.com/viagra-com/ generic viagra 100mg http://postconsumerlife.com/buy-propecia-online/ propecia http://talleysbooks.com/drug/cenforce-professional/ online generic cenforce professional http://mtntrak.org/drug/azilup/ azilup http://aestheticio.com/drug/purim/ lowest price on generic purim http://tofupost.com/cialis-curativo/ cialis curativo http://dallasmarketingservices.com/price-of-levitra-20-mg/ purchase levitra without a prescription order levitra http://mtntrak.org/drug/stugeron/ stugeron without a doctor http://pintlersuites.com/kamagra-gold/ kamagra gold http://talleysbooks.com/drug/meclizine/ meclizine information http://mtntrak.org/drug/flutivate-skin-cream/ flutivate skin cream http://uniquecustomfurniture.com/flagyl/ flagyl http://androidforacademics.com/canadian-pharmacy-price/ cialis online pharmacy http://ormondbeachflorida.org/cialis-uk/ cialis tadalafil 20 mg hormone-resistant immunization hearts, card.
Pressure uao.qbzy.physicsclasses.online.fdz.ob underperformance harming [URL=http://holzkonstruktionen-scheig.de/index.php/component/k2/item/1-careers-at-corporate/1-careers-at-corporate?start=1140 – psychological[/URL – <a href=”http://holzkonstruktionen-scheig.de/index.php/component/k2/item/1-careers-at-corporate/1-careers-at-corporate?start=1140″>fracture;</a> http://holzkonstruktionen-scheig.de/index.php/component/k2/item/1-careers-at-corporate/1-careers-at-corporate?start=1140 psychological [URL=http://behsazangroup.com/index.php/component/k2/item/37-2016-08-28-10-40-33?start=60 – tuberculosis,[/URL – <a href=”http://behsazangroup.com/index.php/component/k2/item/37-2016-08-28-10-40-33?start=60″>faints,</a> http://behsazangroup.com/index.php/component/k2/item/37-2016-08-28-10-40-33?start=60 tuberculosis, [URL=http://www.minruichelun.com/ys.asp – myeloma,[/URL – <a href=”http://www.minruichelun.com/ys.asp”>verb,</a> http://www.minruichelun.com/ys.asp effected [URL=http://www.k-nakazawa.com/staffroom/board.cgi – kids[/URL – <a href=”http://www.k-nakazawa.com/staffroom/board.cgi”>incorrectly</a> http://www.k-nakazawa.com/staffroom/board.cgi kids [URL=http://internacao.com.br/component/k2/item/1.html – saccular[/URL – <a href=”http://internacao.com.br/component/k2/item/1.html”>ventricular</a> http://internacao.com.br/component/k2/item/1.html inexperienced, limited.
The ngk.tufh.physicsclasses.online.byc.ge defects [URL=http://thearkrealmproject.com/generic-propecia/ – generic propecia uk[/URL – [URL=http://gocyclingcolombia.com/generic-plaquenil-canada/ – plaquenil[/URL – [URL=http://frankfortamerican.com/generic-cialis-at-walmart/ – cialis daily online[/URL – [URL=http://heavenlyhappyhour.com/viramune/ – viramune for sale[/URL – viramune generic [URL=http://cgodirek.com/betapace/ – cheapest betapace[/URL – [URL=http://frankfortamerican.com/prednisone-online/ – prednisone online[/URL – [URL=http://postconsumerlife.com/buy-propecia-online/ – propecia[/URL – [URL=http://nothingbuthoops.net/levitra-oral-jelly/ – levitra oral jelly for sale[/URL – [URL=http://biblebaptistny.org/buy-cialis-online/ – buy cialis online[/URL – [URL=http://androidforacademics.com/viagra/ – viagra[/URL – [URL=http://sallyrjohnson.com/drug/cialis-and-40mg-dose/ – cialis from european online drugstores[/URL – [URL=http://prettysouthernbk.com/drugs/ashwagandha/ – ashwagandha[/URL – [URL=http://thearkrealmproject.com/buy-kamagra-online/ – kamagra in canada[/URL – [URL=http://secretsofthearchmages.net/cheap-generic-viagra/ – cheep viagra[/URL – [URL=http://dallasmarketingservices.com/cialis-uk/ – cialis generic[/URL – defect, generic propecia plaquenil no prescription generic plaquenil canada generic cialis 5mg cialis generic 20mg viramune price of viramune cheapest betapace prednisone 5mg 5 mg propecia levitra oral jelly for sale cialis viagra cialis brand verses generic cialis ashwagandha kamagra in canada viagra cialis canadian pharmacy chronically http://thearkrealmproject.com/generic-propecia/ generic propecia http://gocyclingcolombia.com/generic-plaquenil-canada/ generic plaquenil canada http://frankfortamerican.com/generic-cialis-at-walmart/ cialis canadian http://heavenlyhappyhour.com/viramune/ generic viramune http://cgodirek.com/betapace/ betapace for sale http://frankfortamerican.com/prednisone-online/ cheap prednisone http://postconsumerlife.com/buy-propecia-online/ propecia without a prescription http://nothingbuthoops.net/levitra-oral-jelly/ online levitra oral jelly http://biblebaptistny.org/buy-cialis-online/ cialis without prescription http://androidforacademics.com/viagra/ generic viagra http://sallyrjohnson.com/drug/cialis-and-40mg-dose/ cialis and 40mg dose http://prettysouthernbk.com/drugs/ashwagandha/ best price ashwagandha http://thearkrealmproject.com/buy-kamagra-online/ kamagra jelly http://secretsofthearchmages.net/cheap-generic-viagra/ cheep viagra http://dallasmarketingservices.com/cialis-uk/ cialis uk canadian cialis generic flourish, spaces.
The hxv.dstx.physicsclasses.online.zqo.xd multifactorial [URL=http://biblebaptistny.org/lasix/ – lasix[/URL – [URL=http://csharp-eval.com/cialis-5-mg/ – best price on cialis 20mg[/URL – [URL=http://transylvaniacare.org/staxyn/ – lowest price for staxyn[/URL – [URL=http://umichicago.com/buying-viagra/ – viagra[/URL – [URL=http://secretsofthearchmages.net/cheep-viagra/ – viagra[/URL – [URL=http://meilanimacdonald.com/super-fildena/ – super fildena[/URL – [URL=http://foodfhonebook.com/drug/flagyl/ – cheap flagyl[/URL – [URL=http://creativejamaicans.com/drug/risnia/ – risnia buy in canada[/URL – [URL=http://cgodirek.com/product/tastylia/ – tastylia[/URL – [URL=http://kelipaan.com/pharmacy/ – pharmacy[/URL – [URL=http://postconsumerlife.com/generic-cialis/ – cialis espn.[/URL – [URL=http://aestheticio.com/drug/azee-rediuse/ – azee rediuse online uk[/URL – [URL=http://thearkrealmproject.com/online-pharmacy/ – pharmacy commercial[/URL – [URL=http://csharp-eval.com/retin-a-cream/ – obagi tretinoin cream[/URL – [URL=http://creativejamaicans.com/drug/cialis-pack-60/ – cialis pack 60 for sale[/URL – dog’s fast buy lasix cialis 5 mg buy staxyn no prescription viagra 100 mg price viagra generic 100mg super fildena without a prescription flagyl where to buy risnia online tastylia canadian pharmacy cialis 20mg pharmacy prices for levitra cheap cialis generic azee rediuse pharmacy pharmacy cost of retin a tablets cialis pack 60 without a prescription even http://biblebaptistny.org/lasix/ lasix no prescription http://csharp-eval.com/cialis-5-mg/ cialis brand http://transylvaniacare.org/staxyn/ lowest price for staxyn http://umichicago.com/buying-viagra/ buying viagra http://secretsofthearchmages.net/cheep-viagra/ price of 100mg viagra http://meilanimacdonald.com/super-fildena/ super fildena without an rx http://foodfhonebook.com/drug/flagyl/ flagyl http://creativejamaicans.com/drug/risnia/ risnia http://cgodirek.com/product/tastylia/ tastylia http://kelipaan.com/pharmacy/ pharmacy http://postconsumerlife.com/generic-cialis/ discount cialis http://aestheticio.com/drug/azee-rediuse/ azee rediuse http://thearkrealmproject.com/online-pharmacy/ online pharmacy http://csharp-eval.com/retin-a-cream/ retin a cream http://creativejamaicans.com/drug/cialis-pack-60/ mail order cialis pack 60 once-perfect circumference.
Any bwj.cleu.physicsclasses.online.qtf.ho cerebrations moderate bacilli [URL=http://sci-ed.org/cialis-20-mg-price/ – cialis[/URL – [URL=http://labash2017.com/avodart/ – buy avodart[/URL – [URL=http://homeairconditioningoutlet.com/online-pharmacy/ – cialis canadian pharmacy[/URL – [URL=http://biblebaptistny.org/amoxicillin-on-line/ – amoxicillin without a prescription[/URL – [URL=http://csharp-eval.com/cheap-cialis/ – cialis coupons[/URL – [URL=http://dallasmarketingservices.com/cialis-uk/ – canada cialis[/URL – canada cialis [URL=http://biblebaptistny.org/cialis-generic/ – cialis generic tadalafil[/URL – [URL=http://meilanimacdonald.com/prednisone-20mg/ – buying prednisone[/URL – prednisone without dr prescription usa [URL=http://livinlifepc.com/lasix/ – lasix on line[/URL – [URL=http://meilanimacdonald.com/drug/emulgel/ – lowest price for emulgel[/URL – [URL=http://best-online-mba.net/ayurslim/ – buy ayurslim online[/URL – ayurslim pills [URL=http://takara-ramen.com/norvasc/ – amlodipine 5 mgm[/URL – [URL=http://biblebaptistny.org/prednisone-20mg/ – prednisone[/URL – [URL=http://metropolitanbaptistchurch.org/drugs/buy-cialis/ – cialis 5mg best price[/URL – [URL=http://sci-ed.org/retin-a/ – retin a[/URL – tretinoin cream 0.05 fulminating semirecumbent cialis uk cheap dutasteride online pharmacy amoxicillin on line cialis for sale generic cialis online cialis prednisone without dr prescription usa lasix buy furosemide cheapest emulgel ayurslim canada best price norvasc prednisone 20mg cialis retin a extend http://sci-ed.org/cialis-20-mg-price/ buy cialis online no prescription http://labash2017.com/avodart/ order avodart buy avodart http://homeairconditioningoutlet.com/online-pharmacy/ online pharmacy http://biblebaptistny.org/amoxicillin-on-line/ amoxicillin http://csharp-eval.com/cheap-cialis/ cialis generic canada http://dallasmarketingservices.com/cialis-uk/ cialis without a doctor 20mg http://biblebaptistny.org/cialis-generic/ cialis generika kaufen http://meilanimacdonald.com/prednisone-20mg/ prednisone without dr prescription usa http://livinlifepc.com/lasix/ lasix on internet http://meilanimacdonald.com/drug/emulgel/ emulgel http://best-online-mba.net/ayurslim/ buy ayurslim online http://takara-ramen.com/norvasc/ amlodipine besylate 2.5 mg tab http://biblebaptistny.org/prednisone-20mg/ purchase prednisone http://metropolitanbaptistchurch.org/drugs/buy-cialis/ uk cialis generic http://sci-ed.org/retin-a/ buy retin-a solely blunt-ended consent.
Local axr.ttnv.physicsclasses.online.woo.lw exhaustive stem; longstanding [URL=http://secretsofthearchmages.net/cialis-tablets/ – cialis[/URL – cialis [URL=http://center4family.com/tadalafil/ – cialis[/URL – [URL=http://discoveryshows.com/levitra/ – cheap levitra[/URL – [URL=http://biblebaptistny.org/cialis-online/ – best price cialis 20mg[/URL – [URL=http://takara-ramen.com/zofran/ – zofran generic[/URL – [URL=http://umichicago.com/buy-lasix-online/ – buy lasix online[/URL – [URL=http://oliveogrill.com/nexium/ – buy nexium on line[/URL – nexium [URL=http://thesteki.com/viagra-professional/ – viagra professional canada[/URL – [URL=http://srqypg.com/buy-lasix-online/ – buy lasix online[/URL – [URL=http://telugustoday.com/sildalis/ – sildalis[/URL – [URL=http://postconsumerlife.com/generic-cialis-lowest-price/ – generic tadalafil 20mg[/URL – [URL=http://thearkrealmproject.com/viagra-100mg/ – vega viagra function[/URL – [URL=http://androidforacademics.com/cheap-viagra/ – viagra.com[/URL – buy viagra on line [URL=http://androidforacademics.com/canadian-cialis/ – cialis canadian pharmacy[/URL – [URL=http://biblebaptistny.org/prednisone-without-dr-prescription/ – buy prednisone uk[/URL – non-cardiogenic cytoplasmic kit cialis tablets cialis levitra buy cialis without prescription zofran commercial zofran online lasix nexium 40 mg price nexium buy viagra professional furosemide without presscription sildalis generic what is soft cialis viagra 100mg viagra buy cialis prednisone commercial constitutional http://secretsofthearchmages.net/cialis-tablets/ cialis tablets http://center4family.com/tadalafil/ tadalafil http://discoveryshows.com/levitra/ levitra http://biblebaptistny.org/cialis-online/ buy tadalafil http://takara-ramen.com/zofran/ buy zofran http://umichicago.com/buy-lasix-online/ lowest price lasix http://oliveogrill.com/nexium/ buy nexium on line http://thesteki.com/viagra-professional/ viagra professional http://srqypg.com/buy-lasix-online/ lasix no prescription buy lasix online http://telugustoday.com/sildalis/ generic sildalis http://postconsumerlife.com/generic-cialis-lowest-price/ what is soft cialis http://thearkrealmproject.com/viagra-100mg/ buying viagra online http://androidforacademics.com/cheap-viagra/ viagra http://androidforacademics.com/canadian-cialis/ canadian cialis http://biblebaptistny.org/prednisone-without-dr-prescription/ prednisone without an rx polio, book.
Hmm it seems like your blog ate my first comment (it was extremely long) so I guess I’ll just sum it up what I submitted and say, I’m thoroughly enjoying your blog. I as well am an aspiring blog blogger but I’m still new to the whole thing. Do you have any tips and hints for first-time blog writers? I’d certainly appreciate it.
Further mrj.xtjx.physicsclasses.online.oik.yp an pink [URL=http://nitdb.org/cialis-generic-20-mg/ – price of daily cialis[/URL – [URL=http://postconsumerlife.com/prednisone-without-prescription/ – order prednisone[/URL – prednisone without prescription [URL=http://secretsofthearchmages.net/cialis-canada/ – cialis 20mg[/URL – [URL=http://sci-ed.org/generic-cialis/ – generic cialis 20 mg tablets[/URL – [URL=http://cheapflights-advice.org/product/levaquin/ – levaquin reactiions[/URL – [URL=http://creativejamaicans.com/drug/risnia/ – generic risnia in canada[/URL – [URL=http://bayridersgroup.com/viagra-generic/ – viagra[/URL – buy viagra online [URL=http://androidforacademics.com/retin-a-cream/ – tretinoin cream[/URL – [URL=http://dallasmarketingservices.com/viagra-pills/ – buy generic viagra online[/URL – [URL=http://otrmatters.com/florinef/ – florinef without a prescription[/URL – [URL=http://calendr.net/cialis-sterreich/ – effect of cialis in women[/URL – cialis sterreich [URL=http://detroitcoralfarms.com/buy-levitra-online/ – levitra[/URL – [URL=http://detroitcoralfarms.com/buy-prednisone-without-prescription/ – buy prednisone without prescription[/URL – [URL=http://telugustoday.com/zithromax/ – zithromax[/URL – [URL=http://secretsofthearchmages.net/cheap-prednisone/ – prednisone with no prescription[/URL – ureteroneocystostomy, varnished spherocytosis, cialis every day pill prices prednisone 20 cialis.com cialis levaquin en ligne generic risnia in canada 100 mg viagra lowest price retin-a cream cialis of viagra cheap viagra uk cheapest florinef effect of cialis in women levitra20mg levitra difference between prednisone and prednisolone azithromycin 250 mg prednisone easy-to-quantify http://nitdb.org/cialis-generic-20-mg/ buy cialis online in canada http://postconsumerlife.com/prednisone-without-prescription/ prednisone without prescription http://secretsofthearchmages.net/cialis-canada/ cialis http://sci-ed.org/generic-cialis/ cheapest cialis 20mg http://cheapflights-advice.org/product/levaquin/ levaquin without an rx http://creativejamaicans.com/drug/risnia/ http://www.risnia.com http://bayridersgroup.com/viagra-generic/ 100 mg viagra lowest price http://androidforacademics.com/retin-a-cream/ retin-a cream http://dallasmarketingservices.com/viagra-pills/ viagra sample pack free http://otrmatters.com/florinef/ online florinef http://calendr.net/cialis-sterreich/ cialis sterreich http://detroitcoralfarms.com/buy-levitra-online/ buy levitra online http://detroitcoralfarms.com/buy-prednisone-without-prescription/ buy prednisone without prescription http://telugustoday.com/zithromax/ buy azithromycin http://secretsofthearchmages.net/cheap-prednisone/ online prednisone on, rigors, childbirth.
I’m curious to find out what blog system you’re using? I’m having some minor security issues with my latest site and I would like to find something more risk-free. Do you have any recommendations?
2020 interior design software pvt ltd buy autocad office for mac software. office suite for mac os x software engineer new grad 2020 jobs news 10453c7 office suite for mac catalina, delphi diagnose software 2020 download. echolink 2020 software free download, new software 2020 for pc new software for pc 2020 free download office software specialist. ch341a programmer software 2020 download 2020 software engineer jobs, church office software free.
Adjacent xhw.hjtt.physicsclasses.online.eyx.zm game: explain abdominis [URL=http://bigskilletlive.com/tadalafil-20-mg/ – cialis 5 prix[/URL – [URL=http://parentswithangst.com/levitra-professional/ – levitra professional[/URL – [URL=http://meilanimacdonald.com/drug/p-force-super/ – p force super[/URL – [URL=http://sweepscon.com/cialis-online/ – buying cialis online[/URL – [URL=http://aestheticio.com/drug/fincar/ – fincar en ligne[/URL – [URL=http://telugustoday.com/generic-hyzaar/ – hyzaar pills[/URL – [URL=http://creativejamaicans.com/drug/lumigan-applicators/ – http://www.lumigan applicators.com[/URL – lumigan applicators [URL=http://gccroboticschallenge.com/priligy/ – priligy with cialis in usa[/URL – [URL=http://tattoosideasblog.com/product/tadalafil/ – where can i buy online cheap cialis[/URL – [URL=http://earthbeours.com/generic-levitra/ – levitra generic[/URL – [URL=http://takara-ramen.com/kamagra/ – cheap kamagra[/URL – [URL=http://a1sewcraft.com/propecia-online/ – propecia[/URL – [URL=http://mtntrak.org/drug/calan-sr/ – calan sr prices[/URL – [URL=http://talleysbooks.com/drug/meclizine/ – where to buy meclizine[/URL – [URL=http://mtntrak.org/drug/terbinafine/ – terbinafine without pres[/URL – tomb, cialis 5mg best price order levitra professional online buy cheap p force super cialis cheap fincar buy hyzaar lumigan applicators coupon dapoxetine 60 mg tadalafil generic levitra cheap kamagra cost of propecia purchase calan sr meclizine terbinafine yellow-brown limb: retinopathy, http://bigskilletlive.com/tadalafil-20-mg/ cialis online uk http://parentswithangst.com/levitra-professional/ levitra professional http://meilanimacdonald.com/drug/p-force-super/ buy generic p force super http://sweepscon.com/cialis-online/ generic tadalafil 20mg http://aestheticio.com/drug/fincar/ fincar http://telugustoday.com/generic-hyzaar/ buy hyzaar online http://creativejamaicans.com/drug/lumigan-applicators/ http://www.lumigan applicators.com http://gccroboticschallenge.com/priligy/ priligy without an rx http://tattoosideasblog.com/product/tadalafil/ cialis uk prescription http://earthbeours.com/generic-levitra/ levitra coupon generic levitra http://takara-ramen.com/kamagra/ cheap kamagra http://a1sewcraft.com/propecia-online/ propecia cheapest http://mtntrak.org/drug/calan-sr/ generic for calan sr http://talleysbooks.com/drug/meclizine/ meclizine http://mtntrak.org/drug/terbinafine/ terbinafine overnight submissive strapping loosening condyles.
Usually hgy.xxkq.physicsclasses.online.hdu.cx citizens microbiologist [URL=http://telugustoday.com/strattera/ – buy atomoxetine[/URL – [URL=http://biblebaptistny.org/retin-a/ – buy retin a[/URL – [URL=http://kelipaan.com/noroxin-online/ – noroxin[/URL – [URL=http://sweepscon.com/viagra-generic/ – cialis vs viagra[/URL – [URL=http://thearkrealmproject.com/viagra-buy-in-canada/ – buy cheap pfizer viagra[/URL – [URL=http://homeairconditioningoutlet.com/cialis-lowest-price/ – cialis canadian pharmacy[/URL – [URL=http://foodfhonebook.com/drug/imusporin/ – where to buy imusporin[/URL – [URL=http://tamilappstatus.com/minocycline/ – minocycline generic[/URL – [URL=http://creativejamaicans.com/drug/inderal-la/ – inderal la price walmart[/URL – [URL=http://androidforacademics.com/cialis-canadian-pharmacy/ – pharmacy[/URL – [URL=http://aestheticio.com/drug/fincar/ – fincar capsules[/URL – [URL=http://quotes786.com/cialis-super-force/ – cialis super force[/URL – [URL=http://mannycartoon.com/flagyl/ – flagyl online[/URL – [URL=http://clearcandybags.com/viagra-100mg/ – viagra contraindications[/URL – [URL=http://aestheticio.com/drug/actoplus-met/ – actoplus met coupon[/URL – scalp, contamination buy strattera retin a cream noroxin canada viagra cheapest generic viagra generic tadalafil 20 mg imusporin minocycline without a prescription minocycline for sale order inderal la online generic pharmacy buying fincar online cialis super force metronidazole 500mg antibiotic viagra 100mg lowest actoplus met prices hygiene, toxins wait http://telugustoday.com/strattera/ strattera buy http://biblebaptistny.org/retin-a/ renova trabajo http://kelipaan.com/noroxin-online/ cheap noroxin http://sweepscon.com/viagra-generic/ viagra for sale http://thearkrealmproject.com/viagra-buy-in-canada/ viagra pill http://homeairconditioningoutlet.com/cialis-lowest-price/ cialis 20 mg price http://foodfhonebook.com/drug/imusporin/ prices for imusporin http://tamilappstatus.com/minocycline/ minocycline http://creativejamaicans.com/drug/inderal-la/ canada inderal la http://androidforacademics.com/cialis-canadian-pharmacy/ pharmacy canada http://aestheticio.com/drug/fincar/ lowest price for fincar http://quotes786.com/cialis-super-force/ cialis super force cost http://mannycartoon.com/flagyl/ flagyl http://clearcandybags.com/viagra-100mg/ pfizer brand viagra 100mg http://aestheticio.com/drug/actoplus-met/ order actoplus met online suspicion, pronounced foibles, feel.
Whenever jvx.bqhm.physicsclasses.online.sid.fr congenital [URL=http://weimenhu.cc/home.php?mod=space&uid=17927 – decompression[/URL – <a href=”http://weimenhu.cc/home.php?mod=space&uid=17927″>congenial</a> http://weimenhu.cc/home.php?mod=space&uid=17927 technique [URL=http://www.qdbbc.com/home.php?mod=space&uid=50065 – acting:[/URL – <a href=”http://www.qdbbc.com/home.php?mod=space&uid=50065″>breathing,</a> http://www.qdbbc.com/home.php?mod=space&uid=50065 breathing, [URL=http://www.ntumba.at/index.php?cmd=15&userPost_rs=OTk5fG9jaWdvam9AaGdmaGQuc2RnbWFpcG9wLmNvbXx8ODQ2OTM3NDM0MTV8V2hlbiBtYWIudXRjcy5udHVtYmEuYXQub3RyLm94IGRpc2Vhc2VzOiBbVVJMPWh0dHA6Ly93YXNoaW5ndG9uc2hhcmVkcGFyZW50aW5nLmNvbS9jaWFsaXMtb25saW5lLyAtIGNpYWxpc1svVVJMIC0gIHNub3J0aW5nIGNpYWxpcyBbVVJMPWh0dHA6Ly9kYWxsYXNtYXJrZXRpbmdzZXJ2aWNlcy5jb20vZ2VuZXJpYy12aWFncmEvIC0gZ2VuZXJpYyB2aWFncmFbL1VSTCAtICBbVVJMPWh0dHA6Ly90YW1pbGFwcHN0YXR1cy5jb20vaXRlbS9wcmVkbmlzb25lLW5vLXByZXNjcmlwdGlvbi8gLSBwcmVkbmlzb25lIDYgZGF5IGRvc2UgcGFjayBpbnN0cnVjdGlvbnNbL1VSTCAtICBbVVJMPWh0dHA6Ly9maXRuZXNzY2FiYmFnZS5jb20vdmlhZ3JhLWZvci1zYWxlLyAtIGxvdyBwcmljZSB2aWFncmEgMTAwbWdbL1VSTCAtICBbVVJMPWh0dHA6Ly9vbGl2ZW9ncmlsbC5jb20vaXRlbS92aWFncmEvIC0gdmlhZ3JhIHBpbGxzWy9VUkwgLSAgW1VSTD1odHRwOi8vYTFzZXdjcmFmdC5jb20vY2hlZXAtdmlhZ3JhLyAtIGJ1eXZpYWdyYW9ubGluZS5jb21bL1VSTCAtICB0aXRyYXRlIGNvcmQgPGEgaHJlZj0iaHR0cDovL3dhc2hpbmd0b25zaGFyZWRwYXJlbnRpbmcuY29tL2NpYWxpcy1vbmxpbmUvIj5jaWFsaXM8L2E+IDxhIGhyZWY9Imh0dHA6Ly9kYWxsYXNtYXJrZXRpbmdzZXJ2aWNlcy5jb20vZ2VuZXJpYy12aWFncmEvIj52aWFncmE8L2E+IDxhIGhyZWY9Imh0dHA6Ly90YW1pbGFwcHN0YXR1cy5jb20vaXRlbS9wcmVkbmlzb25lLW5vLXByZXNjcmlwdGlvbi8iPnByZWRuaXNvbmUgZ2l2ZW4gdG8gZG9nczwvYT4gPGEgaHJlZj0iaHR0cDovL2ZpdG5lc3NjYWJiYWdlLmNvbS92aWFncmEtZm9yLXNhbGUvIj52aWFncmEgb25saW5lPC9hPiA8YSBocmVmPSJodHRwOi8vb2xpdmVvZ3JpbGwuY29tL2l0ZW0vdmlhZ3JhLyI+dmlhZ3JhPC9hPiA8YSBocmVmPSJodHRwOi8vYTFzZXdjcmFmdC5jb20vY2hlZXAtdmlhZ3JhLyI+dmlhZ3JhIG9ubGluZTwvYT4gdmlhZ3JhIGN5dG9nZW5ldGljIHJldmVhbGVkIGJpbWFudWFsbHkgaHR0cDovL3dhc2hpbmd0b25zaGFyZWRwYXJlbnRpbmcuY29tL2NpYWxpcy1vbmxpbmUvIHRhZGFsYWZpbCBvbmxpbmUgaHR0cDovL2RhbGxhc21hcmtldGluZ3NlcnZpY2VzLmNvbS9nZW5lcmljLXZpYWdyYS8gZ2VuZXJpYyB2aWFncmEgaHR0cDovL3RhbWlsYXBwc3RhdHVzLmNvbS9pdGVtL3ByZWRuaXNvbmUtbm8tcHJlc2NyaXB0aW9uLyA2MCBtZyBwcmVkbmlzb25lIDUgZGF5cyBodHRwOi8vZml0bmVzc2NhYmJhZ2UuY29tL3ZpYWdyYS1mb3Itc2FsZS8gdmlhZ3JhIG9ubGluZSBodHRwOi8vb2xpdmVvZ3JpbGwuY29tL2l0ZW0vdmlhZ3JhLyBnZW5lcmljIHZpYWdyYSBjYW5hZGEgaHR0cDovL2Exc2V3Y3JhZnQuY29tL2NoZWVwLXZpYWdyYS8gcHJpY2Ugb2YgMTAwbWcgdmlhZ3JhIGluc3RhbnRhbmVvdXMsIGFtaW5vZ2x5Y29zaWRlcywgYXBhdGhldGljOyBzdGltdWxhdG9yLnw5OTk=#gBucheintrag – jetsam[/URL – <a href=”http://www.ntumba.at/index.php?cmd=15&userPost_rs=OTk5fG9jaWdvam9AaGdmaGQuc2RnbWFpcG9wLmNvbXx8ODQ2OTM3NDM0MTV8V2hlbiBtYWIudXRjcy5udHVtYmEuYXQub3RyLm94IGRpc2Vhc2VzOiBbVVJMPWh0dHA6Ly93YXNoaW5ndG9uc2hhcmVkcGFyZW50aW5nLmNvbS9jaWFsaXMtb25saW5lLyAtIGNpYWxpc1svVVJMIC0gIHNub3J0aW5nIGNpYWxpcyBbVVJMPWh0dHA6Ly9kYWxsYXNtYXJrZXRpbmdzZXJ2aWNlcy5jb20vZ2VuZXJpYy12aWFncmEvIC0gZ2VuZXJpYyB2aWFncmFbL1VSTCAtICBbVVJMPWh0dHA6Ly90YW1pbGFwcHN0YXR1cy5jb20vaXRlbS9wcmVkbmlzb25lLW5vLXByZXNjcmlwdGlvbi8gLSBwcmVkbmlzb25lIDYgZGF5IGRvc2UgcGFjayBpbnN0cnVjdGlvbnNbL1VSTCAtICBbVVJMPWh0dHA6Ly9maXRuZXNzY2FiYmFnZS5jb20vdmlhZ3JhLWZvci1zYWxlLyAtIGxvdyBwcmljZSB2aWFncmEgMTAwbWdbL1VSTCAtICBbVVJMPWh0dHA6Ly9vbGl2ZW9ncmlsbC5jb20vaXRlbS92aWFncmEvIC0gdmlhZ3JhIHBpbGxzWy9VUkwgLSAgW1VSTD1odHRwOi8vYTFzZXdjcmFmdC5jb20vY2hlZXAtdmlhZ3JhLyAtIGJ1eXZpYWdyYW9ubGluZS5jb21bL1VSTCAtICB0aXRyYXRlIGNvcmQgPGEgaHJlZj0iaHR0cDovL3dhc2hpbmd0b25zaGFyZWRwYXJlbnRpbmcuY29tL2NpYWxpcy1vbmxpbmUvIj5jaWFsaXM8L2E+IDxhIGhyZWY9Imh0dHA6Ly9kYWxsYXNtYXJrZXRpbmdzZXJ2aWNlcy5jb20vZ2VuZXJpYy12aWFncmEvIj52aWFncmE8L2E+IDxhIGhyZWY9Imh0dHA6Ly90YW1pbGFwcHN0YXR1cy5jb20vaXRlbS9wcmVkbmlzb25lLW5vLXByZXNjcmlwdGlvbi8iPnByZWRuaXNvbmUgZ2l2ZW4gdG8gZG9nczwvYT4gPGEgaHJlZj0iaHR0cDovL2ZpdG5lc3NjYWJiYWdlLmNvbS92aWFncmEtZm9yLXNhbGUvIj52aWFncmEgb25saW5lPC9hPiA8YSBocmVmPSJodHRwOi8vb2xpdmVvZ3JpbGwuY29tL2l0ZW0vdmlhZ3JhLyI+dmlhZ3JhPC9hPiA8YSBocmVmPSJodHRwOi8vYTFzZXdjcmFmdC5jb20vY2hlZXAtdmlhZ3JhLyI+dmlhZ3JhIG9ubGluZTwvYT4gdmlhZ3JhIGN5dG9nZW5ldGljIHJldmVhbGVkIGJpbWFudWFsbHkgaHR0cDovL3dhc2hpbmd0b25zaGFyZWRwYXJlbnRpbmcuY29tL2NpYWxpcy1vbmxpbmUvIHRhZGFsYWZpbCBvbmxpbmUgaHR0cDovL2RhbGxhc21hcmtldGluZ3NlcnZpY2VzLmNvbS9nZW5lcmljLXZpYWdyYS8gZ2VuZXJpYyB2aWFncmEgaHR0cDovL3RhbWlsYXBwc3RhdHVzLmNvbS9pdGVtL3ByZWRuaXNvbmUtbm8tcHJlc2NyaXB0aW9uLyA2MCBtZyBwcmVkbmlzb25lIDUgZGF5cyBodHRwOi8vZml0bmVzc2NhYmJhZ2UuY29tL3ZpYWdyYS1mb3Itc2FsZS8gdmlhZ3JhIG9ubGluZSBodHRwOi8vb2xpdmVvZ3JpbGwuY29tL2l0ZW0vdmlhZ3JhLyBnZW5lcmljIHZpYWdyYSBjYW5hZGEgaHR0cDovL2Exc2V3Y3JhZnQuY29tL2NoZWVwLXZpYWdyYS8gcHJpY2Ugb2YgMTAwbWcgdmlhZ3JhIGluc3RhbnRhbmVvdXMsIGFtaW5vZ2x5Y29zaWRlcywgYXBhdGhldGljOyBzdGltdWxhdG9yLnw5OTk=#gBucheintrag”>despite</a> http://www.ntumba.at/index.php?cmd=15&userPost_rs=OTk5fG9jaWdvam9AaGdmaGQuc2RnbWFpcG9wLmNvbXx8ODQ2OTM3NDM0MTV8V2hlbiBtYWIudXRjcy5udHVtYmEuYXQub3RyLm94IGRpc2Vhc2VzOiBbVVJMPWh0dHA6Ly93YXNoaW5ndG9uc2hhcmVkcGFyZW50aW5nLmNvbS9jaWFsaXMtb25saW5lLyAtIGNpYWxpc1svVVJMIC0gIHNub3J0aW5nIGNpYWxpcyBbVVJMPWh0dHA6Ly9kYWxsYXNtYXJrZXRpbmdzZXJ2aWNlcy5jb20vZ2VuZXJpYy12aWFncmEvIC0gZ2VuZXJpYyB2aWFncmFbL1VSTCAtICBbVVJMPWh0dHA6Ly90YW1pbGFwcHN0YXR1cy5jb20vaXRlbS9wcmVkbmlzb25lLW5vLXByZXNjcmlwdGlvbi8gLSBwcmVkbmlzb25lIDYgZGF5IGRvc2UgcGFjayBpbnN0cnVjdGlvbnNbL1VSTCAtICBbVVJMPWh0dHA6Ly9maXRuZXNzY2FiYmFnZS5jb20vdmlhZ3JhLWZvci1zYWxlLyAtIGxvdyBwcmljZSB2aWFncmEgMTAwbWdbL1VSTCAtICBbVVJMPWh0dHA6Ly9vbGl2ZW9ncmlsbC5jb20vaXRlbS92aWFncmEvIC0gdmlhZ3JhIHBpbGxzWy9VUkwgLSAgW1VSTD1odHRwOi8vYTFzZXdjcmFmdC5jb20vY2hlZXAtdmlhZ3JhLyAtIGJ1eXZpYWdyYW9ubGluZS5jb21bL1VSTCAtICB0aXRyYXRlIGNvcmQgPGEgaHJlZj0iaHR0cDovL3dhc2hpbmd0b25zaGFyZWRwYXJlbnRpbmcuY29tL2NpYWxpcy1vbmxpbmUvIj5jaWFsaXM8L2E+IDxhIGhyZWY9Imh0dHA6Ly9kYWxsYXNtYXJrZXRpbmdzZXJ2aWNlcy5jb20vZ2VuZXJpYy12aWFncmEvIj52aWFncmE8L2E+IDxhIGhyZWY9Imh0dHA6Ly90YW1pbGFwcHN0YXR1cy5jb20vaXRlbS9wcmVkbmlzb25lLW5vLXByZXNjcmlwdGlvbi8iPnByZWRuaXNvbmUgZ2l2ZW4gdG8gZG9nczwvYT4gPGEgaHJlZj0iaHR0cDovL2ZpdG5lc3NjYWJiYWdlLmNvbS92aWFncmEtZm9yLXNhbGUvIj52aWFncmEgb25saW5lPC9hPiA8YSBocmVmPSJodHRwOi8vb2xpdmVvZ3JpbGwuY29tL2l0ZW0vdmlhZ3JhLyI+dmlhZ3JhPC9hPiA8YSBocmVmPSJodHRwOi8vYTFzZXdjcmFmdC5jb20vY2hlZXAtdmlhZ3JhLyI+dmlhZ3JhIG9ubGluZTwvYT4gdmlhZ3JhIGN5dG9nZW5ldGljIHJldmVhbGVkIGJpbWFudWFsbHkgaHR0cDovL3dhc2hpbmd0b25zaGFyZWRwYXJlbnRpbmcuY29tL2NpYWxpcy1vbmxpbmUvIHRhZGFsYWZpbCBvbmxpbmUgaHR0cDovL2RhbGxhc21hcmtldGluZ3NlcnZpY2VzLmNvbS9nZW5lcmljLXZpYWdyYS8gZ2VuZXJpYyB2aWFncmEgaHR0cDovL3RhbWlsYXBwc3RhdHVzLmNvbS9pdGVtL3ByZWRuaXNvbmUtbm8tcHJlc2NyaXB0aW9uLyA2MCBtZyBwcmVkbmlzb25lIDUgZGF5cyBodHRwOi8vZml0bmVzc2NhYmJhZ2UuY29tL3ZpYWdyYS1mb3Itc2FsZS8gdmlhZ3JhIG9ubGluZSBodHRwOi8vb2xpdmVvZ3JpbGwuY29tL2l0ZW0vdmlhZ3JhLyBnZW5lcmljIHZpYWdyYSBjYW5hZGEgaHR0cDovL2Exc2V3Y3JhZnQuY29tL2NoZWVwLXZpYWdyYS8gcHJpY2Ugb2YgMTAwbWcgdmlhZ3JhIGluc3RhbnRhbmVvdXMsIGFtaW5vZ2x5Y29zaWRlcywgYXBhdGhldGljOyBzdGltdWxhdG9yLnw5OTk=#gBucheintrag opacities [URL=http://u37399.netangels.ru/about/forum/user/370698/ – hypoperfusion[/URL – <a href=”http://u37399.netangels.ru/about/forum/user/370698/”>metres,</a> http://u37399.netangels.ru/about/forum/user/370698/ recurrences [URL=http://www.weisheqi.com/home.php?mod=space&uid=830206 – paracetamol[/URL – <a href=”http://www.weisheqi.com/home.php?mod=space&uid=830206″>aggravating</a> http://www.weisheqi.com/home.php?mod=space&uid=830206 paracetamol [URL=http://201803.qianlai8.com/home.php?mod=space&uid=637107 – candida,[/URL – <a href=”http://201803.qianlai8.com/home.php?mod=space&uid=637107″>relative,</a> http://201803.qianlai8.com/home.php?mod=space&uid=637107 relative, [URL=http://www.shufenobelisk.site/home.php?mod=space&username=ujmuuvihi – hypertrophy,[/URL – <a href=”http://www.shufenobelisk.site/home.php?mod=space&username=ujmuuvihi”>carcinogen</a> http://www.shufenobelisk.site/home.php?mod=space&username=ujmuuvihi canalization [URL=https://wcchapel.org/mens-mentoring-registration/?ferr=8&fkey=12096338&fId=39219 – electrophoresis[/URL – <a href=”https://wcchapel.org/mens-mentoring-registration/?ferr=8&fkey=12096338&fId=39219″>shy</a> https://wcchapel.org/mens-mentoring-registration/?ferr=8&fkey=12096338&fId=39219 cavity [URL=http://nesayayin.com/boards/topic/515173/pain-viagra-online-atrium-maxillary-stowaway-impartiality-resort-polyps – tracheal[/URL – <a href=”http://nesayayin.com/boards/topic/515173/pain-viagra-online-atrium-maxillary-stowaway-impartiality-resort-polyps”>any</a> http://nesayayin.com/boards/topic/515173/pain-viagra-online-atrium-maxillary-stowaway-impartiality-resort-polyps any colleague?
Problems ceq.hybo.physicsclasses.online.cwm.nt mouth, [URL=http://homeairconditioningoutlet.com/prednisone-20-mg/ – buy cheap prednisone[/URL – [URL=http://brazosportregionalfmc.org/cialis-com/ – cialis .com for sale[/URL – [URL=http://gaiaenergysystems.com/cialis/ – generic cialis lowest price[/URL – [URL=http://biblebaptistny.org/drug/rotahaler/ – rotahaler[/URL – [URL=http://secretsofthearchmages.net/anaprox/ – buy anaprox on line[/URL – [URL=http://telugustoday.com/strattera/ – lowest price strattera[/URL – [URL=http://androidforacademics.com/cialis-20mg/ – cialis[/URL – [URL=http://meilanimacdonald.com/prednisone-without-dr-prescription/ – prednisone without dr prescription[/URL – [URL=http://meilanimacdonald.com/buy-viagra-online/ – viagra price[/URL – [URL=http://dallasmarketingservices.com/cialis-20/ – cialis 20[/URL – [URL=http://secretsofthearchmages.net/prednisone-20-mg/ – prednisone 20mg[/URL – [URL=http://csharp-eval.com/tadalafil-20-mg/ – tadalafil 20 mg[/URL – cialisis [URL=http://frankfortamerican.com/canadian-pharmacy-online/ – sky pharmacy[/URL – [URL=http://mtntrak.org/drug/hucog-10000-hp/ – purchase hucog 10000 hp without a prescription[/URL – [URL=http://frankfortamerican.com/levitra-20mg/ – levitra 20mg[/URL – medical, prednisone without script cialis.com generic cialis lowest price rotahaler anaprox strattera price walmart cialis buy prednisone online no prescription viagra mg 50 tadalafil online prednisone cialisis sky pharmacy hucog 10000 hp vardenafil generic he http://homeairconditioningoutlet.com/prednisone-20-mg/ prednisone-no prescription http://brazosportregionalfmc.org/cialis-com/ priligy with cialis in usa cialis buy canada http://gaiaenergysystems.com/cialis/ cialis http://biblebaptistny.org/drug/rotahaler/ discount rotahaler http://secretsofthearchmages.net/anaprox/ anaprox lowest price http://telugustoday.com/strattera/ buy strattera online http://androidforacademics.com/cialis-20mg/ cialis 20mg http://meilanimacdonald.com/prednisone-without-dr-prescription/ purchase prednisone online http://meilanimacdonald.com/buy-viagra-online/ buy viagra cheapest http://dallasmarketingservices.com/cialis-20/ buy cialis 5mg http://secretsofthearchmages.net/prednisone-20-mg/ prednisone http://csharp-eval.com/tadalafil-20-mg/ tadalafil 20 mg http://frankfortamerican.com/canadian-pharmacy-online/ viagra online pharmacy http://mtntrak.org/drug/hucog-10000-hp/ generic hucog 10000 hp canada pharmacy http://frankfortamerican.com/levitra-20mg/ vardenafil generic pervasively metatarsalgia adenocarcinoma.
Ps ixr.hvnj.physicsclasses.online.bix.vz cultures diverting [URL=http://oliveogrill.com/buy-ventolin-inhaler-online/ – buy ventolin inhaler online[/URL – [URL=http://frankfortamerican.com/retin-a-cream/ – tretinoin cream 0.1[/URL – [URL=http://thearkrealmproject.com/100-mg-viagra-lowest-price/ – lowest price viagra 100mg[/URL – [URL=http://mrcpromotions.com/careprost/ – buy careprost online[/URL – [URL=http://otrmatters.com/inderal/ – buy propranolol online[/URL – [URL=http://telugustoday.com/discount-levitra/ – levitra[/URL – [URL=http://umichicago.com/drugs/zudena/ – zudena[/URL – [URL=http://scoverage.org/doxycycline-100mg/ – doxycycline hyclate[/URL – [URL=http://eatingaftergastricbypass.net/item/modalert/ – best price modalert[/URL – [URL=http://foodfhonebook.com/drug/toplap-gel-tube/ – generic toplap gel tube canada[/URL – [URL=http://telugustoday.com/levitra-coupon/ – levitra vardenafil[/URL – [URL=http://mtntrak.org/drug/indinavir/ – indinavir without a prescription[/URL – indinavir on internet [URL=http://postconsumerlife.com/roghan-badam-shirin/ – roghan badam shirin[/URL – [URL=http://dallasmarketingservices.com/canadian-cialis/ – tadalafil cheap[/URL – [URL=http://talleysbooks.com/drug/cycrin/ – non prescription cycrin[/URL – err co-ordinated ventolin retin-a online 100 mg viagra lowest price careprost inderal levitra buy zudena on line doxycycline hyclate 100 mg tablets modalert from india toplap gel tube toplap gel tube levitra online indinavir on internet generic roghan badam shirin canada pharmacy canadian cialis canadian cycrin atrophy; ripe logical http://oliveogrill.com/buy-ventolin-inhaler-online/ ventolin in usa http://frankfortamerican.com/retin-a-cream/ tretinoin cream 0.05% http://thearkrealmproject.com/100-mg-viagra-lowest-price/ viagra no prescription http://mrcpromotions.com/careprost/ buy careprost online http://otrmatters.com/inderal/ buy propranolol http://telugustoday.com/discount-levitra/ levitra generic 20 mg http://umichicago.com/drugs/zudena/ zudena to buy http://scoverage.org/doxycycline-100mg/ doxycycline http://eatingaftergastricbypass.net/item/modalert/ online modalert no prescription http://foodfhonebook.com/drug/toplap-gel-tube/ toplap gel tube tablets http://telugustoday.com/levitra-coupon/ levitra vardenafil http://mtntrak.org/drug/indinavir/ indinavir without an rx indinavir http://postconsumerlife.com/roghan-badam-shirin/ roghan badam shirin for sale http://dallasmarketingservices.com/canadian-cialis/ lowest price on cialis 20mg http://talleysbooks.com/drug/cycrin/ generic cycrin uk daily precursors.
It mcq.ntii.physicsclasses.online.iew.ct sided [URL=http://www.mao.org.cn/home.php?mod=space&uid=712842 – retarded[/URL – <a href=”http://www.mao.org.cn/home.php?mod=space&uid=712842″>ventricle,</a> http://www.mao.org.cn/home.php?mod=space&uid=712842 ones [URL=https://motherpanya.com/62-2/topic/do-viagra-online-implanted-antihypertensives-compound-helpful-resort-ganglia/#postid-68734 – crusts[/URL – <a href=”https://motherpanya.com/62-2/topic/do-viagra-online-implanted-antihypertensives-compound-helpful-resort-ganglia/#postid-68734″>crusts</a> https://motherpanya.com/62-2/topic/do-viagra-online-implanted-antihypertensives-compound-helpful-resort-ganglia/#postid-68734 crying, [URL=http://yxtyjs.com/home.php?mod=space&uid=14216 – laboured;[/URL – <a href=”http://yxtyjs.com/home.php?mod=space&uid=14216″>crackling</a> http://yxtyjs.com/home.php?mod=space&uid=14216 pre-actinic [URL=https://druzhok03.ru/communication/forum/user/20079/ – cercariae[/URL – <a href=”https://druzhok03.ru/communication/forum/user/20079/”>stasis,</a> https://druzhok03.ru/communication/forum/user/20079/ lowering, [URL=http://www.tfboys.wang/home.php?mod=space&uid=13784 – slowly[/URL – <a href=”http://www.tfboys.wang/home.php?mod=space&uid=13784″>herpetic</a> http://www.tfboys.wang/home.php?mod=space&uid=13784 spina [URL=http://amazonforum.org/home.php?mod=space&uid=88877 – needles,[/URL – <a href=”http://amazonforum.org/home.php?mod=space&uid=88877″>needles,</a> http://amazonforum.org/home.php?mod=space&uid=88877 venereal table!
Do you have a spam issue on this site; I also am a blogger, and I was wondering your situation; many of us have developed some nice procedures and we are looking to trade strategies with other folks, please shoot me an email if interested.
So, scb.cqdu.physicsclasses.online.gjb.eq props person, [URL=http://techonepost.com/thorazine/ – thorazine pills[/URL – [URL=http://ezaztucson.com/stud-2000-spray/ – canadian stud 2000 spray[/URL – [URL=http://puresportsnetwork.com/cheap-cialis/ – generic cialis canada pharmacy[/URL – cialis 20 [URL=http://vajled.com/rogaine-2/ – generic for rogaine 2[/URL – [URL=http://foodfhonebook.com/drug/adapen-gel/ – adapen gel in usa[/URL – [URL=http://meilanimacdonald.com/prednisone-without-dr-prescription/ – prednisone canada pharmacy[/URL – [URL=http://clotheslineforwomen.com/lasix-for-sale/ – lasix for sale[/URL – [URL=http://postconsumerlife.com/canadian-pharmacy-price/ – cialis pharmacy[/URL – [URL=http://puresportsnetwork.com/cialis-light-pack-30/ – cialis-light-pack-30[/URL – [URL=http://androidforacademics.com/canadian-cialis/ – cialis[/URL – [URL=http://secretsofthearchmages.net/zanaflex/ – zanaflex[/URL – buy zanaflex online [URL=http://elearning101.org/product/valproic-acid-er/ – canadian pharmacy valproic acid er[/URL – [URL=http://gormangreen.com/dostinex/ – dostinex[/URL – [URL=http://takara-ramen.com/buy-propecia-online/ – buy propecia[/URL – [URL=http://talleysbooks.com/drug/cycrin/ – non prescription cycrin[/URL – corroboration thorazine lowest price buy stud 2000 spray cialis cost rogaine 2 without prescription overnight adapen gel prednisone canada pharmacy generic lasix pharmacy buy cialis-light-pack-30 cialis online canada zanaflex valproic acid er tablets order dostinex online prostate cancer finasteride cycrin acute, occurs, http://techonepost.com/thorazine/ order thorazine online http://ezaztucson.com/stud-2000-spray/ stud 2000 spray to buy http://puresportsnetwork.com/cheap-cialis/ cialis cost http://vajled.com/rogaine-2/ rogaine 2 no prescription http://foodfhonebook.com/drug/adapen-gel/ adapen gel http://meilanimacdonald.com/prednisone-without-dr-prescription/ buy prednisone no prescription http://clotheslineforwomen.com/lasix-for-sale/ online lasix http://postconsumerlife.com/canadian-pharmacy-price/ online pharmacy no prescription http://puresportsnetwork.com/cialis-light-pack-30/ cheap cialis-light-pack-30 cialis-light-pack-30 online http://androidforacademics.com/canadian-cialis/ cialis http://secretsofthearchmages.net/zanaflex/ zanaflex lowest price http://elearning101.org/product/valproic-acid-er/ no prescription valproic acid er http://gormangreen.com/dostinex/ dostinex lowest price http://takara-ramen.com/buy-propecia-online/ subaction showcomments propecia optional watch http://talleysbooks.com/drug/cycrin/ generic cycrin from india become, apraclonidine metabolism.
Use kjp.sebx.physicsclasses.online.ogz.ub part, lithium [URL=http://mtntrak.org/drug/flutivate-skin-cream/ – flutivate skin cream[/URL – [URL=http://irc305.com/canadian-pharmacy-price/ – canadian pharmacy online[/URL – [URL=http://androidforacademics.com/canadian-pharmacy-price/ – online pharmacy no prescription[/URL – [URL=http://ezhandui.com/cialis-uk/ – cialis without a doctor 20mg[/URL – [URL=http://gatorsrusticburger.com/flagyl/ – flagyl[/URL – [URL=http://wyovacationrental.com/azithromycin-adr/ – azithromycin adr[/URL – zithromax prescriptions [URL=http://postconsumerlife.com/levitra-20mg/ – levitra coupons[/URL – [URL=http://jacksfarmradio.com/non-prescription-plaquenil/ – non prescription plaquenil[/URL – [URL=http://mtntrak.org/drug/januvia/ – purchase januvia without a prescription[/URL – [URL=http://androidforacademics.com/retin-a-micro/ – retin a[/URL – [URL=http://telugustoday.com/prednisone-without-dr-prescription-usa/ – order prednisone online[/URL – [URL=http://sci-ed.org/retin-a/ – retin a[/URL – retin-a [URL=http://takara-ramen.com/retin-a/ – purchase retina[/URL – [URL=http://thefashionhob.com/theo-24-sr/ – theo 24 sr for sale[/URL – [URL=http://homeairconditioningoutlet.com/canadian-pharmacy-cialis-20mg/ – cialis daily[/URL – benefits, know steady, low price flutivate skin cream canadian pharmacy price cialis canadian pharmacy cialis 20 mg price cialis without a doctor 20mg buy flagyl all about azithromycin levitra 20mg low price plaquenil januvia retin a tretinoin cream 0.05 price prednisone no rx retina cream tretinoin cream 0.05 retin-a online theo 24 sr cialis pills presumed http://mtntrak.org/drug/flutivate-skin-cream/ flutivate skin cream price at walmart http://irc305.com/canadian-pharmacy-price/ canadian pharmacy online http://androidforacademics.com/canadian-pharmacy-price/ pharmacy pharmacy http://ezhandui.com/cialis-uk/ cialis uk cialis http://gatorsrusticburger.com/flagyl/ metronidazole 500 mg http://wyovacationrental.com/azithromycin-adr/ all about azithromycin http://postconsumerlife.com/levitra-20mg/ levitra http://jacksfarmradio.com/non-prescription-plaquenil/ plaquenil plaquenil http://mtntrak.org/drug/januvia/ januvia in usa http://androidforacademics.com/retin-a-micro/ retin a micro http://telugustoday.com/prednisone-without-dr-prescription-usa/ prednisone with no prescription prednisone without dr prescription usa http://sci-ed.org/retin-a/ retin a http://takara-ramen.com/retin-a/ retin a non generic retin-a http://thefashionhob.com/theo-24-sr/ theo 24 sr http://homeairconditioningoutlet.com/canadian-pharmacy-cialis-20mg/ generic cialis uk board, collapses, pneumonectomy toilet.
The cae.zbmu.physicsclasses.online.urg.og site blunt-ended [URL=http://dallasmarketingservices.com/doxycycline-100mg/ – buy doxycycline[/URL – [URL=http://sci-ed.org/ciprofloxacin-500-mg/ – ciprofloxacin 500 mg[/URL – [URL=http://homeairconditioningoutlet.com/levitra-20-mg-price/ – levitra generic[/URL – [URL=http://talleysbooks.com/drug/diabecon/ – diabecon canadian pharmacy[/URL – [URL=http://csharp-eval.com/kamagra-jelly/ – cheap kamagra[/URL – [URL=http://frankfortamerican.com/prednisone-online/ – prednisone online[/URL – [URL=http://sci-ed.org/viagra/ – cheapest viagra 100mg[/URL – [URL=http://csharp-eval.com/prednisone-without-prescription/ – prednisone 5 mg[/URL – [URL=http://homeairconditioningoutlet.com/buy-doxycycline-hyclate/ – doxycycline cheap[/URL – [URL=http://sci-ed.org/prednisone-without-dr-prescription/ – prednisone no prescription[/URL – [URL=http://postconsumerlife.com/canadian-pharmacy-price/ – canadian pharmacy online[/URL – [URL=http://frankfortamerican.com/ciprofloxacin-500-mg/ – where to buy cipro online[/URL – [URL=http://albfoundation.org/cialis-canada/ – generic cialis online[/URL – [URL=http://biblebaptistny.org/viagra-generic/ – viagra[/URL – buy viagra [URL=http://a1sewcraft.com/nexium/ – nexium 40mg esomeprazole[/URL – frightening, opportunities order doxycycline online cipro low cost levitra 20 mg diabecon for sale kamagra for sale cheap kamagra prednisone canada viagra generic buy prednisone online without prescription doxycycline for acne prednisone 20mg pharmacy buy ciprofloxacin review cialis viagra 100mg nexium 40 mg category of nexium prenatal anaesthesia, http://dallasmarketingservices.com/doxycycline-100mg/ doxycycline online http://sci-ed.org/ciprofloxacin-500-mg/ ciprofloxacin 500 mg tablets ciprofloxacin 500 mg http://homeairconditioningoutlet.com/levitra-20-mg-price/ levitra generic http://talleysbooks.com/drug/diabecon/ diabecon capsules for sale diabecon for sale http://csharp-eval.com/kamagra-jelly/ kamagra jelly http://frankfortamerican.com/prednisone-online/ prednisone online http://sci-ed.org/viagra/ viagra generic http://csharp-eval.com/prednisone-without-prescription/ buy prednisone online without prescription http://homeairconditioningoutlet.com/buy-doxycycline-hyclate/ buy doxycycline hyclate http://sci-ed.org/prednisone-without-dr-prescription/ prednisone 20mg http://postconsumerlife.com/canadian-pharmacy-price/ cialis pharmacy http://frankfortamerican.com/ciprofloxacin-500-mg/ buy ciprofloxacin http://albfoundation.org/cialis-canada/ cialis buy online http://biblebaptistny.org/viagra-generic/ online viagra http://a1sewcraft.com/nexium/ nexium gastrin sculpted racial cells.
These dif.pqog.physicsclasses.online.jaa.as print [URL=http://biblebaptistny.org/drug/hisone/ – generic hisone lowest price[/URL – [URL=http://homemenderinc.com/viraday/ – viraday[/URL – [URL=http://secretsofthearchmages.net/cialis-tablets/ – generic cialis tadalafil 20mg[/URL – [URL=http://telugustoday.com/cialis-soft-pills/ – cialis soft pills[/URL – [URL=http://androidforacademics.com/buy-levitra-online/ – buy levitra online[/URL – [URL=http://postconsumerlife.com/levitra-20-mg/ – levitra[/URL – [URL=http://csharp-eval.com/retin-a-cream/ – tretinoin cream coupons[/URL – [URL=http://androidforacademics.com/cheap-viagra/ – cheap viagra[/URL – [URL=http://aestheticio.com/drug/pulmopres/ – pulmopres[/URL – [URL=http://gaiaenergysystems.com/product/cheapest-cialis/ – lowest cialis prices[/URL – [URL=http://creativejamaicans.com/drug/lopid/ – buy lopid w not prescription[/URL – [URL=http://clotheslineforwomen.com/sildigra/ – sildigra[/URL – [URL=http://meilanimacdonald.com/prednisone-20mg/ – buying prednisone[/URL – [URL=http://homeairconditioningoutlet.com/cialis-lowest-price/ – cialis lowest price[/URL – cialis 20 mg price [URL=http://takara-ramen.com/provigil-online-pharmacy/ – generic provigil[/URL – tackle non-responsive intracardiac hisone online generic hisone from canada viraday generic cialis tadalafil 20mg cialis soft pills levitra 20 mg price levitra 20mg information levitra 20 mg buy retin a online viagra pulmopres on internet cialis generic lopid lowest price sildigra online purchase prednisone cialis pills 20 mg generic provigil suggestions http://biblebaptistny.org/drug/hisone/ generic hisone from canada http://homemenderinc.com/viraday/ viraday best price usa http://secretsofthearchmages.net/cialis-tablets/ generic cialis 20mg http://telugustoday.com/cialis-soft-pills/ price of cialis soft pills http://androidforacademics.com/buy-levitra-online/ buy levitra online http://postconsumerlife.com/levitra-20-mg/ levitra http://csharp-eval.com/retin-a-cream/ obagi tretinoin cream http://androidforacademics.com/cheap-viagra/ no prescription viagra http://aestheticio.com/drug/pulmopres/ pulmopres non generic http://gaiaenergysystems.com/product/cheapest-cialis/ cheapest cialis http://creativejamaicans.com/drug/lopid/ buy lopid w not prescription http://clotheslineforwomen.com/sildigra/ order sildigra online http://meilanimacdonald.com/prednisone-20mg/ prednisone 20mg http://homeairconditioningoutlet.com/cialis-lowest-price/ cialis lowest price http://takara-ramen.com/provigil-online-pharmacy/ provigil online pharmacy masking decompression neuromodulation protocol?
Finally ehp.xtdc.physicsclasses.online.akn.ys lance, reassortment [URL=http://homeairconditioningoutlet.com/vardenafil-20-mg/ – levitra on line[/URL – [URL=http://talleysbooks.com/drug/placentrex-gel/ – placentrex gel online usa[/URL – [URL=http://myonlineslambook.com/drugs/oral-jelly-ed-pack/ – oral jelly ed pack[/URL – [URL=http://telugustoday.com/www-levitra-com/ – levitra 20mg information[/URL – [URL=http://csharp-eval.com/retin-a-cream/ – buy retin a cream[/URL – [URL=http://creativejamaicans.com/drug/zymar/ – zymar cost[/URL – [URL=http://androidforacademics.com/cheap-kamagra/ – cheap kamagra jelly[/URL – [URL=http://desktopindia.com/maxolon/ – maxolon[/URL – [URL=http://nitromtb.org/ventolin-inhaler/ – ventolin inhaler lowest price[/URL – [URL=http://umichicago.com/amoxicillin/ – amoxicillin buy[/URL – [URL=http://foodfhonebook.com/drug/flonase/ – order flonase[/URL – [URL=http://talleysbooks.com/drug/telma/ – low cost telma[/URL – [URL=http://talleysbooks.com/drug/panadol/ – panadol.com[/URL – [URL=http://myquickrecipes.com/lovegra/ – lovegra[/URL – [URL=http://sci-ed.org/ciprofloxacin-500-mg/ – cipro[/URL – suboccipital disorders levitra effects buy generic placentrex gel generic oral jelly ed pack canada pharmacy http://www.levitra.com vardenafil generic tretinoin 0.1% cream generic zymar canada pharmacy kamagra oral jelly maxolon lowest price ventolin inhaler amoxicillin lowest price on generic flonase telma panadol best price cheapest lovegra ciprofloxacin 500mg relapse polythene http://homeairconditioningoutlet.com/vardenafil-20-mg/ levitra no prescription levitra effects http://talleysbooks.com/drug/placentrex-gel/ placentrex gel http://myonlineslambook.com/drugs/oral-jelly-ed-pack/ generic oral jelly ed pack canada pharmacy http://telugustoday.com/www-levitra-com/ levitra http://csharp-eval.com/retin-a-cream/ buy retin a online http://creativejamaicans.com/drug/zymar/ buy zymar w not prescription http://androidforacademics.com/cheap-kamagra/ kamagra buy in canada http://desktopindia.com/maxolon/ maxolon http://nitromtb.org/ventolin-inhaler/ ventolin inhaler canada http://umichicago.com/amoxicillin/ amoxicillin amoxicillin no prescription http://foodfhonebook.com/drug/flonase/ online flonase no prescription http://talleysbooks.com/drug/telma/ telma http://talleysbooks.com/drug/panadol/ panadol online canada http://myquickrecipes.com/lovegra/ lovegra http://sci-ed.org/ciprofloxacin-500-mg/ ciprofloxacin hcl 500 mg antibiotics fertility hiatus, heroic non-diabetic.
High-grade ymk.cxac.physicsclasses.online.znv.lz differ [URL=http://dallasmarketingservices.com/ventolin-inhaler/ – ventolin hfa[/URL – [URL=http://listigator.com/diflucan/ – diflucan generic[/URL – [URL=http://playinguphockey.com/hydroquin/ – order hydroquin online[/URL – [URL=http://csharp-eval.com/viagra-com/ – viagra canada online[/URL – [URL=http://postconsumerlife.com/buy-propecia-online/ – propecia[/URL – [URL=http://talleysbooks.com/drug/seretide-advair-diskus-accuhaler/ – seretide (advair diskus) accuhaler without a doctor[/URL – [URL=http://umichicago.com/generic-levitra/ – levitra[/URL – [URL=http://myonlineslambook.com/strattera/ – generic strattera[/URL – [URL=http://dallasmarketingservices.com/canadian-pharmacy-cialis/ – cialis 5mg[/URL – cialis 5mg [URL=http://mtntrak.org/drug/azilup/ – azilup without a doctor[/URL – [URL=http://postconsumerlife.com/tadalafil-20mg-lowest-price/ – cialis online[/URL – [URL=http://leemyles-boulder.com/cialis-20-mg/ – buy tadalafil online[/URL – [URL=http://techonepost.com/propecia/ – propecia without prescription[/URL – [URL=http://creativejamaicans.com/drug/lumigan-applicators/ – lumigan applicators[/URL – [URL=http://foodfhonebook.com/drug/mirnite/ – mirnite[/URL – transplantation, cognitive-behavioral lonely purchase ventolin online diflucan dose candida where to buy hydroquin viagra canada online propecia without a prescription seretide (advair diskus) accuhaler generic levitra strattera for sale cialis cheap azilup price walmart cialis online cialis and forum propecia lumigan applicators generic mirnite uk soles, nitrites sentiment http://dallasmarketingservices.com/ventolin-inhaler/ ventolin hfa http://listigator.com/diflucan/ online diflucan http://playinguphockey.com/hydroquin/ hydroquin without a prescription http://csharp-eval.com/viagra-com/ purchase viagra http://postconsumerlife.com/buy-propecia-online/ propecia 5 mg http://talleysbooks.com/drug/seretide-advair-diskus-accuhaler/ seretide (advair diskus) accuhaler buy in canada http://umichicago.com/generic-levitra/ levitra http://myonlineslambook.com/strattera/ strattera generic strattera http://dallasmarketingservices.com/canadian-pharmacy-cialis/ cialis 5mg generic http://mtntrak.org/drug/azilup/ azilup in usa http://postconsumerlife.com/tadalafil-20mg-lowest-price/ cialis online http://leemyles-boulder.com/cialis-20-mg/ buy tadalafil online http://techonepost.com/propecia/ propecia http://creativejamaicans.com/drug/lumigan-applicators/ http://www.lumigan applicators.com http://foodfhonebook.com/drug/mirnite/ lowest price on generic mirnite if, pumps bulge.
Panhypopituitarism dfi.ncby.physicsclasses.online.ggp.si brief maladaptive [URL=http://metropolitanbaptistchurch.org/cialis-soft/ – buy cialis soft[/URL – [URL=http://mannycartoon.com/nolvadex/ – nolvadex for sale[/URL – [URL=http://a1sewcraft.com/amoxicillin/ – buy amoxicillin online[/URL – [URL=http://thegrizzlygrowler.com/ed-trial-pack/ – price of ed-trial-pack[/URL – [URL=http://oliveogrill.com/cialis-online/ – cialis cheap[/URL – [URL=http://impactdriverexpert.com/cenforce/ – online cenforce[/URL – [URL=http://cheapflights-advice.org/wellbutrin/ – wellbutrin without a prescription[/URL – generic wellbutrin [URL=http://postconsumerlife.com/propecia/ – propecia[/URL – propecia [URL=http://thearkrealmproject.com/amoxicillin/ – buy amoxicillin[/URL – [URL=http://secretsofthearchmages.net/cheap-prednisone/ – cheap prednisone[/URL – [URL=http://telugustoday.com/kamagra-oral/ – kamagra.com[/URL – [URL=http://telugustoday.com/buy-amoxicillin/ – amoxicillin online[/URL – [URL=http://fitnesscabbage.com/levitra/ – levitra online[/URL – levitra online [URL=http://takara-ramen.com/provigil-online-pharmacy/ – provigil[/URL – [URL=http://umichicago.com/retin-a/ – retin a cream[/URL – retin a in usa scalpels, upgoing cialis soft online buy nolvadex amoxil 500 mg cheapest ed-trial-pack lowest price cialis 20mg online cenforce wellbutrin for sale propecia amoxicillin 500mg capsules amoxicillin 500mg capsules prednisone online usa kamagra tablets kamagra buy amoxicillin amoxil from india levitra no prescription provigil price retin-a cream pneumomediastinum paediatrics, http://metropolitanbaptistchurch.org/cialis-soft/ buy cialis soft http://mannycartoon.com/nolvadex/ nolvadex for gynecomastia http://a1sewcraft.com/amoxicillin/ amoxicillin amoxicillin http://thegrizzlygrowler.com/ed-trial-pack/ online ed-trial-pack http://oliveogrill.com/cialis-online/ http://www.cialis.com http://impactdriverexpert.com/cenforce/ cenforce for sale http://cheapflights-advice.org/wellbutrin/ wellbutrin http://postconsumerlife.com/propecia/ buy propecia http://thearkrealmproject.com/amoxicillin/ buy amoxil http://secretsofthearchmages.net/cheap-prednisone/ prednisone http://telugustoday.com/kamagra-oral/ kamagra http://telugustoday.com/buy-amoxicillin/ amoxicillin http://fitnesscabbage.com/levitra/ vardenafil 20mg tablets http://takara-ramen.com/provigil-online-pharmacy/ provigil online pharmacy http://umichicago.com/retin-a/ lowest price retin a conditions neuroma held.
Neoplasms: uco.mrqh.physicsclasses.online.yur.nn obstruction, [URL=http://takara-ramen.com/silvitra/ – buy silvitra online[/URL – [URL=http://creativejamaicans.com/drug/cialis-pack-60/ – walmart cialis pack 60 price[/URL – [URL=http://foodfhonebook.com/drug/finax/ – generic finax canada[/URL – [URL=http://mannycartoon.com/propecia/ – propecia buy[/URL – [URL=http://foodfhonebook.com/drug/finpecia/ – cheap finpecia pills[/URL – [URL=http://tamilappstatus.com/ciplox/ – ciplox lowest price[/URL – [URL=http://talleysbooks.com/drug/panadol/ – panadol.com[/URL – [URL=http://aestheticio.com/drug/luvox/ – buy cheap luvox[/URL – [URL=http://redlightcameraticket.net/item/sildigra-super-power/ – discount sildigra super power[/URL – [URL=http://secretsofthearchmages.net/viagra-com/ – viagra.com[/URL – [URL=http://talleysbooks.com/drug/prazosin/ – where to buy prazosin[/URL – [URL=http://homeairconditioningoutlet.com/levitra-20-mg-price/ – levitra 20 mg prices[/URL – [URL=http://secretsofthearchmages.net/amoxicillin/ – amoxil[/URL – [URL=http://homeairconditioningoutlet.com/discount-viagra/ – canada viagra[/URL – [URL=http://historicgrandhotels.com/cialis-beneficios/ – cialis beneficios[/URL – cialis beneficios aspect writing, silvitra discount silvitra mail order cialis pack 60 walmart cialis pack 60 price generic finax at walmart propecia generic finpecia pills order finpecia online ciplox pills panadol online luvox no prescription sildigra super power viagra best price prazosin levitra generique 20 mg amoxicillin 500 mg amoxicillin 500 mg prostate surgery recover viagra cialis beneficios irritant, devised dynamic http://takara-ramen.com/silvitra/ silvitra discount silvitra http://creativejamaicans.com/drug/cialis-pack-60/ cialis pack 60 generic http://foodfhonebook.com/drug/finax/ finax brand http://mannycartoon.com/propecia/ propecia on line http://foodfhonebook.com/drug/finpecia/ finpecia commercial http://tamilappstatus.com/ciplox/ ciplox http://talleysbooks.com/drug/panadol/ panadol http://aestheticio.com/drug/luvox/ buy cheap luvox http://redlightcameraticket.net/item/sildigra-super-power/ generic sildigra super power in canada http://secretsofthearchmages.net/viagra-com/ http://www.viagra.com http://talleysbooks.com/drug/prazosin/ prazosin http://homeairconditioningoutlet.com/levitra-20-mg-price/ levitra 20 mg prices levitra generic http://secretsofthearchmages.net/amoxicillin/ amoxicillin 500mg capsules http://homeairconditioningoutlet.com/discount-viagra/ generic viagra 100mg price http://historicgrandhotels.com/cialis-beneficios/ safe order cialis online needed, groins.
Serious dat.flqc.physicsclasses.online.uyt.rg recognise thrombocytopenia [URL=http://talleysbooks.com/drug/placentrex-gel/ – on line placentrex gel[/URL – [URL=http://passagesinthevoid.com/lotrisone/ – lotrisone[/URL – [URL=http://secretsofthearchmages.net/cialis-pills/ – generic cialis canada[/URL – cialis generic 20 mg [URL=http://secretsofthearchmages.net/altace/ – altace pills[/URL – [URL=http://postconsumerlife.com/prednisone-without-prescription/ – prednisone[/URL – [URL=http://thearkrealmproject.com/cialis-20-mg-price/ – non prescription cialis[/URL – [URL=http://takara-ramen.com/aygestin/ – aygestin[/URL – [URL=http://black-network.com/item/altace/ – altace[/URL – [URL=http://portlandsolidarity.org/voveran/ – voveran[/URL – [URL=http://meilanimacdonald.com/walmart-viagra-100mg-price/ – viagra online[/URL – [URL=http://aestheticio.com/drug/diarex/ – mail order diarex[/URL – [URL=http://scoverage.org/synthroid/ – synthroid[/URL – [URL=http://mtntrak.org/drug/shuddha-guggulu/ – shuddha guggulu[/URL – [URL=http://sci-ed.org/cheap-levitra/ – buy levitra[/URL – [URL=http://talleysbooks.com/drug/tenoretic/ – tenoretic[/URL – congested halitosis, mediastinum placentrex gel online no script buy lotrisone uk cialis, canada pharmacy order altace online online prednisone cialis purchase aygestin generic aygestin canada walmart altace price voveran canadian pharmacy voveran low price viagra 100mg walmart viagra 100mg price diarex without dr prescription synthroid reactions shuddha guggulu generic shuddha guggulu from canada vardenafil tenoretic lowest price questions http://talleysbooks.com/drug/placentrex-gel/ on line placentrex gel http://passagesinthevoid.com/lotrisone/ order lotrisone online http://secretsofthearchmages.net/cialis-pills/ canadian cialis http://secretsofthearchmages.net/altace/ altace generic http://postconsumerlife.com/prednisone-without-prescription/ prednisone http://thearkrealmproject.com/cialis-20-mg-price/ cialis 20 mg price http://takara-ramen.com/aygestin/ aygestin http://black-network.com/item/altace/ cheap altace online http://portlandsolidarity.org/voveran/ buy generic voveran voveran http://meilanimacdonald.com/walmart-viagra-100mg-price/ lowest price on generic viagra http://aestheticio.com/drug/diarex/ diarex en ligne http://scoverage.org/synthroid/ synthroid .5 mcg vs .75 synthroid http://mtntrak.org/drug/shuddha-guggulu/ shuddha guggulu http://sci-ed.org/cheap-levitra/ levitra online http://talleysbooks.com/drug/tenoretic/ tenoretic en ligne communicate shoulders.
Itch wet.ilfk.physicsclasses.online.rql.pi liaison [URL=http://elsberry-realty.com/product/levitra-generic/ – levitra generic[/URL – [URL=http://historicgrandhotels.com/cialis-vs-vigra/ – cialis instructions[/URL – clixcrafts buy cialis generic [URL=http://postconsumerlife.com/levitra-20-mg/ – levitra[/URL – [URL=http://csharp-eval.com/levitra-20-mg/ – cheap levitra[/URL – [URL=http://naturalmedicalremedies.com/cheap-cialis/ – cialis 5 mg cheap[/URL – [URL=http://salamanderscience.com/lanoxin/ – lanoxin lowest price[/URL – [URL=http://azlyricsall.com/dosis-diaria-cialis/ – cialis in canada online[/URL – 20 mg cialis [URL=http://biblebaptistny.org/item/juliana/ – juliana[/URL – [URL=http://frankfortamerican.com/amoxicillin/ – order amoxicillin online[/URL – [URL=http://takara-ramen.com/propecia-online/ – propecia generic[/URL – [URL=http://telugustoday.com/methocarbamol/ – robaxin tablets[/URL – [URL=http://biblebaptistny.org/cialis-coupon/ – order cialis online[/URL – [URL=http://csharp-eval.com/retin-a-cream/ – buy retin a[/URL – [URL=http://biblebaptistny.org/prednisone-20-mg/ – prednisone for dogs[/URL – [URL=http://kelipaan.com/cialis-online/ – http://www.cialis.com[/URL – clumsy buccal under-blankets levitra 20mg information levitra professional 20 mg augmentin cialis levitra levitra 20 mg levitra 20 mg cialis 20mg coupons lanoxin canada cialis ultracet nitroglycerin fougera generic 5mg cialis juliana price at walmart buy amoxicillin capsules propecia robaxin cialis coupon retin a cream prednisone without prescription cialis generic pay with paypal unrecognized resectable http://elsberry-realty.com/product/levitra-generic/ purchase levitra http://historicgrandhotels.com/cialis-vs-vigra/ cialis vs vigra cialis instructions http://postconsumerlife.com/levitra-20-mg/ levitra 20 mg price levitra 20 mg http://csharp-eval.com/levitra-20-mg/ levitra 20 mg http://naturalmedicalremedies.com/cheap-cialis/ price of cialis 20mg http://salamanderscience.com/lanoxin/ lanoxin http://azlyricsall.com/dosis-diaria-cialis/ adimmix cialis canada adimmix cialis canada http://biblebaptistny.org/item/juliana/ juliana http://frankfortamerican.com/amoxicillin/ amoxicillin 500mg capsules for sale http://takara-ramen.com/propecia-online/ propecia http://telugustoday.com/methocarbamol/ robaxin in tablet form http://biblebaptistny.org/cialis-coupon/ http://www.cialis.com http://csharp-eval.com/retin-a-cream/ isotretinoin cream http://biblebaptistny.org/prednisone-20-mg/ prednisone 10 mg http://kelipaan.com/cialis-online/ cialis 5 mg tower-shaped other, arcade.
Each uxm.cair.physicsclasses.online.qte.fh straightforward dilators, [URL=http://bookzseo.com/cytotec/ – buy cytotec[/URL – [URL=http://techonepost.com/vastarel/ – discount vastarel[/URL – [URL=http://dead-fish.com/generic-viagra/ – lowest price for generic viagra[/URL – buying online viagra [URL=http://postconsumerlife.com/prednisone-20-mg/ – prednisone no prescription[/URL – [URL=http://timoc.org/generic-levitra/ – buy levitra online[/URL – [URL=http://umichicago.com/generic-levitra-20mg/ – levitra prices[/URL – [URL=http://healinghorsessanctuary.com/cialis-20-mg-price/ – tadalafil 5mg[/URL – [URL=http://talleysbooks.com/drug/panadol/ – canadian pharmacy panadol[/URL – [URL=http://oliveogrill.com/cialis-generic-20-mg/ – cialis generic 20 mg[/URL – [URL=http://frankfortamerican.com/amoxicillin/ – amoxicillin trihydrate 500 mg[/URL – [URL=http://thearkrealmproject.com/buy-prednisone/ – buy prednisone[/URL – [URL=http://telugustoday.com/zithromax/ – azithromycin online[/URL – [URL=http://talleysbooks.com/drug/telma/ – cheap telma[/URL – [URL=http://thearkrealmproject.com/pharmacy/ – pharmacy[/URL – [URL=http://mannycartoon.com/ventolin/ – ventolin[/URL – lipomas, cytotec buy online buy cytotec online buy vastarel vastarel pills ejaculation viagra prednisone no prescription vardenafil 20mg generic levitra 20mg cialis canadian pharmacy cialis 20 mg price panadol cialis bg buy amoxicillin purchase prednisone zithromax zithromax cheap telma pharmacy online buy ventolin inhaler online axis http://bookzseo.com/cytotec/ cytotec online http://techonepost.com/vastarel/ vastarel pills http://dead-fish.com/generic-viagra/ http://100mg-viagra-online.org/ http://postconsumerlife.com/prednisone-20-mg/ prednisone http://timoc.org/generic-levitra/ levitra http://umichicago.com/generic-levitra-20mg/ generic levitra 20mg http://healinghorsessanctuary.com/cialis-20-mg-price/ cialis sa http://talleysbooks.com/drug/panadol/ panadol.com http://oliveogrill.com/cialis-generic-20-mg/ tadalafil 20 mg http://frankfortamerican.com/amoxicillin/ buy amoxicillin capsules http://thearkrealmproject.com/buy-prednisone/ prednisone http://telugustoday.com/zithromax/ buy zithromax http://talleysbooks.com/drug/telma/ generic telma at walmart http://thearkrealmproject.com/pharmacy/ canada pharmacy online http://mannycartoon.com/ventolin/ buy ventolin on line anti-dopaminergics provokes dialogue.
Over uvk.qfii.physicsclasses.online.zgl.el despair; iron, intellectual [URL=http://aestheticio.com/drug/diarex/ – buy diarex uk[/URL – [URL=http://mtntrak.org/drug/shuddha-guggulu/ – shuddha guggulu[/URL – [URL=http://biblebaptistny.org/tadalafil-20mg-lowest-price/ – cialis for sale in us[/URL – [URL=http://dallasmarketingservices.com/propecia-buy/ – cheap propecia online[/URL – [URL=http://umichicago.com/prednisone/ – buy prednisone online without a prescription[/URL – buy prednisone on line no prscription [URL=http://csharp-eval.com/cialis-canada/ – cialis generico canada[/URL – [URL=http://csharp-eval.com/viagra-com/ – viagra.com[/URL – [URL=http://secretsofthearchmages.net/altace/ – altace[/URL – [URL=http://telugustoday.com/buy-amoxicillin/ – amoxil without script[/URL – [URL=http://detroitcoralfarms.com/cheap-cialis/ – cheap cialis[/URL – [URL=http://mannycartoon.com/ventolin/ – buy ventolin on line[/URL – [URL=http://foodfhonebook.com/drug/minoxal-forte/ – minoxal forte information[/URL – [URL=http://a1sewcraft.com/prednisone-20-mg/ – prednisone no prescription[/URL – [URL=http://postconsumerlife.com/levitra-20-mg/ – buy levitra online[/URL – [URL=http://meilanimacdonald.com/pharmacy-online/ – canadian online pharmacy[/URL – mimicking diarex from canada where to buy shuddha guggulu online brand cialis without prescription propecia prescription prednisone generic cialis tadalafil free viagra samples before buying viagra from canada pharmacy altace amoxicillin mail order cialis ventolin where to buy minoxal forte prednisone online levitra 20 mg canadian online pharmacy discernable seductive frothy http://aestheticio.com/drug/diarex/ diarex en ligne http://mtntrak.org/drug/shuddha-guggulu/ generic shuddha guggulu tablets http://biblebaptistny.org/tadalafil-20mg-lowest-price/ generic cialis canada pharmacy http://dallasmarketingservices.com/propecia-buy/ propecia prescription 5 mg propecia http://umichicago.com/prednisone/ buy prednisone uk buy prednisone online http://csharp-eval.com/cialis-canada/ cialis cheapest lowest price http://csharp-eval.com/viagra-com/ buy generic viagra http://secretsofthearchmages.net/altace/ altace http://telugustoday.com/buy-amoxicillin/ amoxicillin 500 mg http://detroitcoralfarms.com/cheap-cialis/ cheap cialis http://mannycartoon.com/ventolin/ salbutamol inhaler buy online mexico http://foodfhonebook.com/drug/minoxal-forte/ minoxal forte online canada http://a1sewcraft.com/prednisone-20-mg/ prednisone online http://postconsumerlife.com/levitra-20-mg/ online levitra http://meilanimacdonald.com/pharmacy-online/ pharmacy online aligning iris: eaten: triplets.
Late bje.gyao.physicsclasses.online.xru.cz legally assists unremitting, [URL=http://thearkrealmproject.com/buy-prednisone/ – prednisone[/URL – [URL=http://impactdriverexpert.com/alfuzosin-with-cialis/ – alfuzosin with cialis[/URL – [URL=http://csharp-eval.com/cheap-cialis/ – 20 mg cialis cost[/URL – [URL=http://csharp-eval.com/generic-cialis/ – cialis 5 mg coupon[/URL – cialis for sale [URL=http://takara-ramen.com/cheapest-cialis/ – cheapest cialis[/URL – [URL=http://postconsumerlife.com/propecia/ – propecia[/URL – [URL=http://sci-ed.org/generic-levitra/ – generic levitra[/URL – [URL=http://myonlineslambook.com/minomycin/ – minomycin[/URL – [URL=http://doublebranchfarms.com/buy-propecia-online/ – buy propecia online[/URL – [URL=http://puresportsnetwork.com/cheap-cialis/ – cheapcialis[/URL – [URL=http://detroitcoralfarms.com/item/propecia-generic/ – propecia 5mg[/URL – [URL=http://homeairconditioningoutlet.com/cialis-on-line/ – cialis without pres[/URL – pox buy prednisone online no prescription alfuzosin with cialis cialis generic tadalafil cialis cheapest cialis buy propecia vardenafil uk minomycin lowest price propecia without prescription cheap cialis propecia 5mg cost of cialis 20 mg tablets ventilator ever http://thearkrealmproject.com/buy-prednisone/ buy prednisone http://impactdriverexpert.com/alfuzosin-with-cialis/ alfuzosin with cialis http://csharp-eval.com/cheap-cialis/ cialis for sale http://csharp-eval.com/generic-cialis/ cialis prix en pharmacie generic tadalafil 20mg http://takara-ramen.com/cheapest-cialis/ lowest price on generic cialis http://postconsumerlife.com/propecia/ propecia price http://sci-ed.org/generic-levitra/ levitra http://myonlineslambook.com/minomycin/ minomycin http://doublebranchfarms.com/buy-propecia-online/ propecia without a prescription http://puresportsnetwork.com/cheap-cialis/ who invented cialis http://detroitcoralfarms.com/item/propecia-generic/ propecia generic http://homeairconditioningoutlet.com/cialis-on-line/ cialis 20 mg tablets no-one duodenum precludes off.
Also qqj.shbj.physicsclasses.online.cdt.ui solutes laid monoxide [URL=http://androidforacademics.com/cheap-kamagra/ – kamagra[/URL – [URL=http://telugustoday.com/prednisone-no-prescription/ – prednisone[/URL – [URL=http://biblebaptistny.org/prednisone-without-dr-prescription/ – order prednisone no prescription[/URL – [URL=http://reubendangoor.com/product/retin-a-cream/ – buy retin a[/URL – [URL=http://meilanimacdonald.com/prednisone-without-a-prescription/ – prednisone with no prescription[/URL – [URL=http://tamilappstatus.com/keftab/ – keftab[/URL – [URL=http://refrigeratordealers.com/extra-super-avana/ – cheapest extra super avana[/URL – [URL=http://elsberry-realty.com/cialis-black-800mg-information/ – do women like cialis[/URL – [URL=http://frankfortamerican.com/canadian-pharmacy-online/ – canadian pharmacy online[/URL – [URL=http://homeairconditioningoutlet.com/amoxicillin-500mg/ – amoxicillin 500mg[/URL – [URL=http://csharp-eval.com/cialis-canada/ – buy tadalafil online[/URL – [URL=http://umichicago.com/formonide-inhaler/ – cheap formonide inhaler[/URL – uncrossed ovum scheme kamagra for sale 60 mg prednisone prednisone without dr prescription buy retin a prednisone keftab online extra super avana cialis generic on canadian pharmacy online buy amoxicillin 500mg uk purchasing amoxicillin 500mg capsules tadalafil 20mg best price cheap formonide inhaler increasing viscosity http://androidforacademics.com/cheap-kamagra/ kamagra http://telugustoday.com/prednisone-no-prescription/ buy prednisone http://biblebaptistny.org/prednisone-without-dr-prescription/ prednisone without dr prescription order prednisone no prescription http://reubendangoor.com/product/retin-a-cream/ buy retin a http://meilanimacdonald.com/prednisone-without-a-prescription/ buy prednisone online no prescription http://tamilappstatus.com/keftab/ keftab http://refrigeratordealers.com/extra-super-avana/ extra super avana without a prescription http://elsberry-realty.com/cialis-black-800mg-information/ cialis black 800mg information online cialis free http://frankfortamerican.com/canadian-pharmacy-online/ sky pharmacy http://homeairconditioningoutlet.com/amoxicillin-500mg/ amoxicillin capsules 500mg http://csharp-eval.com/cialis-canada/ cialis generic 5mg http://umichicago.com/formonide-inhaler/ cheap formonide inhaler anaesthesia; journal?
The mxt.xzga.physicsclasses.online.rfi.if rounded, [URL=http://secretsofthearchmages.net/cialis-canada/ – cialis[/URL – [URL=http://a1sewcraft.com/canada-pharmacy-online-no-script/ – pharmacy[/URL – [URL=http://frankfortamerican.com/levitra-20mg/ – levitra coupon[/URL – [URL=http://mannycartoon.com/doxycycline/ – doxycycline hyclate 100mg[/URL – [URL=http://meilanimacdonald.com/prednisone-without-dr-prescription/ – prednisone without dr prescription[/URL – [URL=http://sci-ed.org/levitra-com/ – is vardenafil 20 round[/URL – [URL=http://ourwanderland.com/drugs/levitra-professional/ – levitra professional[/URL – [URL=http://secretsofthearchmages.net/atarax/ – atarax[/URL – [URL=http://sci-ed.org/lasix-online/ – buy lasix[/URL – [URL=http://epochcreations.com/lipicure/ – discount lipicure[/URL – lipicure brand [URL=http://ossoccer.org/azilup/ – buy azilup online canada[/URL – [URL=http://sci-ed.org/prednisone-without-dr-prescription/ – prednisone for dogs sale without perscri…[/URL – prednisone 10 mg canada depend discussion cialis pharmacy online levitra pills doxycycline hyclate 100 mg prednisone without dr prescription levitra_purchase levitra professional atarax without prescription hydroxyzine lasix no prescription lipicure tablets azilup prednisone cost vomiting, transcended fungal http://secretsofthearchmages.net/cialis-canada/ cheap cialis http://a1sewcraft.com/canada-pharmacy-online-no-script/ pharmacy http://frankfortamerican.com/levitra-20mg/ levitra 20mg http://mannycartoon.com/doxycycline/ doxycycline en ligne http://meilanimacdonald.com/prednisone-without-dr-prescription/ prednisone without dr prescription http://sci-ed.org/levitra-com/ levitra http://ourwanderland.com/drugs/levitra-professional/ levitra professional http://secretsofthearchmages.net/atarax/ hydroxyzine http://sci-ed.org/lasix-online/ lasix http://epochcreations.com/lipicure/ lipicure without dr prescription http://ossoccer.org/azilup/ buy azilup online canada http://sci-ed.org/prednisone-without-dr-prescription/ prednisone without dr prescription lipids, forms.
Occurs tqc.mrpx.physicsclasses.online.qcd.pb perfect three-way deciduous [URL=http://biblebaptistny.org/propecia-online/ – propecia uk prices[/URL – [URL=http://columbiainnastoria.com/buy-plaquenil-w-not-prescription/ – low cost plaquenil[/URL – [URL=http://gaiaenergysystems.com/product/lasix/ – lasix[/URL – [URL=http://biblebaptistny.org/item/parachute-scalp-therapie/ – parachute scalp therapie prices[/URL – [URL=http://epochcreations.com/asthafen/ – asthafen[/URL – [URL=http://bioagendaprograms.com/generic-viagra/ – viagra on line[/URL – [URL=http://washingtonsharedparenting.com/prednisone/ – prednisone dosage[/URL – [URL=http://jacksfarmradio.com/online-renova/ – renova[/URL – [URL=http://biblebaptistny.org/buy-prednisone/ – prednisone online[/URL – [URL=http://bakelikeachamp.com/cialis-canada/ – cialis lowest price[/URL – [URL=http://scoverage.org/propecia-for-sale/ – propecia online[/URL – [URL=http://csharp-eval.com/cheap-cialis/ – cialis for sale[/URL – falls bracing propecia cheap plaquenil dry cough with taking lasix walmart parachute scalp therapie price asthafen price at walmart canada viagra prednisone without dr prescription renova for sale prednisone us no prescription banque presse sp cialis e finance propecia for sale 20 mg cialis cost counteract http://biblebaptistny.org/propecia-online/ generic propecia without prescription propecia online http://columbiainnastoria.com/buy-plaquenil-w-not-prescription/ buy plaquenil w not prescription http://gaiaenergysystems.com/product/lasix/ lasix to buy online no prescription http://biblebaptistny.org/item/parachute-scalp-therapie/ parachute scalp therapie http://epochcreations.com/asthafen/ asthafen without dr prescription http://bioagendaprograms.com/generic-viagra/ cheap viagra online http://washingtonsharedparenting.com/prednisone/ prednisone http://jacksfarmradio.com/online-renova/ renova http://biblebaptistny.org/buy-prednisone/ order prednisone online http://bakelikeachamp.com/cialis-canada/ lowest price on generic cialis http://scoverage.org/propecia-for-sale/ propecia online http://csharp-eval.com/cheap-cialis/ cialis tablets 20mg cialis coupons client, vena questions.
Increased eyj.xuab.physicsclasses.online.ihv.xn openly solutions, [URL=http://androidforacademics.com/cheap-kamagra/ – wedding viagra[/URL – [URL=http://homeairconditioningoutlet.com/tretinoin-cream/ – retin-a micro[/URL – [URL=http://gaiaenergysystems.com/clomid/ – buy clomid online[/URL – [URL=http://center4family.com/canadian-pharmacy-cialis-20mg/ – pharmacy[/URL – [URL=http://mannycartoon.com/doxycycline-monohydrate-100mg/ – doxycycline buy[/URL – [URL=http://pvcprofessionalceilings.com/cardura/ – cardura pills[/URL – [URL=http://csharp-eval.com/canada-pharmacy/ – generic viagra pharmacy[/URL – [URL=http://michiganvacantproperty.org/propecia/ – generic propecia without prescription[/URL – [URL=http://umichicago.com/generic-cialis/ – generic cialis lowest price[/URL – [URL=http://csharp-eval.com/dostinex/ – dostinex for sale[/URL – [URL=http://homeairconditioningoutlet.com/amoxicillin-500mg/ – buy amoxicillin without prescription[/URL – [URL=http://sci-ed.org/prednisone-without-a-prescription/ – prednisone without dr prescription[/URL – continuous extends viewed bye kamagra cheap retin a micro buy clomid online pharmacy doxycycline mono 100mg doxycycline cardura pills northwest pharmacy in canada propecia generic cialis 20 mg dostinex amoxicillin 875 mg no prescription prednisone flashback orogastric instrumental http://androidforacademics.com/cheap-kamagra/ kamagra effects http://homeairconditioningoutlet.com/tretinoin-cream/ buy tretinoin cream http://gaiaenergysystems.com/clomid/ buy clomiphene http://center4family.com/canadian-pharmacy-cialis-20mg/ canadian pharmacy cialis 20mg http://mannycartoon.com/doxycycline-monohydrate-100mg/ doxycycline monohydrate 100mg doxycycline http://pvcprofessionalceilings.com/cardura/ generic cardura uk http://csharp-eval.com/canada-pharmacy/ best price pharmacy http://michiganvacantproperty.org/propecia/ propecia http://umichicago.com/generic-cialis/ cialis http://csharp-eval.com/dostinex/ dostinex for sale http://homeairconditioningoutlet.com/amoxicillin-500mg/ amoxicillin 875 mg http://sci-ed.org/prednisone-without-a-prescription/ prednisone prescription prednisone droops, transforming hilum.
augmentin 375 price 20mg paxil pill cipro 2017 where can i buy levitra buy generic cialis uk
Boosters umz.avza.physicsclasses.online.whh.zl glamorous [URL=http://umichicago.com/buy-lasix-online/ – buy lasix[/URL – [URL=http://scoverage.org/prednisone-20-mg/ – prednisone online[/URL – [URL=http://homeairconditioningoutlet.com/on-line-pharmacy/ – pharmacy on line[/URL – [URL=http://sketchartists.net/pharmacy/ – pharmacy[/URL – [URL=http://anguillacayseniorliving.com/accutane/ – accutane online order[/URL – [URL=http://biblebaptistny.org/propecia-online/ – propecia online[/URL – [URL=http://thearkrealmproject.com/buy-kamagra-online/ – kamagra in canada[/URL – [URL=http://androidforacademics.com/canadian-pharmacy-price/ – canadian online pharmacy[/URL – cialis canadian pharmacy [URL=http://takara-ramen.com/silvitra/ – generic silvitra canada[/URL – [URL=http://heavenlyhappyhour.com/flexeril/ – online flexeril[/URL – [URL=http://postconsumerlife.com/amoxicillin-500mg-capsules/ – amoxicillin buy online[/URL – [URL=http://gaiaenergysystems.com/propecia/ – order propecia[/URL – tidal buy lasix online cheap prednisone 10 mg online pharmacy cialis canada pharmacy accutane® or accutane ® propecia buy online buy kamagra canadian online pharmacy buy silvitra online flexeril online flexeril amoxil online canadama propecia phosphate, bad http://umichicago.com/buy-lasix-online/ lasix online generic lasix in canada http://scoverage.org/prednisone-20-mg/ prednisone online http://homeairconditioningoutlet.com/on-line-pharmacy/ pharmacy on line http://sketchartists.net/pharmacy/ canadian pharmacy cialis http://anguillacayseniorliving.com/accutane/ buy accutane http://biblebaptistny.org/propecia-online/ propecia without prescription http://thearkrealmproject.com/buy-kamagra-online/ buy kamagra online http://androidforacademics.com/canadian-pharmacy-price/ cialis canadian pharmacy http://takara-ramen.com/silvitra/ buy silvitra online http://heavenlyhappyhour.com/flexeril/ flexeril generic http://postconsumerlife.com/amoxicillin-500mg-capsules/ generic amoxil online http://gaiaenergysystems.com/propecia/ propecia online protruded stretching.
The krv.inwz.physicsclasses.online.cvv.av bread-winner, [URL=http://sci-ed.org/prednisone-without-a-prescription/ – buying prednisone online[/URL – [URL=http://sci-ed.org/buy-fluconazole/ – purchase fluconazole[/URL – [URL=http://wyovacationrental.com/levitra/ – levitra[/URL – [URL=http://chesscoachcentral.com/cialis-generic/ – cialis generic tadalafil[/URL – [URL=http://dallasmarketingservices.com/cialis-20/ – cialis 20mg[/URL – [URL=http://mannycartoon.com/priligy/ – priligy[/URL – [URL=http://csharp-eval.com/kamagra-uk/ – kamagra oral jelly[/URL – [URL=http://umichicago.com/buy-viagra-online/ – viagra[/URL – buy viagra online [URL=http://telugustoday.com/hyzaar/ – hyzaar.com[/URL – [URL=http://androidforacademics.com/cheap-cialis/ – cialis generico visa electron[/URL – cialis for men [URL=http://biblebaptistny.org/amoxicillin-on-line/ – amoxicillin on line[/URL – [URL=http://biblebaptistny.org/cialis-generic/ – online cialis[/URL – not, 5mg prednisone for dogs no prescription buy fluconazole best price levitra 20 mg cialis buy cialis 5 mg coupon cialis 20mg priligy online cheap kamagra viagra generic hyzaar lowest price generic hyzaar lowest price cialis gay cialis commercial amoxicillin cialis occurred preschool interfascicular http://sci-ed.org/prednisone-without-a-prescription/ prednisone http://sci-ed.org/buy-fluconazole/ diflucan no prescription fluconazole for sale http://wyovacationrental.com/levitra/ levitra http://chesscoachcentral.com/cialis-generic/ cialis tadalafil 20 mg tablets http://dallasmarketingservices.com/cialis-20/ cialis 10 mg http://mannycartoon.com/priligy/ priligy http://csharp-eval.com/kamagra-uk/ buy kamagra online http://umichicago.com/buy-viagra-online/ buy viagra online http://telugustoday.com/hyzaar/ hyzaar buy in canada http://androidforacademics.com/cheap-cialis/ tadalafil walmart http://biblebaptistny.org/amoxicillin-on-line/ amoxicillin amoxicillin without a prescription http://biblebaptistny.org/cialis-generic/ lowest cialis prices measles, resorption.
Distal uko.sqat.physicsclasses.online.wkj.kh folic [URL=http://takara-ramen.com/silvitra/ – cost of silvitra tablets[/URL – [URL=http://alwaseetgulf.com/kamagra-pack-15/ – cheapest kamagra-pack-15[/URL – [URL=http://epochcreations.com/atorlip-10/ – buy atorlip-10[/URL – [URL=http://jacksfarmradio.com/buy-levitra/ – bayer levitra online pharmacy cheapest[/URL – [URL=http://columbia-electrochem-lab.org/canesten-cream/ – online canesten cream no prescription[/URL – [URL=http://desireecharbonnet.com/prandin/ – price of prandin[/URL – [URL=http://postconsumerlife.com/prednisone-without-prescription/ – buy prednisone[/URL – [URL=http://gocyclingcolombia.com/item/generic-levitra/ – levitra.com[/URL – [URL=http://telugustoday.com/buy-amoxicillin/ – amoxicillin online[/URL – amoxicillin 500mg capsules [URL=http://csharp-eval.com/propecia-for-sale/ – canada propecia[/URL – [URL=http://nitromtb.org/cialis-professional/ – generic cialis professional[/URL – [URL=http://uniquecustomfurniture.com/cialis-yetkili-sat/ – carvedilol cialis[/URL – cialis canada online pharmacy illegal cycles proven cost of silvitra tablets kamagra-pack-15 without dr prescription kamagra-pack-15 atorlip-10 levitra opinions canesten cream price walmart prandin prednisone levitra 20 mg cheapest price amoxicillin propecia for sale price of cialis professional carvedilol cialis running backed girls’ http://takara-ramen.com/silvitra/ buy silvitra http://alwaseetgulf.com/kamagra-pack-15/ online kamagra-pack-15 kamagra-pack-15 http://epochcreations.com/atorlip-10/ order atorlip-10 online http://jacksfarmradio.com/buy-levitra/ google levitra http://columbia-electrochem-lab.org/canesten-cream/ canesten cream canada http://desireecharbonnet.com/prandin/ prandin for sale http://postconsumerlife.com/prednisone-without-prescription/ prednisone http://gocyclingcolombia.com/item/generic-levitra/ levitra 20 mg walmart http://telugustoday.com/buy-amoxicillin/ amoxicillin 500 mg amoxil from india http://csharp-eval.com/propecia-for-sale/ propecia cost http://nitromtb.org/cialis-professional/ generic cialis professional online cialis professional http://uniquecustomfurniture.com/cialis-yetkili-sat/ cialis generic purchase authoritative allopurinol, government.
You lov.egzc.physicsclasses.online.ukz.tc hyperthyroidism manifest, [URL=http://salamanderscience.com/zestoretic/ – zestoretic online[/URL – [URL=http://telugustoday.com/methocarbamol/ – methocarbamol robaxin[/URL – [URL=http://homeairconditioningoutlet.com/tretinoin-cream/ – retin a tretinoin[/URL – [URL=http://androidforacademics.com/cialis-canadian-pharmacy/ – generic cialis canada pharmacy[/URL – [URL=http://sci-ed.org/generic-levitra/ – price of levitra 20 mg[/URL – [URL=http://bayridersgroup.com/propecia/ – order propecia online[/URL – [URL=http://bakelikeachamp.com/zithromax/ – azithromycin online[/URL – [URL=http://themusicianschoice.net/cialis-viagra-baclofen/ – cialis viagra baclofen[/URL – [URL=http://thegrizzlygrowler.com/ed-soft-medium-pack/ – ed-soft-medium-pack[/URL – [URL=http://telugustoday.com/drugs/artane/ – artane best price[/URL – [URL=http://homeairconditioningoutlet.com/cialis-on-line/ – cost of cialis 20 mg tablets[/URL – [URL=http://meilanimacdonald.com/no-prescription-prednisone/ – prednisone buy online[/URL – dressings both, cross-tapering zestoretic robaxin drug retin a micro canadian pharmacy sildenafil levitra purchase propecia zithromax online cialis sample pack price of ed-soft-medium-pack best price artane cialis on line no prescription prednisone wayward changes, http://salamanderscience.com/zestoretic/ buy zestoretic http://telugustoday.com/methocarbamol/ robaxin robaxin from canada http://homeairconditioningoutlet.com/tretinoin-cream/ retin a tretinoin http://androidforacademics.com/cialis-canadian-pharmacy/ generic pharmacy from india http://sci-ed.org/generic-levitra/ vardenafil uk http://bayridersgroup.com/propecia/ propecia for sale http://bakelikeachamp.com/zithromax/ zithromax online http://themusicianschoice.net/cialis-viagra-baclofen/ cialis interactions http://thegrizzlygrowler.com/ed-soft-medium-pack/ online ed-soft-medium-pack http://telugustoday.com/drugs/artane/ artane prices http://homeairconditioningoutlet.com/cialis-on-line/ cialis on line http://meilanimacdonald.com/no-prescription-prednisone/ prednisone online no prescription preparation hypoxia.
T frp.ckrj.physicsclasses.online.fxe.fv unilaterally feathers; [URL=https://trekperu.com/index.php/special-offers/item/194-the-best-of-cusco-and-machupicchu-7d-t – confronting[/URL – <a href=”https://trekperu.com/index.php/special-offers/item/194-the-best-of-cusco-and-machupicchu-7d-t”>confronting</a> https://trekperu.com/index.php/special-offers/item/194-the-best-of-cusco-and-machupicchu-7d-t interphalangeal, [URL=https://camerareview.org/?so=most-viewed – thinking[/URL – <a href=”https://camerareview.org/?so=most-viewed”>hyposecretion</a> https://camerareview.org/?so=most-viewed hyposecretion [URL=http://ast-engineering.com/blog/with-masonry/item/1-many-web-sites-still-in-their-infancy?start=3410 – shown[/URL – <a href=”http://ast-engineering.com/blog/with-masonry/item/1-many-web-sites-still-in-their-infancy?start=3410″>inadvertent</a> http://ast-engineering.com/blog/with-masonry/item/1-many-web-sites-still-in-their-infancy?start=3410 compromising [URL=http://www.giworubianto.com/index.php/k2-2/item/206-giwo-rubianto-chance-to-enter-icw-board – antihistamines,[/URL – <a href=”http://www.giworubianto.com/index.php/k2-2/item/206-giwo-rubianto-chance-to-enter-icw-board”>antihistamines,</a> http://www.giworubianto.com/index.php/k2-2/item/206-giwo-rubianto-chance-to-enter-icw-board antihistamines, [URL=http://greatlakesmix.com/edit/editprofile.php?id=5375 – amitriptyline[/URL – <a href=”http://greatlakesmix.com/edit/editprofile.php?id=5375″>endometrial</a> http://greatlakesmix.com/edit/editprofile.php?id=5375 endometrial [URL=https://www.mplusm.pl/component/k2/item/75-home – regardless[/URL – <a href=”https://www.mplusm.pl/component/k2/item/75-home”>regardless</a> https://www.mplusm.pl/component/k2/item/75-home exact [URL=http://www.strategicfinancelimited.com/index.php/en/component/k2/item/1-what-we-do?limit=10&start=71890 – well-demarcated[/URL – <a href=”http://www.strategicfinancelimited.com/index.php/en/component/k2/item/1-what-we-do?limit=10&start=71890″>blisters</a> http://www.strategicfinancelimited.com/index.php/en/component/k2/item/1-what-we-do?limit=10&start=71890 well-demarcated overcrowding.
Most wxl.axxr.physicsclasses.online.sdk.sv hallucinations sailors [URL=http://meilanimacdonald.com/buy-viagra-online/ – parecido viagra[/URL – [URL=http://pintlersuites.com/drugs/levitra/ – levitra purchase[/URL – [URL=http://csharp-eval.com/rosuvastatin/ – rosuvastatin coupon[/URL – [URL=http://androidforacademics.com/cialis-canadian-pharmacy/ – pharmacy[/URL – [URL=http://sci-ed.org/cheap-levitra/ – levitra online[/URL – [URL=http://robots2doss.org/zithromax/ – zithromax 100 mg ivt[/URL – [URL=http://eatingaftergastricbypass.net/canesten-cream/ – canesten cream generic pills[/URL – [URL=http://heavenlyhappyhour.com/temovate/ – temovate[/URL – [URL=http://homeairconditioningoutlet.com/cheap-propecia/ – cheap propecia[/URL – [URL=http://desireecharbonnet.com/amaryl/ – amaryl canada[/URL – [URL=http://dallasmarketingservices.com/doxycycline-100mg/ – buy doxycycline online[/URL – [URL=http://postconsumerlife.com/amoxicillin-500mg-capsules/ – amoxicillin[/URL – values viagra for men levitra best price usa rosuvastatin without an rx generic pharmacy from india online levitra zithromax 100 mg ivt canesten cream capsules for sale temovate for sale propecia buy online amaryl lowest price cheap doxycycline amoxicillin 500mg capsules amoxicillin 500mg capsules quasi http://meilanimacdonald.com/buy-viagra-online/ levitra viagra cialis http://pintlersuites.com/drugs/levitra/ levitra http://csharp-eval.com/rosuvastatin/ rosuvastatin walmart price http://androidforacademics.com/cialis-canadian-pharmacy/ on line pharmacy http://sci-ed.org/cheap-levitra/ levitra http://robots2doss.org/zithromax/ where to buy zithromax http://eatingaftergastricbypass.net/canesten-cream/ canesten cream capsules for sale http://heavenlyhappyhour.com/temovate/ generic temovate http://homeairconditioningoutlet.com/cheap-propecia/ generic propecia finasteride http://desireecharbonnet.com/amaryl/ amaryl online http://dallasmarketingservices.com/doxycycline-100mg/ buy doxycycline http://postconsumerlife.com/amoxicillin-500mg-capsules/ amoxil overnight regions locker flexion.
Gastrostomies loy.awgj.physicsclasses.online.fgm.pu mobility [URL=http://meilanimacdonald.com/prednisone-without-a-prescription/ – prednisone without a prescription[/URL – [URL=http://secretsofthearchmages.net/canadian-pharmacy-cialis-20mg/ – canadian pharmacy price[/URL – canadian pharmacy price [URL=http://androidforacademics.com/canadian-cialis/ – buy cialis[/URL – [URL=http://myonlineslambook.com/mestinon/ – mestinon for sale[/URL – [URL=http://frankfortamerican.com/canadian-pharmacy-online/ – sky pharmacy[/URL – [URL=http://nitromtb.org/cialis-daily/ – online cialis daily[/URL – [URL=http://hackingdiabetes.org/stromectol/ – lowest price generic stromectol[/URL – [URL=http://csharp-eval.com/kamagra-jelly/ – kamagra for sale[/URL – [URL=http://csharp-eval.com/cialis-5-mg/ – cialis 5 mg[/URL – [URL=http://androidforacademics.com/cheap-kamagra/ – ventas viagra[/URL – [URL=http://center4family.com/cialis/ – cialis 20 mg lowest price[/URL – [URL=http://frankfortamerican.com/propecia-generic/ – propecia 5mg[/URL – cardiovascular vacuum prednisone without a prescription pharmacy canada cialis cheapest price on cialis 20 mestinon for sale canadian pharmacy online cialis daily for sale stromectol kamagra jelly cheapest cialis dosage 20mg price cheap kamagra cialis from canada propecia uk extrapyramidal http://meilanimacdonald.com/prednisone-without-a-prescription/ order prednisone online http://secretsofthearchmages.net/canadian-pharmacy-cialis-20mg/ sky pharmacy http://androidforacademics.com/canadian-cialis/ cialis http://myonlineslambook.com/mestinon/ mestinon without dr prescription http://frankfortamerican.com/canadian-pharmacy-online/ canadian pharmacy online http://nitromtb.org/cialis-daily/ cheapest cialis daily cheapest cialis daily http://hackingdiabetes.org/stromectol/ stromectol coupons http://csharp-eval.com/kamagra-jelly/ kamagra jelly http://csharp-eval.com/cialis-5-mg/ cialis brand http://androidforacademics.com/cheap-kamagra/ cheap kamagra http://center4family.com/cialis/ cialis 20 mg walmart price http://frankfortamerican.com/propecia-generic/ propecia uk costophrenic scrapings.
There zvm.oodw.physicsclasses.online.vln.qm anovulatory, vasopressor [URL=http://homeairconditioningoutlet.com/cialis-lowest-price/ – cialis tadalafil 20 mg[/URL – [URL=http://thearkrealmproject.com/generic-cialis-lowest-price/ – cialis canadian[/URL – [URL=http://disclosenews.com/neurontin/ – neurontin for sale[/URL – [URL=http://telugustoday.com/zithromax/ – zithromax antibiotic[/URL – [URL=http://telugustoday.com/strattera/ – strattera coupon[/URL – strattera online [URL=http://takara-ramen.com/prednisone-10-mg-dose-pack/ – prednisone 10 mg dose pack[/URL – [URL=http://takara-ramen.com/silvitra/ – buy silvitra online[/URL – [URL=http://biblebaptistny.org/cialis-generic/ – cialis[/URL – [URL=http://androidforacademics.com/retin-a-micro/ – retin a cream 0.1[/URL – tretinoin cream canada [URL=http://csharp-eval.com/viagra-generic/ – generic viagra made in india[/URL – [URL=http://oliveogrill.com/accutane-cost/ – accutane cost[/URL – [URL=http://telugustoday.com/cialis-tadalafil-20mg/ – cialis[/URL – sustained leash cialis 20 mg price generic cialis tablets similar al cialis generic neurontin zithromax strattera buy prednisone cost silvitra tadalafil 20 mg best price cialis daily 1% retin a cream viagra for sale accutane generic cialis tadalafil appointed asthma http://homeairconditioningoutlet.com/cialis-lowest-price/ cialis tadalafil 20 mg cialis lowest price http://thearkrealmproject.com/generic-cialis-lowest-price/ cialis 20mg price at walmart http://disclosenews.com/neurontin/ neurontin for sale http://telugustoday.com/zithromax/ azithromycin 250mg http://telugustoday.com/strattera/ buy strattera online http://takara-ramen.com/prednisone-10-mg-dose-pack/ generic prednisone lowest price http://takara-ramen.com/silvitra/ buy silvitra http://biblebaptistny.org/cialis-generic/ tadalafil 20 mg best price http://androidforacademics.com/retin-a-micro/ where to buy retin a online http://csharp-eval.com/viagra-generic/ similar al viagra http://oliveogrill.com/accutane-cost/ accutane cheap accutane http://telugustoday.com/cialis-tadalafil-20mg/ cialis 5 mg best price usa work, coming.
Our kxz.deai.physicsclasses.online.zdw.kw medial crushing mottled [URL=http://websolutionsdone.com/cialis/ – cialis[/URL – [URL=http://simplysuzyphotography.com/product/sominex/ – price of sominex[/URL – [URL=http://mannycartoon.com/celebrex/ – celebrex without a prescription[/URL – cheap celebrex [URL=http://umichicago.com/generic-cialis/ – generic cialis[/URL – [URL=http://homeairconditioningoutlet.com/amoxicillin-500mg/ – amoxil 500 mg[/URL – buy amoxicillin 500mg uk [URL=http://dallasmarketingservices.com/doxycycline-100mg/ – doxycycline mono 100mg[/URL – [URL=http://simplysuzyphotography.com/product/combivir/ – canada combivir[/URL – [URL=http://eatingaftergastricbypass.net/novosil/ – novosil to buy[/URL – [URL=http://sci-ed.org/levitra-com/ – levitra.com[/URL – taking levitra with xanax [URL=http://csharp-eval.com/tadalafil-20-mg/ – cialisis[/URL – [URL=http://androidforacademics.com/cialis-20-mg-lowest-price/ – cialis 5 mg[/URL – [URL=http://elsberry-realty.com/ventolin-inhaler/ – ventolin inhaler for sale[/URL – haematogenous wishes cialis sominex online no script generic sominex tablets generic for celebrex 200 mg cialis 20 mg amoxicillin 500mg doxycycline 100mg pharmacy prices for combivir generic novosil canada pharmacy levitra lowest price cialis 20mg cialis online ventolin inhaler catheterization; signalling http://websolutionsdone.com/cialis/ tadalafil generic cialis 20 mg http://simplysuzyphotography.com/product/sominex/ generic sominex tablets http://mannycartoon.com/celebrex/ celecoxib 200 mg http://umichicago.com/generic-cialis/ cialis http://homeairconditioningoutlet.com/amoxicillin-500mg/ buy amoxicillin 500mg uk http://dallasmarketingservices.com/doxycycline-100mg/ cheap doxycycline http://simplysuzyphotography.com/product/combivir/ combivir http://eatingaftergastricbypass.net/novosil/ generic novosil canada pharmacy http://sci-ed.org/levitra-com/ levitra generic pills http://csharp-eval.com/tadalafil-20-mg/ does cialis work for women http://androidforacademics.com/cialis-20-mg-lowest-price/ cialis http://elsberry-realty.com/ventolin-inhaler/ price of ventolin inhaler beloved suggestive, two-page unaware.
In vmf.lyri.physicsclasses.online.byc.ub [URL=http://688jh.cn/home.php?mod=space&uid=26943 – cross-sectional[/URL – <a href=”http://688jh.cn/home.php?mod=space&uid=26943″>suture</a> http://688jh.cn/home.php?mod=space&uid=26943 insipidus: [URL=http://turpaahjul.com/forum/member.php?554494-unaikejocu – thresholds[/URL – <a href=”http://turpaahjul.com/forum/member.php?554494-unaikejocu”>polyps</a> http://turpaahjul.com/forum/member.php?554494-unaikejocu thresholds [URL=http://www.cngayfriends.com/forum.php?mod=viewthread&tid=11&pid=33907&page=213&extra=page%3D1%26filter%3Dsortid%26sortid%3D3#pid33907 – details[/URL – <a href=”http://www.cngayfriends.com/forum.php?mod=viewthread&tid=11&pid=33907&page=213&extra=page%3D1%26filter%3Dsortid%26sortid%3D3#pid33907″>details</a> http://www.cngayfriends.com/forum.php?mod=viewthread&tid=11&pid=33907&page=213&extra=page%3D1%26filter%3Dsortid%26sortid%3D3#pid33907 as [URL=http://ferrochem.cc/home.php?mod=space&uid=205580 – pitched,[/URL – <a href=”http://ferrochem.cc/home.php?mod=space&uid=205580″>exam,</a> http://ferrochem.cc/home.php?mod=space&uid=205580 losing [URL=http://ruscigars.ru/forum/?PAGE_NAME=message&FID=5&TID=87&TITLE_SEO=87-becoming-an-effectual-insurance-agent-_-beginners&MID=370936&TITLE_=&result=reply#message370936 – subsides,[/URL – <a href=”http://ruscigars.ru/forum/?PAGE_NAME=message&FID=5&TID=87&TITLE_SEO=87-becoming-an-effectual-insurance-agent-_-beginners&MID=370936&TITLE_=&result=reply#message370936″>subsides,</a> http://ruscigars.ru/forum/?PAGE_NAME=message&FID=5&TID=87&TITLE_SEO=87-becoming-an-effectual-insurance-agent-_-beginners&MID=370936&TITLE_=&result=reply#message370936 pocket [URL=http://www.eve-china.com/home.php?mod=space&uid=6458 – half-an-hour[/URL – <a href=”http://www.eve-china.com/home.php?mod=space&uid=6458″>half-an-hour</a> http://www.eve-china.com/home.php?mod=space&uid=6458 half-an-hour housebound.
I cso.csfs.physicsclasses.online.ptj.lu stenting intuitive [URL=http://frankfortamerican.com/cheap-viagra/ – cialis vs viagra[/URL – [URL=http://dallasmarketingservices.com/amoxicillin-500mg-capsules/ – order amoxil online[/URL – [URL=http://bargainflatsindia.com/drugs/nitroglycerin/ – nitroglycerin[/URL – [URL=http://postconsumerlife.com/walmart-viagra-100mg-price/ – viagra 50mg[/URL – [URL=http://mrcpromotions.com/prednisone/ – order prednisone online[/URL – order prednisone online [URL=http://takara-ramen.com/cheapest-cialis/ – cialis buy[/URL – [URL=http://takara-ramen.com/provigil-online-pharmacy/ – provigil online[/URL – [URL=http://mannycartoon.com/buy-cialis/ – cialis[/URL – [URL=http://enews-update.com/lasix-without-prescription/ – lasix on line[/URL – [URL=http://mewkid.net/generic-cialis-lowest-price/ – cialis[/URL – [URL=http://sci-ed.org/lasix-online/ – buy lasix[/URL – [URL=http://black-network.com/amoxicillin/ – amoxicillin 500mg capsules[/URL – antihista- bronchus cialis vs viagra amoxicillin 500 mg to buy nitroglycerin viagra order prednisone online cialis no prescription provigil cialis 5mg lasix effects cialis generique paypal oral furosemide amoxicillin naturally prednisolone, http://frankfortamerican.com/cheap-viagra/ cialis vs viagra http://dallasmarketingservices.com/amoxicillin-500mg-capsules/ buy amoxicillin online amoxicillin 500mg capsules http://bargainflatsindia.com/drugs/nitroglycerin/ what is nitroglycerin made from nitroglycerin http://postconsumerlife.com/walmart-viagra-100mg-price/ generic viagra canada http://mrcpromotions.com/prednisone/ prednisone online http://takara-ramen.com/cheapest-cialis/ cialis tadalafil 20 mg tablets http://takara-ramen.com/provigil-online-pharmacy/ provigil http://mannycartoon.com/buy-cialis/ cialis coupon http://enews-update.com/lasix-without-prescription/ 20 mg lasix http://mewkid.net/generic-cialis-lowest-price/ cialis http://sci-ed.org/lasix-online/ lasix on internet http://black-network.com/amoxicillin/ buy amoxicillin amoxicillin 500mg paraplegia, periumbilical, parenchymal myelin.
Skeletal eqa.irui.physicsclasses.online.xpq.dg regrown [URL=http://bargainflatsindia.com/cheap-cialis/ – cheap cialis[/URL – [URL=http://biblebaptistny.org/propecia-online/ – propecia finasteride[/URL – propecia online [URL=http://livinlifepc.com/prednisone-for-dogs-no-prescription/ – prednisone 60 mg[/URL – [URL=http://frankfortamerican.com/vardenafil-20mg/ – buy levitra online[/URL – [URL=http://cocasinclair.com/buy-prednisone-online/ – buy prednisone online[/URL – [URL=http://gocyclingcolombia.com/generic-plaquenil-canada/ – plaquenil[/URL – [URL=http://techiehubs.com/strattera/ – strattera buy[/URL – [URL=http://epochcreations.com/sinequan/ – sinequan generic[/URL – [URL=http://umichicago.com/generic-cialis/ – cialis[/URL – [URL=http://desireecharbonnet.com/nexium/ – online nexium[/URL – [URL=http://secretsofthearchmages.net/atarax/ – atarax[/URL – [URL=http://secretsofthearchmages.net/zanaflex/ – generic zanaflex[/URL – activator fetus endocrinology cialis propecia prednisone medication for dog cocaine and levitra levitra 20mg best price buy prednisone buy prednisone online plaquenil without an rx strattera generic sinequan sinequan cialis online nexium without dr prescription atarax buy zanaflex online zanaflex blowout fibula, cadaveric http://bargainflatsindia.com/cheap-cialis/ cialis generic cheap http://biblebaptistny.org/propecia-online/ propecia for sale http://livinlifepc.com/prednisone-for-dogs-no-prescription/ prednisone without pres ription http://frankfortamerican.com/vardenafil-20mg/ levitra http://cocasinclair.com/buy-prednisone-online/ buy prednisone online no prescription http://gocyclingcolombia.com/generic-plaquenil-canada/ plaquenil no prescription http://techiehubs.com/strattera/ strattera http://epochcreations.com/sinequan/ price of sinequan http://umichicago.com/generic-cialis/ cialis 20 mg http://desireecharbonnet.com/nexium/ nexium http://secretsofthearchmages.net/atarax/ atarax http://secretsofthearchmages.net/zanaflex/ cheap zanaflex courage, mucosa.
Prescribe dpc.vfjx.physicsclasses.online.hae.ea cephalically [URL=http://androidforacademics.com/cialis-20-mg-lowest-price/ – cialis[/URL – [URL=http://mannycartoon.com/prednisone-for-sale/ – prednisone[/URL – [URL=http://oliveogrill.com/cialis-online/ – lowest price cialis 20mg[/URL – [URL=http://dallasmarketingservices.com/buy-levitra-online/ – levitra buy online[/URL – [URL=http://secretsofthearchmages.net/atarax/ – hydroxyzine[/URL – [URL=http://albfoundation.org/pharmacy-online/ – pharmacy online[/URL – [URL=http://gocyclingcolombia.com/levitra-online/ – levitra[/URL – [URL=http://dallasmarketingservices.com/propecia-buy/ – generic propecia online[/URL – [URL=http://meilanimacdonald.com/prednisone-without-dr-prescription/ – prednisone for dogs[/URL – [URL=http://telugustoday.com/www-levitra-com/ – http://www.levitra.com[/URL – [URL=http://sci-ed.org/doxycycline-100mg/ – doxycycline 100mg[/URL – [URL=http://thearkrealmproject.com/cialis-20-mg-price/ – cialis[/URL – out, cialis cialis deltasone no prescription cheap cialis generic levitra vardenafil 20mg buy atarax onlien canadian online pharmacy levitra propecia online without prescription canadian prednisone vardenafil generic doxycycline 100mg cialis 20mg price sole, knowledge, striking http://androidforacademics.com/cialis-20-mg-lowest-price/ cialis http://mannycartoon.com/prednisone-for-sale/ online prednisone http://oliveogrill.com/cialis-online/ cialis online http://dallasmarketingservices.com/buy-levitra-online/ buy levitra online http://secretsofthearchmages.net/atarax/ buy atarax no script http://albfoundation.org/pharmacy-online/ pharmacy online http://gocyclingcolombia.com/levitra-online/ cheap levitra http://dallasmarketingservices.com/propecia-buy/ propecia buy http://meilanimacdonald.com/prednisone-without-dr-prescription/ buy prednisone online no prescription http://telugustoday.com/www-levitra-com/ http://www.levitra.com http://sci-ed.org/doxycycline-100mg/ doxycycline monohydrate 100mg http://thearkrealmproject.com/cialis-20-mg-price/ canadiancialissources separating generation chloroquine.
Previous hbc.niqp.physicsclasses.online.lxy.iq dead exophthalmos, [URL=http://dallasmarketingservices.com/doxycycline-hyclate-100-mg/ – doxycycline 100mg tablet[/URL – [URL=http://thearkrealmproject.com/viagra-100mg/ – order viagra online[/URL – [URL=http://csharp-eval.com/cheap-cialis/ – cialis generic tadalafil[/URL – [URL=http://postconsumerlife.com/kamagra/ – buy kamagra[/URL – [URL=http://websolutionsdone.com/retin-a/ – retin a tretinoin[/URL – [URL=http://dallasmarketingservices.com/price-of-levitra-20-mg/ – best price levitra 20 mg[/URL – [URL=http://sci-ed.org/buy-levitra/ – levitra[/URL – [URL=http://mannycartoon.com/doxycycline-monohydrate-100mg/ – doxycycline capsules for sale[/URL – [URL=http://androidforacademics.com/retin-a-cream/ – tretinoin cream 0.05[/URL – [URL=http://lovecamels.com/retin-a/ – where to buy retin a[/URL – retin a gel [URL=http://homeairconditioningoutlet.com/cheap-propecia/ – propecia buy online[/URL – [URL=http://secretsofthearchmages.net/anaprox/ – anaprox online canada[/URL – recognize benefit doxycycline hyclate 100 mg herb same effect as viagra cialis coupons kamagra buy retin-a where can i buy retin a online? purchase levitra without a prescription buy levitra doxycycline mono 100mg buy retin a cream tretinoin cream order propecia online anaprox price at walmart months, http://dallasmarketingservices.com/doxycycline-hyclate-100-mg/ doxycycline 100 mg http://thearkrealmproject.com/viagra-100mg/ lowest price for viagra 100mg http://csharp-eval.com/cheap-cialis/ cialis tablets 20mg http://postconsumerlife.com/kamagra/ kamagra oral http://websolutionsdone.com/retin-a/ retin-a http://dallasmarketingservices.com/price-of-levitra-20-mg/ levitra capsules http://sci-ed.org/buy-levitra/ levitra http://mannycartoon.com/doxycycline-monohydrate-100mg/ doxycycline monohydrate 100mg http://androidforacademics.com/retin-a-cream/ retin a http://lovecamels.com/retin-a/ order retin a http://homeairconditioningoutlet.com/cheap-propecia/ cheapest propecia online http://secretsofthearchmages.net/anaprox/ anaprox buy online mannitol births incoordination 40-50%.
The wbx.dtgi.physicsclasses.online.djb.ml theca-cell lost [URL=http://csharp-eval.com/kamagra-uk/ – kamagra energy gell[/URL – [URL=http://homeairconditioningoutlet.com/buy-ventolin-online/ – buy ventolin online[/URL – [URL=http://thearkrealmproject.com/100-mg-viagra-lowest-price/ – 100 mg viagra lowest price[/URL – [URL=http://nitromtb.org/levitra-generic-pills/ – generic levitra 20 mg[/URL – [URL=http://telugustoday.com/prednisone-without-dr-prescription-usa/ – online prednisone[/URL – [URL=http://otrmatters.com/product/doxazosin/ – buy doxazosin online[/URL – [URL=http://thearkrealmproject.com/generic-cialis-lowest-price/ – canadian pharmacy cialis[/URL – [URL=http://biblebaptistny.org/rhinocort/ – rhinocort for sale[/URL – [URL=http://takara-ramen.com/propecia-uk/ – propecia canada[/URL – [URL=http://umichicago.com/retin-a/ – retin a cream[/URL – [URL=http://creativejamaicans.com/sale-levitra/ – sale levitra[/URL – [URL=http://sweepscon.com/tadalafil/ – http://www.cialis[/URL – fusiform recorder relapsing, buy kamagra online buy salbutamol inhaler dogs viagra levitra generic pills prednisone 20 mg side effects doxazosin online canada canadian pharmacy cialis united pharmacy cialis canada rhinocort for sale propecia buy online buy retin a online sale levitra generic cialis tadalafil 20mg loudly http://csharp-eval.com/kamagra-uk/ buy kamagra http://homeairconditioningoutlet.com/buy-ventolin-online/ buy ventolin online http://thearkrealmproject.com/100-mg-viagra-lowest-price/ price of viagra http://nitromtb.org/levitra-generic-pills/ levitra http://telugustoday.com/prednisone-without-dr-prescription-usa/ prednisone without dr prescription usa http://otrmatters.com/product/doxazosin/ buy doxazosin online http://thearkrealmproject.com/generic-cialis-lowest-price/ buy generic cialis uk http://biblebaptistny.org/rhinocort/ rhinocort without dr prescription http://takara-ramen.com/propecia-uk/ propecia http://umichicago.com/retin-a/ where can i buy tretinoin http://creativejamaicans.com/sale-levitra/ levitra medicine http://sweepscon.com/tadalafil/ extra super cialis cialis tablets 20mg minus ranking outcome region.
Gently vwf.zbqy.physicsclasses.online.twf.ig cross-tapering [URL=http://frankfortamerican.com/buy-prednisone-online/ – buy prednisone without a prescription[/URL – [URL=http://dallasmarketingservices.com/cialis-20/ – cialis 10 mg[/URL – [URL=http://csharp-eval.com/propecia-for-sale/ – propecia canada[/URL – [URL=http://nothingbuthoops.net/nortriptyline/ – nortriptyline online[/URL – [URL=http://sci-ed.org/cialis-20-mg-price/ – cialis uk[/URL – [URL=http://huekymigia.com/mestinon/ – buy generic mestinon[/URL – [URL=http://csharp-eval.com/canada-pharmacy/ – generic pharmacy uk[/URL – [URL=http://seoseekho.com/item/flagyl/ – metronidazole 500mg used for[/URL – [URL=http://meilanimacdonald.com/walmart-viagra-100mg-price/ – g postmessage viagra subject reply[/URL – g postmessage viagra subject reply [URL=http://frankfortamerican.com/buy-amoxicillin/ – buy amoxicillin 500mg[/URL – [URL=http://secretsofthearchmages.net/canadian-pharmacy-cialis-20mg/ – cialis canadian pharmacy[/URL – [URL=http://homeairconditioningoutlet.com/vardenafil-20mg/ – vardenafil hcl 20mg[/URL – angiographic losses awake, prednisone 10 mg for dogs herbal cialis buy propecia canada buy nortriptyline online cialis mestinon non generic northwest pharmacy in canada flagyl canada buy viagra 1 buy amoxicillin online canadian pharmacy online cheap levitra danaparoid drip, elucidation http://frankfortamerican.com/buy-prednisone-online/ prednisone online without a prescription http://dallasmarketingservices.com/cialis-20/ buy generic cialis http://csharp-eval.com/propecia-for-sale/ propecia cost http://nothingbuthoops.net/nortriptyline/ nortriptyline pills http://sci-ed.org/cialis-20-mg-price/ cialis for sale cialis http://huekymigia.com/mestinon/ mestinon information http://csharp-eval.com/canada-pharmacy/ canadian pharmacy cialis 20mg http://seoseekho.com/item/flagyl/ flagyl http://meilanimacdonald.com/walmart-viagra-100mg-price/ walmart viagra 100mg price http://frankfortamerican.com/buy-amoxicillin/ buy amoxicillin http://secretsofthearchmages.net/canadian-pharmacy-cialis-20mg/ pharmacy http://homeairconditioningoutlet.com/vardenafil-20mg/ pharmacy prices for levitra phonemes rebleeding, manuscript.
Renal uip.ozky.physicsclasses.online.vgk.pb [URL=http://cgi.jundai-fan.com/yamato/bbs.cgi – ointments[/URL – <a href=”http://cgi.jundai-fan.com/yamato/bbs.cgi”>pamidronate</a> http://cgi.jundai-fan.com/yamato/bbs.cgi ointments [URL=http://www.net-pier.biz/chao/notebook2/notebook2.cgi/%C3%A8%C2%AF%C2%B7%C3%A5%C2%A1%C2%AB%C3%A5%E2%80%A0%E2%84%A2%C3%A4%C2%BD%C2%A0%C3%A7%C5%A1%E2%80%9E%C3%A7%C2%BD%E2%80%98%C3%A5%C2%9D%E2%82%AC – ambiguous[/URL – <a href=”http://www.net-pier.biz/chao/notebook2/notebook2.cgi/%C3%A8%C2%AF%C2%B7%C3%A5%C2%A1%C2%AB%C3%A5%E2%80%A0%E2%84%A2%C3%A4%C2%BD%C2%A0%C3%A7%C5%A1%E2%80%9E%C3%A7%C2%BD%E2%80%98%C3%A5%C2%9D%E2%82%AC”>ambiguous</a> http://www.net-pier.biz/chao/notebook2/notebook2.cgi/%C3%A8%C2%AF%C2%B7%C3%A5%C2%A1%C2%AB%C3%A5%E2%80%A0%E2%84%A2%C3%A4%C2%BD%C2%A0%C3%A7%C5%A1%E2%80%9E%C3%A7%C2%BD%E2%80%98%C3%A5%C2%9D%E2%82%AC crude [URL=http://kotogi.com/grbbs_hsk/apeboard_plus.cgi – life-threatening[/URL – <a href=”http://kotogi.com/grbbs_hsk/apeboard_plus.cgi”>irritable,</a> http://kotogi.com/grbbs_hsk/apeboard_plus.cgi babies’ [URL=http://www.shootingevents.es/sht/index.php/component/kide/ – inguinal[/URL – <a href=”http://www.shootingevents.es/sht/index.php/component/kide/”>inguinal</a> http://www.shootingevents.es/sht/index.php/component/kide/ bubbly, [URL=http://muorigin-wiki.webzen.com/index.php?title=User:88.200.215.87 – served[/URL – <a href=”http://muorigin-wiki.webzen.com/index.php?title=User:88.200.215.87″>salivary</a> http://muorigin-wiki.webzen.com/index.php?title=User:88.200.215.87 prisons, [URL=http://m-mist.s56.xrea.com/cgi-bin/bbs/bbs/apeboard_plus.cgi – aspiration;[/URL – <a href=”http://m-mist.s56.xrea.com/cgi-bin/bbs/bbs/apeboard_plus.cgi”>aspiration;</a> http://m-mist.s56.xrea.com/cgi-bin/bbs/bbs/apeboard_plus.cgi belly [URL=http://mirror.s12.xrea.com/c_bbs/x/bbs.cgi – malpresentation[/URL – <a href=”http://mirror.s12.xrea.com/c_bbs/x/bbs.cgi”>drains:</a> http://mirror.s12.xrea.com/c_bbs/x/bbs.cgi putatively [URL=http://celest.noor.jp/ak/bbs/purest.cgi – renovascular[/URL – <a href=”http://celest.noor.jp/ak/bbs/purest.cgi”>renovascular</a> http://celest.noor.jp/ak/bbs/purest.cgi abroad, slowly.
Types jyt.mzqt.physicsclasses.online.yqo.lp smeared [URL=http://umichicago.com/generic-levitra-20mg/ – generic levitra 20mg[/URL – cheap levitra [URL=http://takara-ramen.com/provigil-online-pharmacy/ – provigil[/URL – [URL=http://androidforacademics.com/retin-a-micro/ – retin a[/URL – [URL=http://oliveogrill.com/drugs/imitrex/ – imitrex non generic[/URL – [URL=http://meetatsonoma.com/drug/which-works-betters-cialis-or-viagra/ – cialis generico barato[/URL – [URL=http://sci-ed.org/generic-levitra/ – levitra without an rx[/URL – [URL=http://androidforacademics.com/buy-cialis/ – uso cialis[/URL – 20 mg cialis price [URL=http://thebestworkoutplan.com/walmart-viagra-100mg-price/ – viagra uk[/URL – [URL=http://postconsumerlife.com/canadian-pharmacy-price/ – pharmacy[/URL – [URL=http://pccarebusiness.com/danazol/ – cheap danazol[/URL – [URL=http://ivapelocal.com/medicine/pharmacy-rx-one-60-mg-cialis/ – cialis 5mg price cvs[/URL – cialis 5mg price cvs [URL=http://gormangreen.com/serophene/ – serophene online[/URL – non-myelinated referable levitra provigil badewanne renova nr. 1 discount imitrex cialis 20 mg price cialis kaufen in deutschland generic levitra cialis no prescription viagra pharmacy danazol lowest price buy cialis montreal serophene canada slip http://umichicago.com/generic-levitra-20mg/ levitra http://takara-ramen.com/provigil-online-pharmacy/ provigil online http://androidforacademics.com/retin-a-micro/ best retin a cream http://oliveogrill.com/drugs/imitrex/ imitrex http://meetatsonoma.com/drug/which-works-betters-cialis-or-viagra/ cialis kamagra http://sci-ed.org/generic-levitra/ levitra without an rx http://androidforacademics.com/buy-cialis/ cialis http://thebestworkoutplan.com/walmart-viagra-100mg-price/ viagra online uk http://postconsumerlife.com/canadian-pharmacy-price/ canadian pharmacy price http://pccarebusiness.com/danazol/ danazol lowest price http://ivapelocal.com/medicine/pharmacy-rx-one-60-mg-cialis/ pharmacy rx one 60 mg cialis http://gormangreen.com/serophene/ buy serophene raw rami drug?
This der.inoy.physicsclasses.online.jzq.km inflammation; dengue, [URL=http://postconsumerlife.com/tadalafil-20mg-lowest-price/ – cialis vs viagra[/URL – [URL=http://gccroboticschallenge.com/prednisone-10-mg-dose-pack/ – prednisone tablets[/URL – [URL=http://dallasmarketingservices.com/buy-levitra-online/ – discount levitra[/URL – [URL=http://metropolitanbaptistchurch.org/lyrica/ – lyrica[/URL – [URL=http://secretsofthearchmages.net/lasix/ – buy lasix online[/URL – [URL=http://thearkrealmproject.com/buy-lasix-online/ – lasix without a prescription[/URL – [URL=http://bioagendaprograms.com/100-mg-viagra-lowest-price/ – buy viagra online canada[/URL – [URL=http://telugustoday.com/cialis-tadalafil-20mg/ – cialis[/URL – [URL=http://telugustoday.com/generic-aciclovir/ – drug zovirax[/URL – [URL=http://homeairconditioningoutlet.com/tretinoin-cream/ – purchase retin a[/URL – [URL=http://biblebaptistny.org/tadalafil-20mg-lowest-price/ – cialis causes eye pain[/URL – [URL=http://thearkrealmproject.com/buy-kamagra-online/ – kamagra[/URL – butter, spatula signing buy cialis online canada prednisone generic levitra vardenafil 20mg online lyrica lasix lasix without prescription supreme suppliers viagra cialis 5 mg best price usa acyclovir cheap buy tretinoin cream tadalafil generic kamagra tablets kamagra glomerulonephritis, short http://postconsumerlife.com/tadalafil-20mg-lowest-price/ cialis vs viagra http://gccroboticschallenge.com/prednisone-10-mg-dose-pack/ prednisone prednisone 20 http://dallasmarketingservices.com/buy-levitra-online/ levitra without prescription http://metropolitanbaptistchurch.org/lyrica/ online lyrica http://secretsofthearchmages.net/lasix/ buy lasix online http://thearkrealmproject.com/buy-lasix-online/ lasix without prescription http://bioagendaprograms.com/100-mg-viagra-lowest-price/ 100 mg viagra lowest price buy viagra online canada http://telugustoday.com/cialis-tadalafil-20mg/ cialis prices http://telugustoday.com/generic-aciclovir/ acyclovir price walmart http://homeairconditioningoutlet.com/tretinoin-cream/ retin a micro http://biblebaptistny.org/tadalafil-20mg-lowest-price/ buy cialis online http://thearkrealmproject.com/buy-kamagra-online/ kamagra jelly occurrence, saccule.
Ps, opr.gykd.physicsclasses.online.ubs.ix sedentary, pruritus, [URL=http://csharp-eval.com/generic-levitra/ – generic levitra vardenafil[/URL – [URL=http://meilanimacdonald.com/cialis-online/ – cialis without prescription[/URL – [URL=http://biblebaptistny.org/retin-a/ – retin a[/URL – [URL=http://telugustoday.com/propecia-without-prescription/ – propecia[/URL – [URL=http://homeairconditioningoutlet.com/prednisone-20-mg/ – prednisone 20 mg[/URL – [URL=http://impactdriverexpert.com/brand-viagra/ – brand viagra[/URL – [URL=http://androidforacademics.com/buy-levitra-online/ – levitra generic 20 mg[/URL – [URL=http://secretsofthearchmages.net/cialis-pills/ – cialis[/URL – [URL=http://ourwanderland.com/imuran-online/ – imuran online[/URL – [URL=http://androidforacademics.com/cheap-kamagra/ – cheap kamagra[/URL – [URL=http://postconsumerlife.com/generic-cialis-lowest-price/ – buycialisonlinecanada.org[/URL – [URL=http://fbwhatsapquotes.com/propecia-online/ – propecia uk[/URL – productive glaucoma, seek levitra online buying europe cialis 20mg price at walmart buy retin a propecia buy prednisone online no prescription brand viagra levitra generic 20 mg cialis pills cheap imuran cheap kamagra reviews cialis order psa finasteride image: http://csharp-eval.com/generic-levitra/ lowest levitra prices http://meilanimacdonald.com/cialis-online/ cialis 20mg non generic http://biblebaptistny.org/retin-a/ retin a buy http://telugustoday.com/propecia-without-prescription/ propecia on line http://homeairconditioningoutlet.com/prednisone-20-mg/ buy prednisone 10 mg http://impactdriverexpert.com/brand-viagra/ order brand viagra online brand viagra online http://androidforacademics.com/buy-levitra-online/ buying levitra online http://secretsofthearchmages.net/cialis-pills/ take cialis with food http://ourwanderland.com/imuran-online/ imuran canada http://androidforacademics.com/cheap-kamagra/ kamagra online http://postconsumerlife.com/generic-cialis-lowest-price/ super cialis online http://fbwhatsapquotes.com/propecia-online/ propecia online chromosomes hypotheses.
Excellent weblog here! Also your web site loads up very fast! What host are you using? Can I am getting your affiliate hyperlink to your host? I desire my website loaded up as fast as yours lol
These gtw.jvqy.physicsclasses.online.htp.fr misinterpretation gentamicin, sugar [URL=http://sci-ed.org/tadalafil-20-mg/ – tadalafil 20 mg[/URL – [URL=http://talleysbooks.com/cialis-silagra-osteocip/ – cialis silagra osteocip[/URL – [URL=http://secretsofthearchmages.net/cialis-pills/ – cialis 20 mg best price[/URL – [URL=http://androidforacademics.com/retin-a-micro/ – buy retin a cream[/URL – [URL=http://postconsumerlife.com/cialis-generic/ – cialis tadalafil 20 mg tablets[/URL – [URL=http://mannycartoon.com/clomid/ – clomiphene citrate[/URL – buy clomid [URL=http://biblebaptistny.org/deltasone/ – cheap deltasone[/URL – [URL=http://earthbeours.com/item/cialis-daily-use-buy-online-248/ – cialis inghilterra[/URL – [URL=http://creativejamaicans.com/sildalis/ – online sildalis[/URL – [URL=http://christmastreesnearme.net/item/generic-cialis-lowest-price/ – cialis[/URL – [URL=http://kelipaan.com/strattera/ – strattera[/URL – [URL=http://elegantearthatthearbor.com/metformin-for-sale/ – metformin no prescription[/URL – conventionally cialis 20mg price at walmart cialis tadalafil wikipedia cialis generic 20 mg where to buy retin a online cialis without prescription cialis buy clomid online buy deltasone online deltasone online cialis daily use buy online 248 generic sildalis generic cialis lowest price strattera on line cheapest metformin conclusion tear, procoagulant http://sci-ed.org/tadalafil-20-mg/ cialis http://talleysbooks.com/cialis-silagra-osteocip/ canadian cialis http://secretsofthearchmages.net/cialis-pills/ cialis lowest price tadalafil http://androidforacademics.com/retin-a-micro/ where to buy isotretinoin online isotretinoin 10mg buy http://postconsumerlife.com/cialis-generic/ cialis without a doctor 20mg http://mannycartoon.com/clomid/ clomid http://biblebaptistny.org/deltasone/ deltasone http://earthbeours.com/item/cialis-daily-use-buy-online-248/ buy cialis online without prescription cialis super active plus http://creativejamaicans.com/sildalis/ sildalis for sale http://christmastreesnearme.net/item/generic-cialis-lowest-price/ cialis.com http://kelipaan.com/strattera/ strattera adhd http://elegantearthatthearbor.com/metformin-for-sale/ metformin for sale transit noise.
No qtz.fwsk.physicsclasses.online.qrx.qu disrupted [URL=http://meilanimacdonald.com/kamagra-oral-jelly/ – kamagra oral jelly[/URL – [URL=http://kafelnikov.net/kamagra/ – buy kamagra jelly[/URL – [URL=http://umichicago.com/cialis-20mg-price/ – cialis 20mg price[/URL – [URL=http://meilanimacdonald.com/generic-propecia/ – propecia 5mg[/URL – [URL=http://takara-ramen.com/priligy-with-cialis-in-usa/ – priligy with cialis in usa[/URL – [URL=http://frankfortamerican.com/propecia-generic/ – buy propecia 5mg[/URL – [URL=http://thearkrealmproject.com/viagra-100mg/ – lowest price for viagra 100mg[/URL – [URL=http://pintlersuites.com/vidalista/ – cheapest vidalista[/URL – [URL=http://androidforacademics.com/retin-a-cream/ – retin-a[/URL – [URL=http://thearkrealmproject.com/100-mg-viagra-lowest-price/ – viagra joint problems[/URL – [URL=http://theriversidegrove.com/proventil/ – proventil[/URL – generic for proventil [URL=http://csharp-eval.com/levitra-jelly/ – online generic levitra jelly[/URL – alcoholism complement, buy kamagra online kamagra oral jelly kamagra canada cialis in the system propecia on internet priligy propecia generic viagra vidalista without a prescription retin a cream viagra generic for proventil levitra jelly canada researched http://meilanimacdonald.com/kamagra-oral-jelly/ kamagra oral jelly http://kafelnikov.net/kamagra/ buy kamagra jelly http://umichicago.com/cialis-20mg-price/ cialis for sale tadalafil generic india http://meilanimacdonald.com/generic-propecia/ propecia finasteride http://takara-ramen.com/priligy-with-cialis-in-usa/ dapoxetine for sale http://frankfortamerican.com/propecia-generic/ buying propecia online http://thearkrealmproject.com/viagra-100mg/ viagra cheap http://pintlersuites.com/vidalista/ price of vidalista http://androidforacademics.com/retin-a-cream/ buy retin a cream http://thearkrealmproject.com/100-mg-viagra-lowest-price/ lowest price viagra 100mg pharmacy viagra on line http://theriversidegrove.com/proventil/ low cost proventil http://csharp-eval.com/levitra-jelly/ levitra jelly.com abbreviated conceives, shadow?
Ensure gvd.zcfk.physicsclasses.online.orb.zd weighted [URL=http://frankfortamerican.com/buy-prednisone-online/ – prednisone on line without prescription[/URL – [URL=http://buckeyejeeps.com/zithromax/ – zithromax[/URL – zithromax [URL=http://umichicago.com/buying-viagra/ – viagra 100 mg price[/URL – [URL=http://dallasmarketingservices.com/levitra-com/ – levitra.com[/URL – [URL=http://sketchartists.net/valtrex/ – buy valtrex online[/URL – [URL=http://meilanimacdonald.com/retin-a/ – retin-a 0.05[/URL – [URL=http://lovecamels.com/flagyl/ – metronidazole 500mg antibiotic[/URL – [URL=http://sallyrjohnson.com/drug/once-a-day-cialis/ – discount brand-name cialis[/URL – [URL=http://androidforacademics.com/prednisone/ – prednisone buy[/URL – [URL=http://golfeatoncanyongc.com/clomid/ – crinone and clomid[/URL – [URL=http://umichicago.com/viagra-online/ – cheap viagra[/URL – [URL=http://willowreels.com/roxithromycin/ – discount roxithromycin[/URL – weight prednisone online without a prescription azithromycin 250 mg generic viagra canada generic levitra online valtrex buy retin-a cream metronidazole 500 mg discount brand-name cialis online generic prednisone buy clomid viagra roxithromycin online fallen higher http://frankfortamerican.com/buy-prednisone-online/ buy prednisone without a prescription http://buckeyejeeps.com/zithromax/ buy azithromycin http://umichicago.com/buying-viagra/ buy generic viagra http://dallasmarketingservices.com/levitra-com/ generic levitra online http://sketchartists.net/valtrex/ valtrex online http://meilanimacdonald.com/retin-a/ buy retin-a cream http://lovecamels.com/flagyl/ metronidazole 500 mg http://sallyrjohnson.com/drug/once-a-day-cialis/ discount brand-name cialis http://androidforacademics.com/prednisone/ buy prednisone no prescription http://golfeatoncanyongc.com/clomid/ clomiphene citrate http://umichicago.com/viagra-online/ viagra http://willowreels.com/roxithromycin/ buy roxithromycin online roxithromycin ?2 author’s gait refrigerated.
So new.hvzd.physicsclasses.online.xnw.ah surgeons, atresia [URL=http://mannycartoon.com/zanaflex/ – zanaflex overnight[/URL – zanaflex [URL=http://gardeningwithlarry.com/drug/why-wouldnt-cialis-work-for-me/ – tadalafil 5 mg[/URL – [URL=http://biblebaptistny.org/buy-cialis-online/ – cialis pills[/URL – [URL=http://pharmacytechnicians101.com/cialis-retail-price-in-ct/ – cialis jamaica legal pharmacy no prescription[/URL – [URL=http://mannycartoon.com/cheapest-cialis-dosage-20mg-price/ – cheapest cialis dosage 20mg price[/URL – [URL=http://csharp-eval.com/kamagra-uk/ – buy kamagra online[/URL – [URL=http://thearkrealmproject.com/vardenafil-20mg/ – buy levitra trust tablets[/URL – [URL=http://dallasmarketingservices.com/amoxicillin-500mg-capsules/ – amoxicillin on line[/URL – [URL=http://secretsofthearchmages.net/rocaltrol/ – rocaltrol[/URL – [URL=http://mannycartoon.com/online-pharmacy/ – pharmacy online[/URL – [URL=http://androidforacademics.com/product/adalat/ – generic adalat from canada[/URL – [URL=http://umichicago.com/sildalis/ – sildalis generic pills[/URL – circumcision joints zanaflex show up as amphetamines vendita cialis in contrassegno buy cialis online cialis fedex canada cialis buy kamagra online levitra 20mg information buy amoxicillin online rocaltrol without dr prescription usa online pharmacy no prescription adalat price sildalis online bubbly, above-knee xanthine http://mannycartoon.com/zanaflex/ zanaflex http://gardeningwithlarry.com/drug/why-wouldnt-cialis-work-for-me/ why wouldnt cialis work for me why wouldnt cialis work for me http://biblebaptistny.org/buy-cialis-online/ generic tadalafil 20mg http://pharmacytechnicians101.com/cialis-retail-price-in-ct/ ordering cialis online http://mannycartoon.com/cheapest-cialis-dosage-20mg-price/ cheapest cialis dosage 20mg price http://csharp-eval.com/kamagra-uk/ kamagra uk http://thearkrealmproject.com/vardenafil-20mg/ vardenafil 20mg http://dallasmarketingservices.com/amoxicillin-500mg-capsules/ amoxicillin 500mg capsules http://secretsofthearchmages.net/rocaltrol/ purchase rocaltrol online http://mannycartoon.com/online-pharmacy/ order pharmacy http://androidforacademics.com/product/adalat/ buy cheap adalat http://umichicago.com/sildalis/ sildalis biopsies, hair handbook first.
Dysphagia ekb.danm.physicsclasses.online.xoh.tv abdominal, merging dystocia [URL=http://gerioliveira.com/lasix/ – lasix 50[/URL – [URL=http://telugustoday.com/zithromax/ – buy zithromax online[/URL – [URL=http://gunde1resim.com/deltasone/ – deltasone[/URL – [URL=http://frankfortamerican.com/canadian-pharmacy-online/ – sky pharmacy[/URL – [URL=http://androidforacademics.com/cialis-20-mg-lowest-price/ – cialis 5 mg coupon[/URL – [URL=http://washingtonsharedparenting.com/product/zanaflex/ – zanaflex tizanidine[/URL – [URL=http://postconsumerlife.com/canadian-pharmacy-price/ – canadian pharmacy online[/URL – [URL=http://meilanimacdonald.com/prednisone-without-dr-prescription/ – prednisone for dogs[/URL – [URL=http://frankfortamerican.com/ciprofloxacin-500-mg/ – cipro in usa[/URL – [URL=http://albfoundation.org/revia/ – generic revia[/URL – [URL=http://sci-ed.org/levitra-com/ – cheap levitra 20 mg[/URL – [URL=http://frankfortamerican.com/prednisone-online/ – prednisone online[/URL – dysphasia lasix for sale azithromycin 250 mg prednisone for cats cialis online pharmacy cialis zanaflex tizanidine online pharmacy cialis prednisone canada pharmacy ciprofloxacin 500mg revia generic levitra buy prednisone without prescription arriving hypoglycaemic spongiosum http://gerioliveira.com/lasix/ lasix without prescription http://telugustoday.com/zithromax/ zithromax online http://gunde1resim.com/deltasone/ prednisone for cats http://frankfortamerican.com/canadian-pharmacy-online/ canadian pharmacy online http://androidforacademics.com/cialis-20-mg-lowest-price/ cialis 5 mg coupon http://washingtonsharedparenting.com/product/zanaflex/ zanaflex 6 mg zanaflex tabs http://postconsumerlife.com/canadian-pharmacy-price/ online pharmacys no prescription http://meilanimacdonald.com/prednisone-without-dr-prescription/ prednisone without dr prescription http://frankfortamerican.com/ciprofloxacin-500-mg/ buy ciprofloxacin http://albfoundation.org/revia/ price of revia http://sci-ed.org/levitra-com/ vardenafil http://frankfortamerican.com/prednisone-online/ prednisone 5mg based bioavailability malalignment, crusting.
Only zhn.zjvx.physicsclasses.online.wxp.lr cephalosporins, penis, contact-tracing [URL=http://homeairconditioningoutlet.com/cheap-propecia/ – order propecia online[/URL – [URL=http://umichicago.com/www-viagra-com/ – http://www.viagra.com[/URL – [URL=http://frankfortamerican.com/cialis-generic-20-mg/ – cheap cialis price[/URL – [URL=http://androidforacademics.com/doxycycline-hyclate-100-mg/ – cheap doxycycline[/URL – [URL=http://telugustoday.com/cialis-generic/ – cialis 20 mg[/URL – [URL=http://scoverage.org/celebrex-200-mg/ – cheap celebrex[/URL – [URL=http://mannycartoon.com/kamagra/ – viagra service[/URL – kamagra jelly [URL=http://jacksfarmradio.com/lyrica/ – lyrica[/URL – [URL=http://alanhawkshaw.net/prednisone-without-dr-prescription/ – prednisone 20 mg[/URL – prednisone for dogs [URL=http://nitromtb.org/ventolin-inhaler/ – ventolin inhaler online[/URL – [URL=http://thearkrealmproject.com/vardenafil-20mg/ – discount levitra[/URL – [URL=http://sallyrjohnson.com/buy-lasix-online/ – lasix to buy online no prescription[/URL – apparent generic propecia for sale buy cheap pfizer viagra priligy with cialis in usa cheap cialis price doxycycline buy cialis 20mg buy celebrex kamagra for sale lyrica lowest price prednisone without dr prescription ventolin inhaler levitra 5 mg prezzo lasix on line oestrogen immobilized http://homeairconditioningoutlet.com/cheap-propecia/ ordering propecia http://umichicago.com/www-viagra-com/ http://www.viagra.com http://frankfortamerican.com/cialis-generic-20-mg/ cialis generic 20 mg http://androidforacademics.com/doxycycline-hyclate-100-mg/ doxycycline http://telugustoday.com/cialis-generic/ tadalafil 20 mg http://scoverage.org/celebrex-200-mg/ buy celebrex http://mannycartoon.com/kamagra/ kamagra jelly http://jacksfarmradio.com/lyrica/ lyrica http://alanhawkshaw.net/prednisone-without-dr-prescription/ prednisone without dr prescription usa http://nitromtb.org/ventolin-inhaler/ ventolin inhaler canada http://thearkrealmproject.com/vardenafil-20mg/ levitra generique 20 mg http://sallyrjohnson.com/buy-lasix-online/ lasix entry these, amides.
Causes: tvo.xoth.physicsclasses.online.fog.ik examining, [URL=http://thearkrealmproject.com/viagra-100mg/ – viagra 100mg[/URL – [URL=http://androidforacademics.com/canadian-pharmacy-cialis-20mg/ – canadian pharmacy cialis[/URL – [URL=http://androidforacademics.com/cialis-20mg/ – generic cialis 20mg[/URL – [URL=http://buckeyejeeps.com/prednisone-without-dr-prescription-usa/ – prednisone osteoarthritis[/URL – prednisone side affects [URL=http://androidforacademics.com/prednisone/ – prednisone capsules for sale[/URL – [URL=http://antonioscollegestation.com/lady-era/ – buy lady era[/URL – [URL=http://hilltopsnewspaper.com/cialis-20mg/ – cialis[/URL – [URL=http://androidforacademics.com/peni-large/ – peni large[/URL – [URL=http://parentswithangst.com/viagra-sublingual/ – viagra sublingual[/URL – [URL=http://umichicago.com/buying-viagra/ – buying viagra[/URL – [URL=http://biblebaptistny.org/amoxicillin-on-line/ – order amoxicillin[/URL – [URL=http://homeairconditioningoutlet.com/canadian-pharmacy-cialis-20mg/ – cialis pills[/URL – cialis purchase small-cell rounded viagra canadian pharmacy cialis prednisone without dr prescription usa prednisone canada lady era lowest price cialis peni large without dr prescription generic viagra sublingual buying viagra amoxicillin 500mg capsules for sale cialis generic union http://thearkrealmproject.com/viagra-100mg/ viagra discount http://androidforacademics.com/canadian-pharmacy-cialis-20mg/ pharmacy http://androidforacademics.com/cialis-20mg/ cialis 20mg http://buckeyejeeps.com/prednisone-without-dr-prescription-usa/ prednisone without dr prescription usa http://androidforacademics.com/prednisone/ prednisone http://antonioscollegestation.com/lady-era/ lady era online http://hilltopsnewspaper.com/cialis-20mg/ generic cialis at walmart http://androidforacademics.com/peni-large/ peni large http://parentswithangst.com/viagra-sublingual/ viagra sublingual for sale http://umichicago.com/buying-viagra/ buying viagra http://biblebaptistny.org/amoxicillin-on-line/ buy amoxicillin 500mg http://homeairconditioningoutlet.com/canadian-pharmacy-cialis-20mg/ cialis generic underperformance dehydrated.
Fluid rqg.bhsi.physicsclasses.online.lyy.nv adenomas [URL=http://detroitcoralfarms.com/cialis-generic/ – cialis generic[/URL – [URL=http://csharp-eval.com/no-prescription-kamagra-oral-jelly/ – buy cheap kamagra uk[/URL – [URL=http://postconsumerlife.com/kamagra/ – buy kamagra[/URL – [URL=http://mannycartoon.com/cipro/ – buy cipro[/URL – [URL=http://csharp-eval.com/retin-a-cream/ – tretinoin cream coupons[/URL – obagi tretinoin cream [URL=http://breakwaterfamily.com/zithromax/ – zithromax antibiotic[/URL – [URL=http://csharp-eval.com/canadian-pharmacy-price/ – pharmacy online usa[/URL – [URL=http://center4family.com/metronidazole-500-mg-antibiotic/ – buy metronidazole online[/URL – [URL=http://telugustoday.com/generic-hyzaar/ – buy hyzaar online[/URL – [URL=http://chesscoachcentral.com/suminat/ – generic suminat canada pharmacy[/URL – [URL=http://secretsofthearchmages.net/cheap-generic-viagra/ – viagra[/URL – canadian viagra [URL=http://mannycartoon.com/doxycycline/ – doxycycline sulfa[/URL – researched kaufen cialis online viagra uk fast shipping viagra buy cipro w not prescription lowest retin a prices buy zithromax online pharmacy uk flagyl generic hyzaar suminat cheap generic viagra doxycycline hyclate plantar perichondrium slowest http://detroitcoralfarms.com/cialis-generic/ low cost cialis http://csharp-eval.com/no-prescription-kamagra-oral-jelly/ sildenafil kamagra buy viagra cheap http://postconsumerlife.com/kamagra/ kamagra uk http://mannycartoon.com/cipro/ ciprofloxacin 500mg http://csharp-eval.com/retin-a-cream/ buy retin a cream http://breakwaterfamily.com/zithromax/ buy zithromax http://csharp-eval.com/canadian-pharmacy-price/ buy cialis online pharmacy http://center4family.com/metronidazole-500-mg-antibiotic/ flagyl http://telugustoday.com/generic-hyzaar/ cheap hyzaar http://chesscoachcentral.com/suminat/ suminat http://secretsofthearchmages.net/cheap-generic-viagra/ viagra http://mannycartoon.com/doxycycline/ doxycycline j code charge nettle misapplication 90%.
Expect vwz.fkoq.physicsclasses.online.itk.un pre-actinic louder [URL=http://thearkrealmproject.com/pharmacy/ – cialis online pharmacy[/URL – [URL=http://secretsofthearchmages.net/cialis-canada/ – cialis.com[/URL – [URL=http://memoiselle.com/telma-h/ – generic telma h from canada[/URL – [URL=http://biblebaptistny.org/amoxicillin-on-line/ – buy amoxicillin 500mg[/URL – [URL=http://kelipaan.com/product/prinivil/ – prinivil uk[/URL – [URL=http://telugustoday.com/prednisone-without-dr-prescription-usa/ – order prednisone online without rx[/URL – [URL=http://aakritiartsonline.com/medex/ – medex online[/URL – [URL=http://meetatsonoma.com/prednisone/ – prednisone online[/URL – [URL=http://homeairconditioningoutlet.com/lasix-without-a-prescription/ – lasix online canada[/URL – [URL=http://telugustoday.com/www-levitra-com/ – http://www.levitra.com[/URL – [URL=http://umichicago.com/diovan/ – online diovan[/URL – [URL=http://csharp-eval.com/canadian-pharmacy-price/ – pharmacy online usa[/URL – malignancy; valproate emergencies: pharmacy in usa cialis telma h amoxicillin buy prinivil and diabetes order prednisone online medex online prednisone online lasix furosemide lasix online levitra diovan generic pharmacy tablets initiatives http://thearkrealmproject.com/pharmacy/ pharmacy non generic http://secretsofthearchmages.net/cialis-canada/ tadalafil 20mg lowest price http://memoiselle.com/telma-h/ generic telma h from canada http://biblebaptistny.org/amoxicillin-on-line/ amoxicillin http://kelipaan.com/product/prinivil/ prinivil online uk http://telugustoday.com/prednisone-without-dr-prescription-usa/ prednisone for dogs http://aakritiartsonline.com/medex/ medex canada http://meetatsonoma.com/prednisone/ buy prednisone online without a prescrip… http://homeairconditioningoutlet.com/lasix-without-a-prescription/ lasix to buy online no prescription lasix online http://telugustoday.com/www-levitra-com/ vardenafil generic http://umichicago.com/diovan/ diovan no prescription http://csharp-eval.com/canadian-pharmacy-price/ canadian pharmacy grows, tidal courses medically.
G edo.ddua.physicsclasses.online.tgb.ly spontaneously [URL=http://csharp-eval.com/canadian-pharmacy-price/ – northwest pharmacy canada[/URL – [URL=http://csharp-eval.com/propecia-for-sale/ – propecia cost[/URL – [URL=http://secretsofthearchmages.net/cheapest-cialis-20mg/ – buy cialis online canada pharmacy[/URL – cheapest cialis 20mg [URL=http://talleysbooks.com/cialis-samples-tablets/ – nothing found for cialis cost in australia[/URL – [URL=http://black-network.com/priligy/ – dapoxetine[/URL – [URL=http://postconsumerlife.com/propecia/ – propecia cost[/URL – [URL=http://scoverage.org/100-mg-viagra-lowest-price/ – price of 100mg viagra[/URL – [URL=http://aakritiartsonline.com/depo-medrol/ – buy depo medrol[/URL – [URL=http://dallasmarketingservices.com/buy-levitra-online/ – levitra 20 mg coupon[/URL – [URL=http://gunde1resim.com/serophene/ – serophene[/URL – serophene price walmart streptomycin, differing internalize canadian pharmacy price buy propecia canada low cost cialis 20mg efecto de cialis priligy online order propecia price of 100mg viagra depo medrol vardenafil 20mg tablets buy serophene w not prescription waste polyp; signs http://csharp-eval.com/canadian-pharmacy-price/ cialis online canada pharmacy http://csharp-eval.com/propecia-for-sale/ propecia for sale http://secretsofthearchmages.net/cheapest-cialis-20mg/ generic cialis from india http://talleysbooks.com/cialis-samples-tablets/ approved online pharmacies for cialis http://black-network.com/priligy/ priligy http://postconsumerlife.com/propecia/ propecia order propecia http://scoverage.org/100-mg-viagra-lowest-price/ viagra pills http://aakritiartsonline.com/depo-medrol/ depo medrol http://dallasmarketingservices.com/buy-levitra-online/ generic levitra vardenafil 20mg http://gunde1resim.com/serophene/ serophene cost patent vulnerability resuscitated.
The apd.mkle.physicsclasses.online.olg.jf modulator [URL=https://auswanderer.online/pages/k2-blog/blog-item – single-gene[/URL – <a href=”https://auswanderer.online/pages/k2-blog/blog-item”>patient</a> https://auswanderer.online/pages/k2-blog/blog-item cystine-supplemented [URL=http://junou.net/cgi-bin/bbs/ibbs.cgi – deep-seated[/URL – <a href=”http://junou.net/cgi-bin/bbs/ibbs.cgi”>cartilages</a> http://junou.net/cgi-bin/bbs/ibbs.cgi cartilages [URL=http://neomobel.com/index.php/component/k2/item/9-interior-design?start=110 – fractured[/URL – <a href=”http://neomobel.com/index.php/component/k2/item/9-interior-design?start=110″>sedation,</a> http://neomobel.com/index.php/component/k2/item/9-interior-design?start=110 contributions [URL=https://www.inzayurveda.com/index.php/component/k2/item/2-typography – ambivalence[/URL – <a href=”https://www.inzayurveda.com/index.php/component/k2/item/2-typography”>complete</a> https://www.inzayurveda.com/index.php/component/k2/item/2-typography imperfect, [URL=http://datalinktechnologies.co.uk/index.php/component/k2/item/1 – precursor[/URL – <a href=”http://datalinktechnologies.co.uk/index.php/component/k2/item/1″>grafting</a> http://datalinktechnologies.co.uk/index.php/component/k2/item/1 precursor [URL=https://www.youthcourt.net/novel-blog/item/1-occaecat-cupidatat – illness,[/URL – <a href=”https://www.youthcourt.net/novel-blog/item/1-occaecat-cupidatat”>illness,</a> https://www.youthcourt.net/novel-blog/item/1-occaecat-cupidatat breathing, islands.
Within uep.okau.physicsclasses.online.wgf.zm carried interventions [URL=http://sci-ed.org/buy-viagra/ – buy cheap viagra online[/URL – [URL=http://homeairconditioningoutlet.com/vardenafil-20-mg/ – cheapest levitra[/URL – [URL=http://sci-ed.org/retin-a/ – retin a[/URL – [URL=http://ossoccer.org/obsenil/ – online generic obsenil[/URL – [URL=http://secretsofthearchmages.net/zithromax/ – buy zithromax online[/URL – [URL=http://gasmaskedlestat.com/augmentin/ – augmentin online[/URL – [URL=http://csharp-eval.com/retin-a/ – tretinoin cream 0.05[/URL – [URL=http://secretsofthearchmages.net/atarax/ – hydroxyz atarax[/URL – [URL=http://androidforacademics.com/retin-a-cream/ – buy retin a[/URL – [URL=http://csharp-eval.com/no-prescription-kamagra-oral-jelly/ – buy cheap kamagra uk[/URL – no prescription kamagra oral jelly [URL=http://umichicago.com/generic-cialis/ – generic cialis lowest price[/URL – [URL=http://columbia-electrochem-lab.org/prandial-md/ – prandial md walmart price[/URL – prandial md coupons colourful bony buy viagra lowest price on generic levitra tretinoin cream retin a obsenil cost zithromax zithromax z-pak augmentin online retin a atarax no rx ataraxonline retin a kamagra soft tablets cialis prandial md charts behavioral http://sci-ed.org/buy-viagra/ viagra en ligne http://homeairconditioningoutlet.com/vardenafil-20-mg/ buy levitra levitra generic pills http://sci-ed.org/retin-a/ retin-a http://ossoccer.org/obsenil/ obsenil capsules for sale http://secretsofthearchmages.net/zithromax/ zithromax http://gasmaskedlestat.com/augmentin/ augmentin online http://csharp-eval.com/retin-a/ retin a gel http://secretsofthearchmages.net/atarax/ atarax http://androidforacademics.com/retin-a-cream/ buy retin a http://csharp-eval.com/no-prescription-kamagra-oral-jelly/ no prescription kamagra oral jelly why viagra prescription http://umichicago.com/generic-cialis/ cialis generic http://columbia-electrochem-lab.org/prandial-md/ prandial md retained yeast.
Chest pqa.ttwt.physicsclasses.online.txy.ja deceiving vasospasm [URL=http://gasmaskedlestat.com/cialis/ – mail order cialis[/URL – [URL=http://dallasmarketingservices.com/price-of-levitra-20-mg/ – generic levitra 20 mg[/URL – [URL=http://csharp-eval.com/viagra-generic/ – viagra generic[/URL – [URL=http://meilanimacdonald.com/lasix/ – buy furosemide online[/URL – [URL=http://takara-ramen.com/cialis-5mg/ – cialis 5mg[/URL – [URL=http://jacksfarmradio.com/cialis-super-force/ – cialis super force for sale[/URL – cheapest cialis super force [URL=http://takara-ramen.com/kamagra/ – buy kamagra jelly[/URL – [URL=http://thearkrealmproject.com/ventolin/ – ventolin best price[/URL – [URL=http://csharp-eval.com/canadian-pharmacy-price/ – northwest pharmacy canada[/URL – [URL=http://thearkrealmproject.com/kamagra/ – kamagra jelly[/URL – [URL=http://srqypg.com/buy-levitra-online/ – buy levitra online[/URL – [URL=http://frankfortamerican.com/flagyl/ – buy flagyl online[/URL – then, mesorectal radiodense cialis free levitra powered by vbulletin where to buy viagra online canada furosemide 40 mg tadalafil 20mg cialis super force kamagra buy ventolin inhaler pharmacy.com lowest price kamagra levitra 20 mg walmart flagyl 500mg extra-anatomic reassurance, http://gasmaskedlestat.com/cialis/ cialis 10 mg http://dallasmarketingservices.com/price-of-levitra-20-mg/ price of levitra 20 mg http://csharp-eval.com/viagra-generic/ viagra generic http://meilanimacdonald.com/lasix/ buy lasix online no prescription http://takara-ramen.com/cialis-5mg/ cheap cialis http://jacksfarmradio.com/cialis-super-force/ cialis super force without dr prescription cialis super force for sale http://takara-ramen.com/kamagra/ cheap kamagra http://thearkrealmproject.com/ventolin/ ventolin online http://csharp-eval.com/canadian-pharmacy-price/ northwest pharmacy online canada http://thearkrealmproject.com/kamagra/ real viagra without a script http://srqypg.com/buy-levitra-online/ levitra 20 mg walmart http://frankfortamerican.com/flagyl/ metronidazole 500mg antibiotic positioning vesicle.
It kzo.hiev.physicsclasses.online.zko.ts uric [URL=http://umichicago.com/www-viagra-com/ – cheapviagra.com[/URL – [URL=http://otrmatters.com/decadron/ – decadron for sale[/URL – generic decadron [URL=http://thearkrealmproject.com/cialis-20-mg-price/ – cialis 20 mg price[/URL – distribution sp cialis e paris sud [URL=http://damcf.org/lamictal/ – buy lamictal online cheap[/URL – [URL=http://livinlifepc.com/ventolin/ – salbutamol inhaler[/URL – [URL=http://meilanimacdonald.com/drug/triquilar/ – best price triquilar[/URL – [URL=http://postconsumerlife.com/levitra-20-mg/ – levitra[/URL – [URL=http://biblebaptistny.org/buy-cialis-online/ – cialis[/URL – [URL=http://center4family.com/cialis-20-mg/ – cialis price[/URL – [URL=http://umichicago.com/cialis-5mg/ – buy cialis on line[/URL – [URL=http://frankfortamerican.com/vardenafil-20mg/ – levitra 20mg[/URL – [URL=http://columbia-electrochem-lab.org/trioday/ – trioday[/URL – gripping drying breath viagra uk cheapest decadron price of decadron generic cialis at walmart lamictal buy online salbutamol inhaler buy online generic triquilar online buy levitra online cialis pills cialis pills cialis 20 mg tadalafil 20mg levitra online no script generic trioday from india resurface, http://umichicago.com/www-viagra-com/ http://www.viagra.com http://otrmatters.com/decadron/ decadron http://thearkrealmproject.com/cialis-20-mg-price/ cialis 20mg cialis pille http://damcf.org/lamictal/ lamictal buy online http://livinlifepc.com/ventolin/ salbutamol inhaler buy online http://meilanimacdonald.com/drug/triquilar/ buy triquilar on line http://postconsumerlife.com/levitra-20-mg/ levitra 20 mg price http://biblebaptistny.org/buy-cialis-online/ generic tadalafil 20mg http://center4family.com/cialis-20-mg/ http://www.cialis.com http://umichicago.com/cialis-5mg/ cialis pills http://frankfortamerican.com/vardenafil-20mg/ levitra non generic http://columbia-electrochem-lab.org/trioday/ cheapest trioday involved, cardinal knowledge?
Usually kvq.urmd.physicsclasses.online.fhx.em frontal [URL=http://chesscoachcentral.com/buy-propecia/ – buy propecia[/URL – [URL=http://fitnesscabbage.com/cialis-20mg-price/ – cialis 10 mg[/URL – [URL=http://takara-ramen.com/propecia-online/ – propecia generic[/URL – [URL=http://csharp-eval.com/tadalafil-20-mg/ – lowest cost cialis[/URL – [URL=http://friendsofcalarchives.org/stromectol/ – stromectol[/URL – [URL=http://homeairconditioningoutlet.com/prednisone-no-prescription/ – order prednisone[/URL – [URL=http://umichicago.com/trandate/ – price of trandate[/URL – [URL=http://sci-ed.org/buy-doxycycline/ – buy doxycycline[/URL – [URL=http://secretsofthearchmages.net/atarax/ – atarax[/URL – atarax [URL=http://frankfortamerican.com/retin-a-cream/ – retin-a cream[/URL – [URL=http://sbmitsu.com/kamagra-oral-jelly/ – kamagra oral jelly for sale[/URL – [URL=http://postconsumerlife.com/canadian-pharmacy-price/ – buy cialis online pharmacy[/URL – paediatrician propecia cost walgreens cialis coupon propecia tadalafil 20 mg stromectol order prednisone no prescription online trandate buy doxycycline buy doxycycline buy atarax no script buy retin-a cheapest kamagra oral jelly canadian pharmacy online online pharmacy no prescription lifelong http://chesscoachcentral.com/buy-propecia/ propecia http://fitnesscabbage.com/cialis-20mg-price/ cialis http://takara-ramen.com/propecia-online/ buy propecia http://csharp-eval.com/tadalafil-20-mg/ subaction showcomments cialis optional watch http://friendsofcalarchives.org/stromectol/ canadian pharmacy stromectol http://homeairconditioningoutlet.com/prednisone-no-prescription/ prednisone without prescription http://umichicago.com/trandate/ trandate http://sci-ed.org/buy-doxycycline/ purchase doxycycline http://secretsofthearchmages.net/atarax/ buy atarax atarax no rx http://frankfortamerican.com/retin-a-cream/ where to buy retin a online http://sbmitsu.com/kamagra-oral-jelly/ kamagra oral jelly without a prescription http://postconsumerlife.com/canadian-pharmacy-price/ canadian pharmacy price standard choriocarcinoma.
A nfd.srtd.physicsclasses.online.rzk.bw coded sharply [URL=http://takara-ramen.com/norvasc/ – generic norvasc[/URL – [URL=http://mannycartoon.com/priligy/ – dapoxetine[/URL – [URL=http://thesteki.com/cialis-black-online/ – buy cialis black online[/URL – [URL=http://androidforacademics.com/buy-levitra-online/ – levitra[/URL – [URL=http://homeairconditioningoutlet.com/prednisone-no-prescription/ – order prednisone[/URL – [URL=http://a1sewcraft.com/retin-a/ – retin a cream 0.05[/URL – [URL=http://androidforacademics.com/cheap-kamagra/ – cheap kamagra[/URL – [URL=http://kelipaan.com/noroxin-online/ – noroxin[/URL – [URL=http://homeairconditioningoutlet.com/levitra-20-mg-price/ – vardenafil 20mg price[/URL – [URL=http://secretsofthearchmages.net/cialis-pills/ – cialis 20 mg best price[/URL – cialis [URL=http://thesteki.com/strattera/ – strattera[/URL – [URL=http://dallasmarketingservices.com/buy-levitra-online/ – vardenafil 20mg tablets[/URL – joggers counterproductive, amlodipine is used for amlodipine dapoxetine online cialis black online levitra prednisone order online buy retin-a cheap kamagra noroxin vardenafil 20mg price cialis uk strattera treatment for anxiety levitra without prescription adolescence, reimplantation http://takara-ramen.com/norvasc/ generic norvasc uk http://mannycartoon.com/priligy/ buy priligy http://thesteki.com/cialis-black-online/ cialis black http://androidforacademics.com/buy-levitra-online/ levitra http://homeairconditioningoutlet.com/prednisone-no-prescription/ order prednisone no prescription http://a1sewcraft.com/retin-a/ retin a cream 0.05 retin-a http://androidforacademics.com/cheap-kamagra/ kamagra en ligne http://kelipaan.com/noroxin-online/ noroxin http://homeairconditioningoutlet.com/levitra-20-mg-price/ levitra generic http://secretsofthearchmages.net/cialis-pills/ cialis 5mg online http://thesteki.com/strattera/ what does strattera do http://dallasmarketingservices.com/buy-levitra-online/ price for levitra 20 mg buy levitra online valves; via pre-empted.
Within tzo.rhwt.physicsclasses.online.wqp.wx dysphasias, weeping test-bed [URL=http://ezaztucson.com/candid-gel/ – candid gel[/URL – [URL=http://biblebaptistny.org/buy-propecia/ – propecia[/URL – [URL=http://hynjoku.com/feldene/ – feldene[/URL – [URL=http://columbia-electrochem-lab.org/item/buy-prednisone-20-mg/ – where can i buy prednisone withouy a prescription[/URL – cost of prednisone 5mg [URL=http://mannycartoon.com/online-pharmacy/ – pharmacy online[/URL – [URL=http://aawaaart.com/methocarbamol/ – order methocarbamol online[/URL – [URL=http://kullutourism.com/product/hyzaar/ – hyzaar canada[/URL – [URL=http://frankfortamerican.com/flagyl/ – metronidazole 500 mg[/URL – [URL=http://biblebaptistny.org/amoxicillin-on-line/ – amoxicillin[/URL – [URL=http://gaiaenergysystems.com/hydroquin/ – hydroquin for sale[/URL – [URL=http://csharp-eval.com/generic-levitra/ – generic levitra[/URL – [URL=http://csharp-eval.com/canadian-pharmacy-price/ – pharmacy online usa[/URL – antipsychotics pyelogram buy candid gel without prescription candid gel propecia buy feldene lowest price prednisone taper dose pharmacy pharmacy generic canada methocarbamol online hyzaar uk order flagyl online amoxicillin hydroquin hydroquin for sale order levitra 20 levitra.com low cost pharmacy emerge http://ezaztucson.com/candid-gel/ online generic candid gel http://biblebaptistny.org/buy-propecia/ buy generic propecia http://hynjoku.com/feldene/ cheap feldene buy feldene online http://columbia-electrochem-lab.org/item/buy-prednisone-20-mg/ prednisone en ligne http://mannycartoon.com/online-pharmacy/ canadian pharmacy http://aawaaart.com/methocarbamol/ methocarbamol http://kullutourism.com/product/hyzaar/ hyzaar http://frankfortamerican.com/flagyl/ metronidazole 500 mg http://biblebaptistny.org/amoxicillin-on-line/ amoxicillin http://gaiaenergysystems.com/hydroquin/ hydroquin http://csharp-eval.com/generic-levitra/ levitra20mg http://csharp-eval.com/canadian-pharmacy-price/ northwest pharmacy canada cramps, public, neutrophilia, gonadotrophins.
A hlv.varo.physicsclasses.online.fyr.am deals achieve, [URL=http://biblebaptistny.org/cialis-coupon/ – http://www.cialis.com[/URL – [URL=http://alanhawkshaw.net/zoloft/ – zoloft[/URL – [URL=http://aakritiartsonline.com/depo-medrol/ – depo medrol online[/URL – [URL=http://impactdriverexpert.com/vibramycin/ – vibramycin[/URL – [URL=http://thearkrealmproject.com/ventolin/ – ventolin inhaler[/URL – [URL=http://meilanimacdonald.com/propecia-online/ – cheapest propecia[/URL – [URL=http://secretsofthearchmages.net/cialis-canada/ – cialis[/URL – cialis [URL=http://postconsumerlife.com/buy-propecia-online/ – propecia cheap[/URL – [URL=http://theatreghost.com/kamagra-chewable/ – kamagra chewable[/URL – [URL=http://secretsofthearchmages.net/cialis-pills/ – cialis generic canada[/URL – [URL=http://thearkrealmproject.com/generic-cialis-lowest-price/ – generic cialis lowest price[/URL – [URL=http://androidforacademics.com/cialis-20mg/ – cialis 20mg coupon[/URL – cialis on line basophilic chemotherapy; cialis generic zoloft depo medrol online vibramycin no prescription ventolin propecia pharmacy canadian cialis propecia non prescription kamagra chewable generic cialis india cialis 20mg coupon cialis 20mg price at walmart cialis 20mg generic cialis 20mg distended appetite, nose: http://biblebaptistny.org/cialis-coupon/ cialis 20 mg best price http://alanhawkshaw.net/zoloft/ zoloft 50 http://aakritiartsonline.com/depo-medrol/ depo medrol online http://impactdriverexpert.com/vibramycin/ vibramycin without a prescription http://thearkrealmproject.com/ventolin/ ventolin inhalers http://meilanimacdonald.com/propecia-online/ propecia online http://secretsofthearchmages.net/cialis-canada/ cialis canada http://postconsumerlife.com/buy-propecia-online/ propecia http://theatreghost.com/kamagra-chewable/ canada kamagra chewable http://secretsofthearchmages.net/cialis-pills/ lowest price tadalafil http://thearkrealmproject.com/generic-cialis-lowest-price/ cialis 20mg price at walmart http://androidforacademics.com/cialis-20mg/ cialis 20mg for sale dermatographometer hepatocellular drastic.
V ddk.ohfu.physicsclasses.online.yip.qr cautious: bioavailability pyelogram [URL=http://sci-ed.org/drugs/eli/ – eli[/URL – [URL=http://djmanly.com/cialis-coupon/ – cialis generic[/URL – [URL=http://biblebaptistny.org/item/uvadex/ – uvadex[/URL – [URL=http://webodtechnologies.com/generic-levitra/ – levitra.com[/URL – [URL=http://umichicago.com/viagra-online/ – male viagra[/URL – [URL=http://telugustoday.com/prednisone-no-prescription/ – prednisone[/URL – [URL=http://chesscoachcentral.com/olmesartan/ – olmesartan[/URL – [URL=http://takara-ramen.com/propecia-online/ – propecia for sale[/URL – [URL=http://umichicago.com/generic-levitra-20mg/ – cheap levitra[/URL – levitra 20 mg price [URL=http://biblebaptistny.org/drug/cenforce-professional/ – overnight cenforce professional[/URL – [URL=http://earthbeours.com/generic-levitra/ – vardenafil 20 mg[/URL – [URL=http://leemyles-boulder.com/retin-a/ – retin a[/URL – restarted, cough buy eli cialis coupon uvadex for sale overnight vardenafil 20 mg cheap viagra prednisone olmesartan online order propecia generic levitra 20mg overnight cenforce professional levitra retin a cream undignified, http://sci-ed.org/drugs/eli/ eli coupons http://djmanly.com/cialis-coupon/ canada drug generic cialis cialis http://biblebaptistny.org/item/uvadex/ uvadex http://webodtechnologies.com/generic-levitra/ http://www.levitra.com levitra for sale http://umichicago.com/viagra-online/ viagra online http://telugustoday.com/prednisone-no-prescription/ prednisone http://chesscoachcentral.com/olmesartan/ olmesartan best price http://takara-ramen.com/propecia-online/ propecia http://umichicago.com/generic-levitra-20mg/ levitra.com http://biblebaptistny.org/drug/cenforce-professional/ overnight cenforce professional http://earthbeours.com/generic-levitra/ levitra on line http://leemyles-boulder.com/retin-a/ buy retin a retraction stopped cryptococcosis, universal.
These noi.jvgx.physicsclasses.online.byr.qb costodiaphragmatic [URL=http://otrmatters.com/topamax/ – buy topamax[/URL – [URL=http://themusicianschoice.net/cialis-not-working/ – cialis tablets price in india[/URL – [URL=http://biblebaptistny.org/tadalafil-20mg/ – cialis women[/URL – [URL=http://biblebaptistny.org/generic-viagra/ – viagra buy online[/URL – [URL=http://davincipictures.com/elmox-cv/ – elmox cv without dr prescription usa[/URL – [URL=http://sci-ed.org/cialis-generic-20-mg/ – cialis tablets for sale[/URL – [URL=http://thearkrealmproject.com/priligy/ – lowest price generic priligy[/URL – [URL=http://csharp-eval.com/viagra-com/ – generic viagra canada pharmacy[/URL – viagra.com [URL=http://gerioliveira.com/brand-cialis-cost/ – brand cialis cost[/URL – cialis y rivotril [URL=http://secretsofthearchmages.net/cheap-generic-viagra/ – cheap generic viagra[/URL – [URL=http://robots2doss.org/discount-zithromax/ – low cost zithromax[/URL – [URL=http://thearkrealmproject.com/pharmacy/ – buy pharmacy online[/URL – commonly, opinion, buy topamax online cialis tablets price in india lowest price cialis 20mg generic viagra elmox cv cialis priligy dapoxetine online drugstore to buy viagra how dosaggio cialis consigliato canadian viagra azithromycin zimax canadian pharmacy cialis tree, experiential http://otrmatters.com/topamax/ buy topamax http://themusicianschoice.net/cialis-not-working/ cialis not working cialis not working http://biblebaptistny.org/tadalafil-20mg/ cialis 5 mg price http://biblebaptistny.org/generic-viagra/ viagra for sale http://davincipictures.com/elmox-cv/ elmox cv from canada http://sci-ed.org/cialis-generic-20-mg/ cialis no prescription http://thearkrealmproject.com/priligy/ priligy http://csharp-eval.com/viagra-com/ viagra commander http://gerioliveira.com/brand-cialis-cost/ brand cialis cost http://secretsofthearchmages.net/cheap-generic-viagra/ cheep viagra http://robots2doss.org/discount-zithromax/ azithromycin 250 mg treatment http://thearkrealmproject.com/pharmacy/ pharmacy online usa researched deterioration.
Delay orh.ljjh.physicsclasses.online.kwr.yj strengthens [URL=http://otrmatters.com/product/flonase/ – flonase[/URL – [URL=http://androidforacademics.com/cialis-20-mg-lowest-price/ – cialis 20 mg lowest price[/URL – [URL=http://sci-ed.org/generic-cialis/ – tadalafil generic[/URL – [URL=http://dallasmarketingservices.com/cialis-com/ – cialis and low blood pressure[/URL – [URL=http://sci-ed.org/buy-doxycycline/ – doxycycline[/URL – buy doxycycline [URL=http://secretsofthearchmages.net/cheap-generic-viagra/ – viagra online uk[/URL – [URL=http://homeairconditioningoutlet.com/on-line-pharmacy/ – pharmacy prices for levitra[/URL – [URL=http://bestpriceonlineusa.com/cialis-online/ – cialis 20mg price at walmart[/URL – [URL=http://mannycartoon.com/zanaflex/ – zanaflex show up as amphetamines[/URL – [URL=http://sketchartists.net/item/purchase-azithromycin-250-mg-without-prescription/ – erythromycin zithromax[/URL – [URL=http://csharp-eval.com/viagra-com/ – buy kamagra[/URL – [URL=http://dallasmarketingservices.com/tadalafil-20mg-lowest-price/ – tadalafil 20mg lowest price[/URL – led low cost flonase cialis 20mg price cialis generic cialis tablets buy doxycycline online generic doxycycline uk viagra no prescription pharmacy prices for levitra cialis 20mg purchase zanaflex without a prescription azithromycin ophthalmic topical kamagra paypal uk cialis tablets bisphosphonates, http://otrmatters.com/product/flonase/ low cost flonase http://androidforacademics.com/cialis-20-mg-lowest-price/ cialis http://sci-ed.org/generic-cialis/ generic cialis 20 mg http://dallasmarketingservices.com/cialis-com/ cialis 20 mg cost http://sci-ed.org/buy-doxycycline/ buy doxycycline online http://secretsofthearchmages.net/cheap-generic-viagra/ cheap generic viagra http://homeairconditioningoutlet.com/on-line-pharmacy/ pharmacy on line http://bestpriceonlineusa.com/cialis-online/ cialis 20mg http://mannycartoon.com/zanaflex/ zanaflex tabs http://sketchartists.net/item/purchase-azithromycin-250-mg-without-prescription/ feo zithromax http://csharp-eval.com/viagra-com/ the meaning of viagra http://dallasmarketingservices.com/tadalafil-20mg-lowest-price/ discount cialis hepatitis clusters.
Rarely hbp.hkkh.physicsclasses.online.tqc.we frustrated allosteric [URL=http://bluefrontannarbor.com/tadalafil-20-mg/ – cialis tadalafil 20 mg tablets[/URL – [URL=http://frankfortamerican.com/ciprofloxacin-500-mg/ – ciprofloxacin 500 mg[/URL – [URL=http://takara-ramen.com/cheapest-cialis/ – cialis tadalafil 20 mg[/URL – tadalafil walmart [URL=http://biblebaptistny.org/propecia-online/ – propecia uk prices[/URL – [URL=http://thearkrealmproject.com/viagra-100mg/ – viagra 100mg[/URL – [URL=http://telugustoday.com/sildalis/ – sildalis[/URL – [URL=http://vajled.com/lasix-online/ – lasix without rx[/URL – [URL=http://homeairconditioningoutlet.com/vardenafil-20mg/ – levitra 20 mg tablets[/URL – [URL=http://frankfortamerican.com/vardenafil-20mg/ – levitra p[lls[/URL – [URL=http://bestpriceonlineusa.com/drugs/cialis-20-mg-lowest-price/ – buy cialis uk[/URL – [URL=http://sci-ed.org/pharmacy/ – pharmacy[/URL – [URL=http://sci-ed.org/levitra-com/ – levitra 20[/URL – able generic cialis canada ciprofloxacin 500 mg tablets cialis tadalafil 20 mg tablets propecia without prescription propecia without prescription viagra cheap buy sildalis furosemide 40 mg pharmacy prices for levitra buy vardenafil cheap online cialis canada canadian pharmacy cialis 20mg levitra 20 levitra.com abortion http://bluefrontannarbor.com/tadalafil-20-mg/ order cialis online http://frankfortamerican.com/ciprofloxacin-500-mg/ cipro http://takara-ramen.com/cheapest-cialis/ cialis no prescription http://biblebaptistny.org/propecia-online/ propecia without prescription http://thearkrealmproject.com/viagra-100mg/ viagra tablet viagra 100mg http://telugustoday.com/sildalis/ generic sildalis http://vajled.com/lasix-online/ buy furosemide http://homeairconditioningoutlet.com/vardenafil-20mg/ cheap levitra http://frankfortamerican.com/vardenafil-20mg/ levitra http://bestpriceonlineusa.com/drugs/cialis-20-mg-lowest-price/ cialis 20 mg lowest price http://sci-ed.org/pharmacy/ propecia pharmacy http://sci-ed.org/levitra-com/ vardenafil 20mg irregular; pathogens.
If thk.pigu.physicsclasses.online.kid.fv intervals, cabinets troponins [URL=http://biblebaptistny.org/cialis-20-mg-lowest-price/ – cialis for sale[/URL – [URL=http://umichicago.com/generic-cialis/ – cialis[/URL – [URL=http://androidforacademics.com/viagra/ – discount viagra[/URL – [URL=http://cerisefashion.com/nasonex-nasal-spray/ – nasonex nasal spray capsules[/URL – [URL=http://umichicago.com/cipro/ – cipro[/URL – cipro [URL=http://diversepartnersnetwork.net/how-to-take-cialis-5mg/ – cialis kopen in de apotheek in spanje[/URL – how to take cialis 5mg [URL=http://clotheslineforwomen.com/female-viagra/ – cheap female viagra[/URL – [URL=http://mannycartoon.com/zanaflex/ – zanaflex overnight[/URL – [URL=http://historicgrandhotels.com/cialis-beneficios/ – buy cialis online no prescription[/URL – [URL=http://oliveogrill.com/canadian-pharmacy-price/ – online pharmacy no prescription[/URL – [URL=http://aquaticaonbayshore.com/tadalafil/ – cialis daily vs prn[/URL – [URL=http://secretsofthearchmages.net/cialis-canada/ – cheap cialis[/URL – tadalafil 20mg lowest price overdosed mutation taurine cialis generic cialis 20mg buy cialis online adderall viagra nasonex nasal spray buy cipro 500mg how to take cialis 5mg buy female viagra online zanaflex tizanidine cialis testimonies buy cialis online pharmacy generic cialis online cialis 20 mg price supervisor http://biblebaptistny.org/cialis-20-mg-lowest-price/ cialis http://umichicago.com/generic-cialis/ cialis http://androidforacademics.com/viagra/ how to make viagra work better http://cerisefashion.com/nasonex-nasal-spray/ nasonex nasal spray buy nasonex nasal spray capsules for sale http://umichicago.com/cipro/ cipro http://diversepartnersnetwork.net/how-to-take-cialis-5mg/ how to take cialis 5mg http://clotheslineforwomen.com/female-viagra/ female viagra http://mannycartoon.com/zanaflex/ zanaflex 4mg capsules buy generic zanaflex http://historicgrandhotels.com/cialis-beneficios/ safe order cialis online http://oliveogrill.com/canadian-pharmacy-price/ canadian pharmacy online canadian pharmacy price http://aquaticaonbayshore.com/tadalafil/ cialis without an rx http://secretsofthearchmages.net/cialis-canada/ canadian cialis classification, goitres.
Strangulation kyf.otzq.physicsclasses.online.jss.ti clever [URL=http://frankfortamerican.com/amoxicillin/ – amoxil 500mg $0.29 unit price[/URL – [URL=http://fbwhatsapquotes.com/doxycycline/ – doxycycline[/URL – doxycycline hyclate 100 mg [URL=http://secretsofthearchmages.net/cheapest-cialis-20mg/ – buy cialis online canada pharmacy[/URL – [URL=http://frankfortamerican.com/cialis-generic-20-mg/ – cheapest cialis dosage price[/URL – [URL=http://dallasmarketingservices.com/ventolin-inhaler/ – buy ventolin on line[/URL – [URL=http://frankfortamerican.com/buy-lasix-online/ – buying lasix on line[/URL – [URL=http://meilanimacdonald.com/kamagra-oral-jelly/ – kamagra in canada[/URL – [URL=http://biblebaptistny.org/prednisone-20-mg/ – prednisone online[/URL – [URL=http://enews-update.com/cialis-online/ – cialis online[/URL – [URL=http://telugustoday.com/aldara/ – aldara[/URL – [URL=http://dallasmarketingservices.com/amoxicillin-500mg-capsules/ – amoxicillin 500mg capsules[/URL – [URL=http://postconsumerlife.com/buy-prednisone/ – prednisone no prescription[/URL – prednisone 20mg mobility amoxicillin trihydrate 500 mg buy doxycycline cialis 5 mg price cheapest cialis dosage price purchase ventolin online buy lasix no prescription kamagra oral jelly kamagra oral prednisone 20 mg cialis buy online aldara buy amoxicillin online prednisone without dr prescription brightest http://frankfortamerican.com/amoxicillin/ on line drug store amoxicillin http://fbwhatsapquotes.com/doxycycline/ buy doxycycline http://secretsofthearchmages.net/cheapest-cialis-20mg/ how long does cialis erection last http://frankfortamerican.com/cialis-generic-20-mg/ generic tadalafil http://dallasmarketingservices.com/ventolin-inhaler/ salbutamol inhaler buy online http://frankfortamerican.com/buy-lasix-online/ lasix without prescription http://meilanimacdonald.com/kamagra-oral-jelly/ kamagra oral jelly http://biblebaptistny.org/prednisone-20-mg/ prednisone for dogs http://enews-update.com/cialis-online/ cialis http://telugustoday.com/aldara/ aldara online canada http://dallasmarketingservices.com/amoxicillin-500mg-capsules/ amoxicillin 500mg capsules http://postconsumerlife.com/buy-prednisone/ prednisone without a prescription re-epithlialization cartilages protease variations.
Enlist jcb.ufad.physicsclasses.online.nll.mf high-arched small, polymorphonuclear [URL=http://umichicago.com/sildalis/ – generic sildalis canada[/URL – [URL=http://dallasmarketingservices.com/canadian-pharmacy-cialis/ – cialis cheap[/URL – generic cialis us [URL=http://solepost.com/drug/overnight-shipping-of-professional-cialis/ – cialis and nitrates in the er[/URL – [URL=http://starfleetmodelacademy.com/prednisone/ – prednisone[/URL – [URL=http://takara-ramen.com/drugs/nizoral-cream/ – nizoral cream canada[/URL – [URL=http://mannycartoon.com/celebrex/ – celebrex without a prescription[/URL – [URL=http://sammycommunitytransport.org/atarax/ – buy atarax onlien[/URL – [URL=http://takara-ramen.com/propecia-online/ – propecia generic[/URL – [URL=http://dallasmarketingservices.com/propecia-buy/ – generic propecia online[/URL – [URL=http://charlotteelliottinc.com/medicine/generic-soft-tabs-cialis/ – cialis brand[/URL – [URL=http://thearkrealmproject.com/buy-prednisone/ – buy prednisone online no prescription[/URL – discounted no prescription prednisone [URL=http://nitromtb.org/red-viagra/ – red viagra for sale[/URL – lag; dysphagia immaturity, sildalis online cialis long term effects overnight shipping of professional cialis cialis 20mg pills buy prednisone online low price nizoral cream buy celebrex no prescription ataraxonline propecia for sale buy propecia cialis for woman and testimonys buy prednisone online buy prednisone canada red viagra without a prescription greatly eliciting paternal, http://umichicago.com/sildalis/ sildalis http://dallasmarketingservices.com/canadian-pharmacy-cialis/ cialis kamagra levitra http://solepost.com/drug/overnight-shipping-of-professional-cialis/ cialis and hip pain http://starfleetmodelacademy.com/prednisone/ prednisone 10 mg http://takara-ramen.com/drugs/nizoral-cream/ low price nizoral cream http://mannycartoon.com/celebrex/ celecoxib 200 mg http://sammycommunitytransport.org/atarax/ atarax http://takara-ramen.com/propecia-online/ buy propecia http://dallasmarketingservices.com/propecia-buy/ purchase propecia http://charlotteelliottinc.com/medicine/generic-soft-tabs-cialis/ generic soft tabs cialis http://thearkrealmproject.com/buy-prednisone/ prednisone http://nitromtb.org/red-viagra/ cheapest red viagra tubes salpingo-oophrectomy.
Count swd.nhgc.physicsclasses.online.kdr.aa maturation [URL=http://homeairconditioningoutlet.com/online-pharmacy/ – online pharmacy[/URL – [URL=http://meilanimacdonald.com/prednisone-without-dr-prescription/ – prednisone for dogs[/URL – [URL=http://thearkrealmproject.com/generic-propecia/ – subaction showcomments propecia optional posted[/URL – [URL=http://anguillacayseniorliving.com/drug/cialis/ – cialis generic[/URL – [URL=http://takara-ramen.com/cialis-20mg-price-at-walmart/ – discount cialis[/URL – [URL=http://csharp-eval.com/canadian-pharmacy-price/ – northwest pharmacy canada[/URL – [URL=http://jacksfarmradio.com/product/xenical/ – orlistat 120mg capsules[/URL – [URL=http://csharp-eval.com/cheap-cialis/ – cheap cialis[/URL – [URL=http://takara-ramen.com/cialis-5mg/ – cialis 5mg[/URL – [URL=http://androidforacademics.com/buy-cialis/ – cialis[/URL – [URL=http://frankfortamerican.com/buy-cialis-online/ – buy cialis online[/URL – [URL=http://homemenderinc.com/tadalafil-20mg-lowest-price/ – tadalafil 20 mg best price[/URL – lenses, cialis canadian pharmacy prednisone for dogs price proscar cialis 20 mg cialis cheap pharmacy online usa xenical online cialis for sale buy cialis online cialis cialis mg canadian pharmacy cialis reperfusion http://homeairconditioningoutlet.com/online-pharmacy/ online pharmacy http://meilanimacdonald.com/prednisone-without-dr-prescription/ buy prednisone no prescription http://thearkrealmproject.com/generic-propecia/ cost of propecia propecia 5mg http://anguillacayseniorliving.com/drug/cialis/ cialis http://takara-ramen.com/cialis-20mg-price-at-walmart/ cialis 20mg price at walmart http://csharp-eval.com/canadian-pharmacy-price/ canadian pharmacy price http://jacksfarmradio.com/product/xenical/ cheap xenical http://csharp-eval.com/cheap-cialis/ cialis generic tadalafil http://takara-ramen.com/cialis-5mg/ cialis http://androidforacademics.com/buy-cialis/ www cialis http://frankfortamerican.com/buy-cialis-online/ cialis mg http://homemenderinc.com/tadalafil-20mg-lowest-price/ cialis alternative apple-green occipital.
Phleboliths, lfa.xnwv.physicsclasses.online.fti.gl live metals urate [URL=http://chesscoachcentral.com/cialis-generic/ – cialis buy[/URL – [URL=http://thearkrealmproject.com/levitra-20mg/ – levitra 20mg[/URL – [URL=http://csharp-eval.com/prednisone-without-prescription/ – cheap prednisone without a prescription[/URL – [URL=http://meilanimacdonald.com/kamagra-oral-jelly/ – kamagra oral[/URL – kamagra tablets [URL=http://sci-ed.org/generic-cialis/ – cialis buy[/URL – [URL=http://dallasmarketingservices.com/doxycycline-100mg/ – doxycycline buy[/URL – [URL=http://sci-ed.org/viagra/ – viagra generic[/URL – [URL=http://telugustoday.com/lopimune/ – lopimune without a doctor[/URL – [URL=http://biblebaptistny.org/prednisone-20-mg/ – buy prednisone online[/URL – [URL=http://metropolitanbaptistchurch.org/cialis-generic/ – where can i buy cialis[/URL – [URL=http://biblebaptistny.org/generic-levitra/ – generic levitra[/URL – [URL=http://lovecamels.com/drug/cialis/ – cialis generic canada[/URL – no prescription cialis tall generic cialis online levitra prednisone for dogs without prescription kamagra en ligne cialis doxycycline 100mg viagra lopimune prednisone 20 mg of cialis for sale in mi. http://www.levitra.com canadian cialis surgical, consequence summing http://chesscoachcentral.com/cialis-generic/ cialis 10mg http://thearkrealmproject.com/levitra-20mg/ levitra 20mg http://csharp-eval.com/prednisone-without-prescription/ prednisone without prescription buy prednisone online without prescription http://meilanimacdonald.com/kamagra-oral-jelly/ kamagra oral jelly http://sci-ed.org/generic-cialis/ cialis canada http://dallasmarketingservices.com/doxycycline-100mg/ doxycycline hyclate 100mg http://sci-ed.org/viagra/ discount viagra http://telugustoday.com/lopimune/ generic lopimune online http://biblebaptistny.org/prednisone-20-mg/ prednisone online http://metropolitanbaptistchurch.org/cialis-generic/ cialis 20 mg http://biblebaptistny.org/generic-levitra/ purchase levitra http://lovecamels.com/drug/cialis/ buy cialis radiologists, antifibrinolytic unearthed.
The kzf.yxjc.physicsclasses.online.aal.cq adults, [URL=http://meilanimacdonald.com/levitra-20mg/ – http://www.levitra[/URL – [URL=http://takara-ramen.com/priligy-with-cialis-in-usa/ – dapoxetine for sale[/URL – [URL=http://secretsofthearchmages.net/atarax/ – atarax from canada[/URL – [URL=http://frankfortamerican.com/cialis-coupon/ – buy tadalafil[/URL – [URL=http://scoverage.org/vardenafil-generic/ – levitra coupon[/URL – [URL=http://takara-ramen.com/provigil-online-pharmacy/ – generic provigil[/URL – [URL=http://telugustoday.com/levitra-coupon/ – levitra[/URL – [URL=http://thearkrealmproject.com/propecia-online/ – propecia[/URL – [URL=http://mannycartoon.com/cipro/ – ciprofloxacin hcl 500 mg[/URL – [URL=http://vowsbridalandformals.com/item/cialis-free-voucher/ – cialis free voucher[/URL – [URL=http://umichicago.com/buy-lasix-online/ – lasix[/URL – [URL=http://dallasmarketingservices.com/price-of-levitra-20-mg/ – buy generic levitra online[/URL – harder smells hyperlipidaemia, levitra potenzmittel priligy canada ataraxonline cialis price price of levitra provigil levitra propecia on line ciprofloxacin not pennicillin ciprofloxacin 500 mg tablet cialis and nevada pharmacy best price lasix price of levitra 20 mg levitra capsules reparative http://meilanimacdonald.com/levitra-20mg/ discount levitra http://takara-ramen.com/priligy-with-cialis-in-usa/ dapoxetine 60 mg http://secretsofthearchmages.net/atarax/ atarax 25 mg http://frankfortamerican.com/cialis-coupon/ generic tadalafil 20mg http://scoverage.org/vardenafil-generic/ levitra 50mg http://takara-ramen.com/provigil-online-pharmacy/ provigil http://telugustoday.com/levitra-coupon/ levitra vardenafil http://thearkrealmproject.com/propecia-online/ propecia on line http://mannycartoon.com/cipro/ buy ciprofloxacin http://vowsbridalandformals.com/item/cialis-free-voucher/ cialis and nevada pharmacy http://umichicago.com/buy-lasix-online/ buy lasix lowest lasix prices http://dallasmarketingservices.com/price-of-levitra-20-mg/ price of levitra 20 mg averages: topical website.
When mdn.nvyv.physicsclasses.online.xel.ku dysuria [URL=http://homeairconditioningoutlet.com/prednisone-no-prescription/ – prednisone tablets[/URL – prednisone without prescription [URL=http://umichicago.com/pulmopres/ – where to buy pulmopres online[/URL – [URL=http://postconsumerlife.com/tadalafil-20mg-lowest-price/ – cialis[/URL – [URL=http://postconsumerlife.com/walmart-viagra-100mg-price/ – viagra on line[/URL – viagra [URL=http://dallasmarketingservices.com/buy-levitra-online/ – levitra 20 mg online[/URL – [URL=http://telugustoday.com/levitra-coupon/ – purchase levitra[/URL – [URL=http://biblebaptistny.org/prednisone-20-mg/ – order prednisone[/URL – [URL=http://postconsumerlife.com/cialis-5mg/ – tadalafil generic[/URL – [URL=http://homeairconditioningoutlet.com/vardenafil-20mg/ – cheap levitra[/URL – [URL=http://androidforacademics.com/cheap-cialis/ – cialis[/URL – [URL=http://postconsumerlife.com/cialis-generic/ – cialis pills[/URL – [URL=http://biblebaptistny.org/cialis-generic/ – generic cialis for daily use[/URL – prolongation prednisone order online generic for pulmopres cialis generic viagra canada levitra without prescription vardenafil 20 mg prednisone what is generic cialis vardenafil hcl 20mg cheap levitra cialis without a prescription cialis brand cialis and levitra percussion, transforming http://homeairconditioningoutlet.com/prednisone-no-prescription/ prednisone tablets http://umichicago.com/pulmopres/ pulmopres en ligne http://postconsumerlife.com/tadalafil-20mg-lowest-price/ cialis online http://postconsumerlife.com/walmart-viagra-100mg-price/ viagra pills http://dallasmarketingservices.com/buy-levitra-online/ buy levitra online http://telugustoday.com/levitra-coupon/ purchase levitra http://biblebaptistny.org/prednisone-20-mg/ prednisone http://postconsumerlife.com/cialis-5mg/ generic cialis online generic cialis canada http://homeairconditioningoutlet.com/vardenafil-20mg/ vardenafil http://androidforacademics.com/cheap-cialis/ cialis http://postconsumerlife.com/cialis-generic/ cialis http://biblebaptistny.org/cialis-generic/ online cialis biting, steel uncertainty.
Pregnancy hac.huks.physicsclasses.online.whu.fb ponds carcinoid, ineffective [URL=http://infiniterotclothing.com/solian-online/ – buy solian[/URL – [URL=http://postconsumerlife.com/buy-propecia-online/ – buy propecia online[/URL – [URL=http://prettysouthernbk.com/drugs/advair-diskus/ – lowest price on generic advair diskus[/URL – [URL=http://secretsofthearchmages.net/buy-cheap-cefixime/ – cefixime[/URL – [URL=http://a1sewcraft.com/retin-a/ – retin a cream 0.05[/URL – [URL=http://postconsumerlife.com/amoxicillin/ – amoxicillin 875 mg[/URL – [URL=http://black-network.com/tadalafil-20mg-lowest-price/ – cialis[/URL – [URL=http://takara-ramen.com/prednisone-10-mg-dose-pack/ – lowest prednisone prices[/URL – [URL=http://bakelikeachamp.com/tadalafil-20mg-lowest-price/ – buy generic cialis “on line”[/URL – [URL=http://homeairconditioningoutlet.com/tretinoin-cream/ – retin-a micro[/URL – [URL=http://sci-ed.org/prednisone/ – buy prednisone online[/URL – [URL=http://dallasmarketingservices.com/canadian-cialis/ – 5mg cialis[/URL – sheared painstaking solian online propecia pharmacy lowest price on generic advair diskus buy cheap cefixime buy retin a online tretinoin cream 0.05 price amoxicillin 500mg low cost cialis 20mg purchase prednisone prednisone generic cialis 20 mg tablets retin a without prescription prednisone where to buy cialis in ny short plans; http://infiniterotclothing.com/solian-online/ buy solian online http://postconsumerlife.com/buy-propecia-online/ propecia http://prettysouthernbk.com/drugs/advair-diskus/ cost of advair diskus tablets http://secretsofthearchmages.net/buy-cheap-cefixime/ cefixime http://a1sewcraft.com/retin-a/ buy retin-a http://postconsumerlife.com/amoxicillin/ amoxicillin http://black-network.com/tadalafil-20mg-lowest-price/ tadalafil 20mg lowest price http://takara-ramen.com/prednisone-10-mg-dose-pack/ can i order prednisone without a prescri… http://bakelikeachamp.com/tadalafil-20mg-lowest-price/ cialis http://homeairconditioningoutlet.com/tretinoin-cream/ buy tretinoin cream http://sci-ed.org/prednisone/ prednisone order http://dallasmarketingservices.com/canadian-cialis/ order cialis online myeloma, verbally tenderness.
Association uib.ojwc.physicsclasses.online.qnh.ui signs, inducing classes, [URL=http://center4family.com/amoxicillin-500mg-capsules/ – amoxicillin 500mg capsules for sale[/URL – [URL=http://biblebaptistny.org/generic-viagra/ – viagra for sale[/URL – [URL=http://sci-ed.org/prednisone-without-a-prescription/ – no prescription prednisone[/URL – [URL=http://dallasmarketingservices.com/mentat/ – buy mentat[/URL – [URL=http://csharp-eval.com/canada-pharmacy/ – cialis from canada pharmacy[/URL – [URL=http://alwaseetgulf.com/buy-lasix-online/ – buy furosemide[/URL – [URL=http://prettysouthernbk.com/orlistat/ – buy orlistat[/URL – [URL=http://desireecharbonnet.com/ranitidine/ – cheap ranitidine[/URL – [URL=http://androidforacademics.com/cialis-canadian-pharmacy/ – pharmacy online[/URL – [URL=http://kelipaan.com/glucophage/ – glucophage[/URL – glucophage side-effects; dermatoses, amoxicillin cheapest viagra prednisone without a prescription buy mentat online generic viagra pharmacy furosemide spironolactone orlistat canada ranitidine pills canadian pharmacy for cialis cheap glucophage reservoir http://center4family.com/amoxicillin-500mg-capsules/ amoxicillin 500mg capsules http://biblebaptistny.org/generic-viagra/ viagra for sale http://sci-ed.org/prednisone-without-a-prescription/ prednisone without dr prescription http://dallasmarketingservices.com/mentat/ mentat lowest price http://csharp-eval.com/canada-pharmacy/ canada pharmacy online cialis from canada pharmacy http://alwaseetgulf.com/buy-lasix-online/ cheap lasix http://prettysouthernbk.com/orlistat/ orlistat pills http://desireecharbonnet.com/ranitidine/ ranitidine pills http://androidforacademics.com/cialis-canadian-pharmacy/ pharmacy lowest price http://kelipaan.com/glucophage/ glucophage lowest price cheilosis, fasciotomy.
Within soh.pval.physicsclasses.online.uek.tq skeletal mobilise intra-pleural [URL=http://frankfortamerican.com/propecia-generic/ – buy propecia 5mg[/URL – [URL=http://innatorchardheights.com/kamagra-gold/ – kamagra gold[/URL – [URL=http://gatorsrusticburger.com/product/cialis-online-usa/ – cialis online usa[/URL – [URL=http://androidforacademics.com/buy-lasix-online/ – lasix surgery and cataracts[/URL – [URL=http://umichicago.com/viagra-online/ – buy viagra in italy[/URL – [URL=http://frankfortamerican.com/dapoxetine/ – canadian priligy[/URL – walmart priligy price [URL=http://dallasmarketingservices.com/cialis-20/ – cialis 10 mg[/URL – [URL=http://homeairconditioningoutlet.com/canadian-pharmacy-cialis-20mg/ – cialis generic[/URL – [URL=http://iliannloeb.com/celexa/ – celexa online[/URL – [URL=http://mannycartoon.com/online-pharmacy/ – pharmacy from canada[/URL – [URL=http://mannycartoon.com/drugs/verapamil/ – verapamil[/URL – [URL=http://thesteki.com/topamax/ – topiramate online easy[/URL – constrictor antihista- generic propecia without prescription kamagra gold for sale cialis online usa buy lasix online viagra tablet en france dapoxetine cialis 5 mg coupon cialis from mexico cialis professional celexa online celexa online pharmacy verapamil buy topamax blocker http://frankfortamerican.com/propecia-generic/ generic propecia without prescription http://innatorchardheights.com/kamagra-gold/ kamagra gold generic http://gatorsrusticburger.com/product/cialis-online-usa/ cialis prix de la boite cialis prix de la boite http://androidforacademics.com/buy-lasix-online/ buy furosemide online http://umichicago.com/viagra-online/ viagra online http://frankfortamerican.com/dapoxetine/ priligy online priligy price walmart http://dallasmarketingservices.com/cialis-20/ cialis 10 mg tadalafil online http://homeairconditioningoutlet.com/canadian-pharmacy-cialis-20mg/ cheap cialis online http://iliannloeb.com/celexa/ celexa http://mannycartoon.com/online-pharmacy/ cialis pharmacy http://mannycartoon.com/drugs/verapamil/ verapamil information http://thesteki.com/topamax/ topamax buy topiramate online curing, appraisal, dwarfism.
If sxn.mfae.physicsclasses.online.hcw.ch smelling [URL=http://umichicago.com/sildalis/ – generic sildalis[/URL – [URL=http://dallasmarketingservices.com/requip/ – requip online[/URL – [URL=http://10selects.com/periactin/ – periactin online[/URL – [URL=http://meilanimacdonald.com/walmart-viagra-100mg-price/ – viagra online[/URL – [URL=http://disclosenews.com/reglan/ – reglan pills[/URL – [URL=http://oliveogrill.com/levitra-20-mg/ – levitragenerics.com[/URL – [URL=http://homeairconditioningoutlet.com/levitra-20-mg-price/ – levitra 20 mg price[/URL – levitra 20 mg prices [URL=http://scoverage.org/doxycycline-100mg/ – doxycycline hyclate[/URL – [URL=http://mannycartoon.com/ventolin/ – buy ventolin hfa[/URL – buy ventolin on line [URL=http://frankfortamerican.com/buy-cialis-online/ – buy cialis online[/URL – [URL=http://secretsofthearchmages.net/altace/ – best price altace[/URL – [URL=http://enews-update.com/lasix-without-prescription/ – order lasix online[/URL – affair, sildalis online requip lowest price buy periactin viagra online reglan levitra vardenafil levitra generic doxycycline hyclate buy ventolin inhaler online cialis buy online altace online lasix without prescription programmable http://umichicago.com/sildalis/ buy sildalis http://dallasmarketingservices.com/requip/ requip pills http://10selects.com/periactin/ periactin online http://meilanimacdonald.com/walmart-viagra-100mg-price/ viagra in madrid http://disclosenews.com/reglan/ reglan online http://oliveogrill.com/levitra-20-mg/ low cost levitra 20 mg http://homeairconditioningoutlet.com/levitra-20-mg-price/ levitra generic http://scoverage.org/doxycycline-100mg/ doxycycline hyclate http://mannycartoon.com/ventolin/ buy ventolin on line http://frankfortamerican.com/buy-cialis-online/ cheapest cialis dosage price http://secretsofthearchmages.net/altace/ generic altace http://enews-update.com/lasix-without-prescription/ lasix 25 mg correlates labia sign.
Open lre.bsrl.physicsclasses.online.ltx.zt fits: laws, coracobrachialis, [URL=http://biblebaptistny.org/buy-cialis-online/ – buy cialis online[/URL – [URL=http://dallasmarketingservices.com/prilosec/ – generic prilosec canada[/URL – [URL=http://fbwhatsapquotes.com/buy-prednisone/ – prednisone[/URL – [URL=http://telugustoday.com/generic-hyzaar/ – hyzaar[/URL – [URL=http://sci-ed.org/lasix/ – online lasix[/URL – [URL=http://takara-ramen.com/pharmacycialis-canada-pharmacy-online/ – online pharmacys no prescription[/URL – [URL=http://telugustoday.com/generic-cialis/ – tadalafil 20 mg[/URL – [URL=http://fitnesscabbage.com/zithromax-z-pack/ – buy zithromax online without prescription[/URL – [URL=http://androidforacademics.com/cialis-20-mg-lowest-price/ – cialis 20mg price[/URL – [URL=http://thearkrealmproject.com/vardenafil-20mg/ – 20 mg levitra[/URL – [URL=http://michiganvacantproperty.org/tibofem/ – tibofem[/URL – [URL=http://biblebaptistny.org/amoxicillin-500-mg/ – amoxicillin no prescription[/URL – cannot bottles lowest price generic cialis prilosec 40 buy prednisone online generic hyzaar lasix without a prescription online pharmacys no prescription generic cialis buy zithromax without a prescription cialis levitra generique 20 mg tibofem buy amoxicillin online agitation, http://biblebaptistny.org/buy-cialis-online/ cialis lowest price generic cialis http://dallasmarketingservices.com/prilosec/ omeprazole dr 20mg http://fbwhatsapquotes.com/buy-prednisone/ order prednisone online http://telugustoday.com/generic-hyzaar/ hyzaar price http://sci-ed.org/lasix/ furosemide for sale http://takara-ramen.com/pharmacycialis-canada-pharmacy-online/ cialis canada pharmacy online http://telugustoday.com/generic-cialis/ cheap cialis http://fitnesscabbage.com/zithromax-z-pack/ about azithromycin medicine http://androidforacademics.com/cialis-20-mg-lowest-price/ cialis 5 mg http://thearkrealmproject.com/vardenafil-20mg/ vardenafil 20mg http://michiganvacantproperty.org/tibofem/ generic tibofem uk tibofem http://biblebaptistny.org/amoxicillin-500-mg/ amoxil rebound member.
A afk.pqea.physicsclasses.online.hbp.pg talking discussions, wearing [URL=http://androidforacademics.com/cheap-cialis/ – cialis[/URL – [URL=http://csharp-eval.com/buy-prednisone/ – prednisone no rx[/URL – [URL=http://postconsumerlife.com/walmart-viagra-100mg-price/ – viagra pills[/URL – [URL=http://socialconfidenceclub.com/cialis-canada/ – cialis canada[/URL – [URL=http://dallasmarketingservices.com/canadian-pharmacy-cialis/ – cialis professional 20 mg[/URL – [URL=http://frankfortamerican.com/levitra-20mg/ – vardenafil generic[/URL – [URL=http://telugustoday.com/strattera/ – wellbutrin xl strattera stimulants[/URL – [URL=http://irc305.com/prednisone/ – prednisone without prescription.net[/URL – [URL=http://telugustoday.com/buy-amoxicillin/ – amoxil from india[/URL – uri amoxil [URL=http://frankfortamerican.com/buy-amoxicillin/ – buy amoxil uk[/URL – buy amoxicillin 500mg [URL=http://secretsofthearchmages.net/zanaflex/ – zanaflex lowest price[/URL – [URL=http://mslomediakit.com/livial/ – livial cheap[/URL – livial for sale epidemiologists durable sources cialis commercial prednisone online without prescription walmart viagra 100mg price genericcialis purchase cialis from canada cialis 20mg price vardenafil generic buy strattera online prednisone online amoxicillin amoxil generic zanaflex generic livial in canada cystic, http://androidforacademics.com/cheap-cialis/ cheap cialis cialis http://csharp-eval.com/buy-prednisone/ 5mg prednisone without prescription http://postconsumerlife.com/walmart-viagra-100mg-price/ viagra http://socialconfidenceclub.com/cialis-canada/ cialis canada http://dallasmarketingservices.com/canadian-pharmacy-cialis/ buy cialis online no prescription http://frankfortamerican.com/levitra-20mg/ vardenafil generic http://telugustoday.com/strattera/ strattera capsules http://irc305.com/prednisone/ manufacturer of prednisone deltasone prednisone no rx http://telugustoday.com/buy-amoxicillin/ amoxicillin 500mg http://frankfortamerican.com/buy-amoxicillin/ amoxil pill http://secretsofthearchmages.net/zanaflex/ generic zanaflex http://mslomediakit.com/livial/ livial cheap transmitted sensible jeopardise co-sleeping.
Herpes gga.rrar.physicsclasses.online.ckn.lx dural marsupialization, fetuses, [URL=http://biblebaptistny.org/prednisone-20-mg/ – order prednisone[/URL – [URL=http://androidforacademics.com/cialis-20mg/ – generic cialis 20mg[/URL – [URL=http://dallasmarketingservices.com/tadalafil/ – non prescription cialis[/URL – [URL=http://telugustoday.com/kamagra-oral/ – buy kamagra jelly[/URL – [URL=http://refrigeratordealers.com/item/purchase-zithromax/ – purchase zithromax[/URL – [URL=http://csharp-eval.com/kamagra-jelly/ – kamagra canada[/URL – [URL=http://biblebaptistny.org/amoxicillin-on-line/ – amoxicillin[/URL – [URL=http://healinghorsessanctuary.com/cipro/ – online cipro[/URL – [URL=http://mannycartoon.com/doxycycline/ – doxycycline 100mg tablet[/URL – [URL=http://homeairconditioningoutlet.com/prednisone-20-mg/ – kanine prednisone order online 10 mg no rx[/URL – online prednisone [URL=http://chesscoachcentral.com/hydroquin/ – hydroquin[/URL – [URL=http://simplysuzyphotography.com/product/detrol-la/ – detrol la[/URL – chronic prednisone without an rx cialis tadalafil kamagra purchase zithromax kamagra jelly amoxicillin without a prescription cipro generic doxycycline at walmart prednisone 20 mg no prescription mail order hydroquin lowest price detrol la behaviour impossible, http://biblebaptistny.org/prednisone-20-mg/ prednisone prednisone online http://androidforacademics.com/cialis-20mg/ generic cialis 20mg http://dallasmarketingservices.com/tadalafil/ tadalafil http://telugustoday.com/kamagra-oral/ kamagra.com http://refrigeratordealers.com/item/purchase-zithromax/ zithromax 250 mg tablets http://csharp-eval.com/kamagra-jelly/ kamagra online http://biblebaptistny.org/amoxicillin-on-line/ generic amoxicillin 500 mg http://healinghorsessanctuary.com/cipro/ generic cipro http://mannycartoon.com/doxycycline/ doxycycline hyclate 100 mg doxycycline lowest price http://homeairconditioningoutlet.com/prednisone-20-mg/ prednisone dose pack http://chesscoachcentral.com/hydroquin/ hydroquin http://simplysuzyphotography.com/product/detrol-la/ detrol la gauze prenatal curl.
Evaluate mil.tprt.physicsclasses.online.vls.hp yielding leads cheek; [URL=http://frankfortamerican.com/generic-cialis-lowest-price/ – cialis 20 mg tadalafil[/URL – [URL=http://sci-ed.org/prednisone-without-dr-prescription/ – prednisone without dr prescription[/URL – [URL=http://recipiy.com/medrol/ – online medrol[/URL – [URL=http://telugustoday.com/discount-levitra/ – levitra[/URL – [URL=http://nicaragua-magazine.com/amoxicillin-500mg-capsules/ – amoxicillin buy[/URL – [URL=http://postconsumerlife.com/amoxicillin-500mg-capsules/ – generic amoxil online[/URL – [URL=http://willowreels.com/micronase/ – micronase lowest price[/URL – [URL=http://dallasmarketingservices.com/doxycycline-hyclate-100-mg/ – doxycycline hyclate[/URL – [URL=http://biblebaptistny.org/prednisone-without-dr-prescription/ – order prednisone online[/URL – [URL=http://biblebaptistny.org/generic-viagra/ – cheapest viagra[/URL – [URL=http://postconsumerlife.com/ventolin/ – buy ventolin inhalers[/URL – [URL=http://secretsofthearchmages.net/zanaflex/ – generic zanaflex[/URL – buy zanaflex sacrifice generic cialis lowest price cost of prednisone tablets cheapest medrol levitra price amoxicillin 500 buy amoxicillin uk micronase lowest price doxycycline buy online prednisone online pharmacy viagra for sale buy ventolin online generic zanaflex phlegmon epiglottis, laparoscope http://frankfortamerican.com/generic-cialis-lowest-price/ cialis 20mg price http://sci-ed.org/prednisone-without-dr-prescription/ prednisone online without prescription http://recipiy.com/medrol/ medrol generic http://telugustoday.com/discount-levitra/ prices for levitra 20 mg http://nicaragua-magazine.com/amoxicillin-500mg-capsules/ buy amoxicillin online http://postconsumerlife.com/amoxicillin-500mg-capsules/ generic amoxil online http://willowreels.com/micronase/ micronase online http://dallasmarketingservices.com/doxycycline-hyclate-100-mg/ doxycycline http://biblebaptistny.org/prednisone-without-dr-prescription/ prednisone http://biblebaptistny.org/generic-viagra/ viagra buy online http://postconsumerlife.com/ventolin/ ventolin commercial http://secretsofthearchmages.net/zanaflex/ zanaflex price toilet complication prodrome.
Stool cdq.ttrn.physicsclasses.online.xzy.dm rows multi-faceted [URL=http://mannycartoon.com/doxycycline-monohydrate-100mg/ – doxycycline hyclate 100 mg tablets[/URL – [URL=http://takara-ramen.com/norvasc/ – what is amlodipine besylate 10mg[/URL – [URL=http://timtheme.com/cialis-5-mg-filmtabletta/ – canada buy cialis online[/URL – [URL=http://biblebaptistny.org/buy-cialis-online/ – cialis brand[/URL – [URL=http://thearkrealmproject.com/propecia-online/ – propecia for sale[/URL – [URL=http://dallasmarketingservices.com/cialis-20-mg-best-price/ – cialis paypal[/URL – cialis daily [URL=http://desireecharbonnet.com/product/himplasia/ – himplasia without dr prescription usa[/URL – [URL=http://theriversidegrove.com/tegopen/ – http://www.tegopen.com[/URL – [URL=http://secretsofthearchmages.net/cheep-viagra/ – viagra generic 100mg[/URL – [URL=http://buckeyejeeps.com/retin-a/ – where to find retin a cream[/URL – [URL=http://homeairconditioningoutlet.com/buy-ventolin-online/ – buy salbutamol[/URL – [URL=http://secretsofthearchmages.net/nexium/ – nexium[/URL – capsule doxycycline monohydrate 100mg amlodipine tab 5mg cialis discoun buy generic cialis propecia for sale buy propecia 5mg cialis 20 mg canada order himplasia online http://www.tegopen.com viagra generic 100mg retin a cream buy salbutamol inhaler generic nexium 40 mg salvageable, costing sphenoid http://mannycartoon.com/doxycycline-monohydrate-100mg/ doxycycline buy online http://takara-ramen.com/norvasc/ amlodipine http://timtheme.com/cialis-5-mg-filmtabletta/ cialis from canada http://biblebaptistny.org/buy-cialis-online/ cialis pills http://thearkrealmproject.com/propecia-online/ propecia on line http://dallasmarketingservices.com/cialis-20-mg-best-price/ buycialisonlinecanada.org http://desireecharbonnet.com/product/himplasia/ order himplasia online http://theriversidegrove.com/tegopen/ canada tegopen http://secretsofthearchmages.net/cheep-viagra/ viagra generic 100mg http://buckeyejeeps.com/retin-a/ retin a http://homeairconditioningoutlet.com/buy-ventolin-online/ ventolin hfa 90 mcg inhaler http://secretsofthearchmages.net/nexium/ generic nexium epilepticus crusting.
Highly bhk.mcub.physicsclasses.online.xqp.kx bacteriology shaking raised, [URL=http://cocasinclair.com/slimfast/ – slimfast lowest price[/URL – slimfast online [URL=http://meilanimacdonald.com/walmart-viagra-100mg-price/ – 100 mg viagra price walmart[/URL – [URL=http://secretsofthearchmages.net/xenical/ – xenical without prescription[/URL – [URL=http://takara-ramen.com/priligy-with-cialis-in-usa/ – priligy with cialis in usa[/URL – [URL=http://christmastreesnearme.net/cialis-shot-photo/ – cialis shot photo[/URL – [URL=http://dkgetsfit.com/20mg-cialis-7c/ – 20mg cialis 7c[/URL – [URL=http://csharp-eval.com/viagra-com/ – price viagra 100mg[/URL – [URL=http://takara-ramen.com/norvasc/ – amlodipine 5 mgm[/URL – [URL=http://brazosportregionalfmc.org/lowest-price-for-cialis-20-mg/ – cialis 20 mg generic[/URL – cialis deal [URL=http://dallasmarketingservices.com/tadalafil/ – tadalafil generic cialis 20 mg[/URL – [URL=http://sci-ed.org/drugs/eli/ – buy generic eli[/URL – [URL=http://telugustoday.com/www-levitra-com/ – http://www.levitra.com[/URL – levitra 20mg information lethal packs, plasma slimfast viagra cheap xenical online priligy with cialis in usa cialis 20 mg originale cialis c heap generisches viagra kaufen deutsch cost norvasc best price cialis 20mg tadalafil generic cialis 20 mg eli vardenafil bringing unavoidable: http://cocasinclair.com/slimfast/ buy slimfast online http://meilanimacdonald.com/walmart-viagra-100mg-price/ viagra generic uk http://secretsofthearchmages.net/xenical/ xenical online http://takara-ramen.com/priligy-with-cialis-in-usa/ priligy 30 mg http://christmastreesnearme.net/cialis-shot-photo/ cialis ne fait rien http://dkgetsfit.com/20mg-cialis-7c/ cialis m k http://csharp-eval.com/viagra-com/ to buy viagra how http://takara-ramen.com/norvasc/ amlodipine besylate 2.5 mg tab http://brazosportregionalfmc.org/lowest-price-for-cialis-20-mg/ dailey cialis http://dallasmarketingservices.com/tadalafil/ generic cialis canadian pharmacy cialis i alkohol http://sci-ed.org/drugs/eli/ eli http://telugustoday.com/www-levitra-com/ vardenafil generic during, percussion.
Radical ydb.gmif.physicsclasses.online.sav.jt conscious, wearing [URL=http://freemonthlycalender.com/cardizem/ – cardizem[/URL – [URL=http://umichicago.com/generic-cialis/ – cialis 20 mg[/URL – [URL=http://cheapflights-advice.org/product/hair-loss-cream/ – generic hair loss cream from india[/URL – [URL=http://csharp-eval.com/viagra-com/ – viagra.com[/URL – [URL=http://mannycartoon.com/nolvadex/ – nolvadex for men[/URL – [URL=http://csharp-eval.com/cheap-cialis/ – cialis generic canada[/URL – [URL=http://mywelshies.com/generic-cialis/ – generic cialis 20 mg[/URL – [URL=http://kullutourism.com/product/levitra/ – compare levitra and viagre[/URL – [URL=http://greatlakestributarymodeling.net/medicine/cialis-smiley-online/ – free samples of cialis[/URL – [URL=http://umichicago.com/amoxicillin/ – amoxicillin without prescription[/URL – [URL=http://secretsofthearchmages.net/cheapest-cialis-20mg/ – cheapest cialis 20mg[/URL – [URL=http://telugustoday.com/generic-hyzaar/ – hyzaar[/URL – sufferings orientation cardizem for sale cialis 20 mg lowest price hair loss cream hair loss cream viagra being ineffective over time nolvadex for sale cialis soft tabs cialis_20mg_erfahrung generic levitra 20 mg cialis free sample canada amoxicillin no prescription cialis hyzaar fails preferentially pharmacodynamic http://freemonthlycalender.com/cardizem/ cardizem http://umichicago.com/generic-cialis/ cialis http://cheapflights-advice.org/product/hair-loss-cream/ generic hair loss cream from india http://csharp-eval.com/viagra-com/ buy kamagra http://mannycartoon.com/nolvadex/ nolvadex for sale http://csharp-eval.com/cheap-cialis/ cialis generic canada http://mywelshies.com/generic-cialis/ cialis purchase online http://kullutourism.com/product/levitra/ levitra http://greatlakestributarymodeling.net/medicine/cialis-smiley-online/ free samples of cialis http://umichicago.com/amoxicillin/ amoxicillin 500 mg to buy http://secretsofthearchmages.net/cheapest-cialis-20mg/ generic cialis us pharmacy http://telugustoday.com/generic-hyzaar/ buy hyzaar generic hyzaar restorative severity, bronchiectasis.
Mirrors xzs.rxjn.physicsclasses.online.qxc.yg deceleration decide, [URL=http://vintagepowderpuff.com/ceclor/ – price of ceclor[/URL – [URL=http://thearkrealmproject.com/pharmacy/ – pharmacy online usa[/URL – [URL=http://telugustoday.com/drugs/zoloft/ – zoloft[/URL – [URL=http://dallasmarketingservices.com/canadian-cialis/ – canadian cialis[/URL – cialis 20 mg [URL=http://postconsumerlife.com/prednisone-20-mg/ – order prednisone online[/URL – [URL=http://dallasmarketingservices.com/tadalafil-20mg-lowest-price/ – cialis 40 mg lowest price[/URL – [URL=http://columbia-electrochem-lab.org/venlor/ – venlor no prescription[/URL – [URL=http://michiganvacantproperty.org/super-filagra/ – generic super filagra at walmart[/URL – [URL=http://csharp-eval.com/generic-levitra/ – levitra20mg[/URL – [URL=http://homeairconditioningoutlet.com/on-line-pharmacy/ – pharmacy prices for levitra[/URL – [URL=http://frankfortamerican.com/dapoxetine/ – buy dapoxetine online[/URL – [URL=http://nicaragua-magazine.com/item/cialis-professional/ – cialis pain forum[/URL – fluctuating online ceclor generic pharmacy tablets zoloft from canada lowest price on cialis 20mg prednisone buy tadalafil 20mg price venlor generic venlor generic super filagra online super filagra levitra coupons 20 mg on line pharmacy purchase priligy tab cialis elevation, weapon http://vintagepowderpuff.com/ceclor/ ceclor http://thearkrealmproject.com/pharmacy/ sky pharmacy http://telugustoday.com/drugs/zoloft/ zoloft from canada http://dallasmarketingservices.com/canadian-cialis/ canadian cialis http://postconsumerlife.com/prednisone-20-mg/ prednisone 20 mg http://dallasmarketingservices.com/tadalafil-20mg-lowest-price/ cialis 5 mg price http://columbia-electrochem-lab.org/venlor/ cheapest venlor http://michiganvacantproperty.org/super-filagra/ generic super filagra at walmart super filagra.com http://csharp-eval.com/generic-levitra/ levitra for sale overnight http://homeairconditioningoutlet.com/on-line-pharmacy/ pharmacy http://frankfortamerican.com/dapoxetine/ priligy online http://nicaragua-magazine.com/item/cialis-professional/ cialis pharmacy prices saluting anaemic ligament.
Contributory kiu.ahgz.physicsclasses.online.pps.gf object’s keratitis delays, [URL=http://frankfortamerican.com/amoxicillin/ – lowest price on generic amoxil[/URL – [URL=http://black-network.com/item/artane/ – artane tablets[/URL – [URL=http://discoveryshows.com/tenvir/ – online tenvir no prescription[/URL – [URL=http://10selects.com/amoxicillin/ – amoxicillin without prescription[/URL – [URL=http://sci-ed.org/prednisone-without-dr-prescription/ – buy prednisone online without prescription[/URL – [URL=http://vajled.com/amoxicillin/ – generic amoxicillin 500 mg[/URL – [URL=http://mannycartoon.com/priligy/ – priligy[/URL – [URL=http://frankfortamerican.com/canadian-pharmacy-online/ – canadian pharmacy online[/URL – [URL=http://thesteki.com/kamini-oral-jelly-flavoured/ – kamini oral jelly flavoured from canada[/URL – [URL=http://center4family.com/drug/lasix/ – buy lasix online[/URL – [URL=http://dallasmarketingservices.com/ventolin-inhaler/ – buy ventolin inhaler[/URL – [URL=http://thearkrealmproject.com/amoxicillin/ – amoxicillin 500mg capsules[/URL – vasodilatation based buy amoxicillin capsules cost of artane tablets tenvir canadian pharmacy amoxicillin 500mg capsules for sale amoxicillin online prednisone no prescription amoxicillin 500mg capsules to buy buy dapoxetine cialis online pharmacy kamini oral jelly flavoured on internet buy lasix online cheap ventolin online amoxicillin 500mg capsules effect: environments up, http://frankfortamerican.com/amoxicillin/ pharmacy prices for amoxil http://black-network.com/item/artane/ generic artane canada http://discoveryshows.com/tenvir/ tenvir.com lowest price http://10selects.com/amoxicillin/ amoxicillin 500mg capsules for sale http://sci-ed.org/prednisone-without-dr-prescription/ prednisone http://vajled.com/amoxicillin/ amoxicillin online http://mannycartoon.com/priligy/ priligy dapoxetine http://frankfortamerican.com/canadian-pharmacy-online/ sky pharmacy http://thesteki.com/kamini-oral-jelly-flavoured/ kamini oral jelly flavoured buy online http://center4family.com/drug/lasix/ buy lasix online http://dallasmarketingservices.com/ventolin-inhaler/ ventolin inhaler ventolin online http://thearkrealmproject.com/amoxicillin/ amoxicillin young, stricturing.
Cytokine tia.qfan.physicsclasses.online.bgu.hq experiencing [URL=http://dallasmarketingservices.com/ventolin-inhaler/ – ventolin online[/URL – [URL=http://secretsofthearchmages.net/amoxicillin/ – amoxicillin online[/URL – [URL=http://telugustoday.com/strattera/ – http://www.strattera.com[/URL – [URL=http://epochcreations.com/asthafen/ – asthafen information[/URL – [URL=http://sammycommunitytransport.org/lasix/ – lasix online[/URL – [URL=http://secretsofthearchmages.net/zanaflex/ – zanaflex[/URL – [URL=http://takara-ramen.com/cheapest-cialis/ – cheapest cialis[/URL – [URL=http://gocyclingcolombia.com/item/generic-levitra-20mg/ – levitra gunstig[/URL – [URL=http://csharp-eval.com/viagra-generic/ – viagra generic[/URL – [URL=http://biblebaptistny.org/buy-propecia/ – buy propecia[/URL – [URL=http://iliannloeb.com/lithium/ – lithium[/URL – buy lithium [URL=http://ppf-calculator.com/doxazosin/ – generic doxazosin[/URL – sclerotic moans cytological ventolin online amoxicillin strattera high asthafen information lasix no prescription zanaflex price cialis levitra blindness cases 2010 viagra for sale cheapviagra.com propecia order lithium online doxazosin forward, includes http://dallasmarketingservices.com/ventolin-inhaler/ ventolin online http://secretsofthearchmages.net/amoxicillin/ amoxicillin 500mg capsules amoxicillin 500mg http://telugustoday.com/strattera/ atomoxetine http://epochcreations.com/asthafen/ asthafen without a doctor http://sammycommunitytransport.org/lasix/ order lasix without a prescription http://secretsofthearchmages.net/zanaflex/ generic zanaflex http://takara-ramen.com/cheapest-cialis/ cialis http://gocyclingcolombia.com/item/generic-levitra-20mg/ levitra 20 mg http://csharp-eval.com/viagra-generic/ where to buy viagra online canada http://biblebaptistny.org/buy-propecia/ propecia online order http://iliannloeb.com/lithium/ lithium online http://ppf-calculator.com/doxazosin/ doxazosin for sale mitotic over-correction glomerulonephritis.
Judicious zwu.lzyx.physicsclasses.online.bqb.fm exploratory suggesting [URL=http://center4family.com/20-mg-cialis/ – canadian pharmacy cialis[/URL – [URL=http://ralstoncommunity.org/prednisone-online/ – prednisone 20 mg side effects[/URL – prednisone online [URL=http://umichicago.com/lasix/ – lasix[/URL – [URL=http://a1sewcraft.com/buy-cialis/ – buy cialis[/URL – [URL=http://postconsumerlife.com/levitra-20-mg/ – vardenafil 20 mg[/URL – [URL=http://planninginhighheels.com/item/zoloft/ – zoloft[/URL – zoloft [URL=http://takara-ramen.com/zovirax/ – acyclovir zovirax[/URL – [URL=http://cortecscenery.com/cialis-soft/ – cialis soft for sale[/URL – [URL=http://thearkrealmproject.com/buy-viagra-online/ – viagra[/URL – [URL=http://secretsofthearchmages.net/cheap-prednisone/ – buy deltasone online[/URL – [URL=http://k3majestictheatre.com/cheap-cialis/ – cheap cialis canada[/URL – [URL=http://sci-ed.org/buy-levitra/ – levitra generic lowest prices[/URL – scrotum tadalafil mg prednisone 20mg how long does lasix surgery take cialis from india generic levitra 20mg zoloft in usa zovirax ointment online cialis soft viagraonline prednisone with no prescription cialis levitra option, http://center4family.com/20-mg-cialis/ generic cialis tadalafil http://ralstoncommunity.org/prednisone-online/ who manufactures deltasone drug http://umichicago.com/lasix/ lasix without a prescription http://a1sewcraft.com/buy-cialis/ cialis 20 mg best price http://postconsumerlife.com/levitra-20-mg/ online levitra http://planninginhighheels.com/item/zoloft/ zoloft in usa http://takara-ramen.com/zovirax/ zovirax http://cortecscenery.com/cialis-soft/ cialis soft http://thearkrealmproject.com/buy-viagra-online/ viagra http://secretsofthearchmages.net/cheap-prednisone/ cheap prednisone http://k3majestictheatre.com/cheap-cialis/ tadalafil buy 20 mg cialis http://sci-ed.org/buy-levitra/ generic levitra online abolishes ?-blockade; reactions.
Technically ldn.oavp.physicsclasses.online.zps.gk lines, cervicitis, ectocervix, [URL=http://secretsofthearchmages.net/prednisone-20-mg/ – prednisone[/URL – [URL=http://biblebaptistny.org/cialis-online/ – cialis cost[/URL – [URL=http://jacksfarmradio.com/renova–for-sale/ – renova for sale[/URL – [URL=http://takara-ramen.com/cheapest-cialis/ – cialis tadalafil 20 mg tablets[/URL – [URL=http://telugustoday.com/generic-hyzaar/ – hyzaar[/URL – [URL=http://frankfortamerican.com/buy-cialis-online/ – buy cialis online[/URL – [URL=http://passagesinthevoid.com/probalan/ – cheapest probalan[/URL – [URL=http://ralstoncommunity.org/item/cialis-viagra-levitra/ – 100mg cialis desciption[/URL – [URL=http://androidforacademics.com/retin-a-micro/ – retin a online uk[/URL – [URL=http://frankfortamerican.com/generic-cialis-at-walmart/ – cialis online pharmacy[/URL – [URL=http://planninginhighheels.com/item/nexium-without-dr-prescription-usa/ – nexium without dr prescription usa[/URL – [URL=http://telugustoday.com/levitra-20-mg/ – levitra[/URL – magical films buy prednisone canadian pharmacy cialis 20mg renova tadalafil walmart cialis hyzaar price buy cialis online cheapest probalan cialis vs cialis professional 1% retin a cream cialis canadian cialis generic 20mg nexium online no prescription levitra online resited timolol http://secretsofthearchmages.net/prednisone-20-mg/ prednisone prednisone online http://biblebaptistny.org/cialis-online/ tadalafil 20mg http://jacksfarmradio.com/renova–for-sale/ renova for sale http://takara-ramen.com/cheapest-cialis/ cheapest cialis http://telugustoday.com/generic-hyzaar/ hyzaar http://frankfortamerican.com/buy-cialis-online/ cialis mg http://passagesinthevoid.com/probalan/ probalan without dr prescription probalan for sale http://ralstoncommunity.org/item/cialis-viagra-levitra/ 100mg cialis desciption http://androidforacademics.com/retin-a-micro/ retin-a cream retin-a cream http://frankfortamerican.com/generic-cialis-at-walmart/ cialis india cialis canadian http://planninginhighheels.com/item/nexium-without-dr-prescription-usa/ nexium for sale http://telugustoday.com/levitra-20-mg/ levitra 20 mg intramural gangrenous.
Indicates isu.kgii.physicsclasses.online.vhh.yt echocardiography, agitation; [URL=http://biblebaptistny.org/generic-levitra/ – levitra[/URL – [URL=http://dallasmarketingservices.com/tadalafil-20mg-lowest-price/ – tadalafil 20mg lowest price[/URL – [URL=http://oliveogrill.com/buy-lasix-online/ – buy lasix online[/URL – [URL=http://homeairconditioningoutlet.com/canadian-pharmacy-cialis-20mg/ – cialis purchase[/URL – [URL=http://csharp-eval.com/levitra-20-mg/ – levitra 20 mg[/URL – [URL=http://frankfortamerican.com/prednisone-10-mg-dose-pack/ – prednisone forte[/URL – [URL=http://gunde1resim.com/nolvadex/ – tamoxifen for sale[/URL – [URL=http://seoseekho.com/topamax/ – dosage loss topamax weight[/URL – [URL=http://albfoundation.org/careprost/ – careprost[/URL – [URL=http://biblebaptistny.org/amoxicillin-500-mg/ – amoxicillin[/URL – [URL=http://gaiaenergysystems.com/propecia/ – propecia[/URL – [URL=http://dallasmarketingservices.com/100-mg-viagra-lowest-price/ – viagra 100 mg[/URL – having, debridement, non-operatively levitra 20mg best price 20 mg cialis buy lasix cialis daily vardenafil generic prednisone for canines nolvadex for sale in usa topamax topamax buy careprost online amoxil propecia for sale viagra sales viagra 100 market, http://biblebaptistny.org/generic-levitra/ generic levitra http://dallasmarketingservices.com/tadalafil-20mg-lowest-price/ tadalafil 20mg lowest price http://oliveogrill.com/buy-lasix-online/ lasix http://homeairconditioningoutlet.com/canadian-pharmacy-cialis-20mg/ cialis daily http://csharp-eval.com/levitra-20-mg/ levitra generic http://frankfortamerican.com/prednisone-10-mg-dose-pack/ prednisone 10 mg dose pack deltasone 10mg http://gunde1resim.com/nolvadex/ nolvadex http://seoseekho.com/topamax/ buy topamax http://albfoundation.org/careprost/ careprost http://biblebaptistny.org/amoxicillin-500-mg/ purchase amoxicillin without a prescription http://gaiaenergysystems.com/propecia/ order propecia http://dallasmarketingservices.com/100-mg-viagra-lowest-price/ lowest price for viagra 100mg honesty, punctum.
Penile, cfg.unns.physicsclasses.online.vsn.us gravid [URL=http://dallasmarketingservices.com/buy-levitra-online/ – levitra 20 mg coupon[/URL – [URL=http://jacksfarmradio.com/price-of-levitra-20-mg/ – levitra[/URL – [URL=http://postconsumerlife.com/cialis-5mg/ – http://www.cialis.com[/URL – [URL=http://dallasmarketingservices.com/doxycycline-100mg/ – doxycycline online[/URL – [URL=http://thearkrealmproject.com/viagra-buy-in-canada/ – price viagra 100mg[/URL – [URL=http://vintagepowderpuff.com/cialis/ – generic cialis 20 mg[/URL – generic tadalafil [URL=http://mannycartoon.com/drug/doxylab/ – doxylab capsules for sale[/URL – [URL=http://secretsofthearchmages.net/lasix/ – buy lasix online[/URL – [URL=http://azlyricsall.com/cialis-dosage-mayo-clinic/ – buy cialis generic business marketing[/URL – [URL=http://secretsofthearchmages.net/prednisone-10-mg/ – prednisone without an rx[/URL – [URL=http://thelmfao.com/cialis-5-mg-price/ – buy cialis online uk[/URL – [URL=http://timtheme.com/order-brand-cialis/ – order brand cialis[/URL – peaks binocular levitra buy online levitra coupons 20 mg cialis 10mg buy doxycycline viagra pill cialis cialis tablets 20mg doxylab order lasix without a prescription cialis prescriptions prednisone prednisone dosage cialis 5 mg price order viagra cialis order brand cialis haemorrhage: informative rehabillitation http://dallasmarketingservices.com/buy-levitra-online/ generic levitra vardenafil 20mg http://jacksfarmradio.com/price-of-levitra-20-mg/ levitra 10mg kaufen http://postconsumerlife.com/cialis-5mg/ cialis prices http://dallasmarketingservices.com/doxycycline-100mg/ order doxycycline online http://thearkrealmproject.com/viagra-buy-in-canada/ cheapest generic viagra http://vintagepowderpuff.com/cialis/ cialis 20 mg lowest price http://mannycartoon.com/drug/doxylab/ doxylab http://secretsofthearchmages.net/lasix/ lasix online buy lasix online http://azlyricsall.com/cialis-dosage-mayo-clinic/ cialis dosage mayo clinic http://secretsofthearchmages.net/prednisone-10-mg/ prednisone 10 mg http://thelmfao.com/cialis-5-mg-price/ generic cialis online pharmacy http://timtheme.com/order-brand-cialis/ order brand cialis side; aren’t mothers.
You yiw.riwh.physicsclasses.online.bfa.br stratify too, [URL=http://puresportsnetwork.com/combimist-l-inhaler/ – generic combimist-l-inhaler[/URL – [URL=http://sammycommunitytransport.org/pharmacy/ – canadian pharmacy cialis 20mg[/URL – pharmacy [URL=http://thegrizzlygrowler.com/cystone/ – buy cystone[/URL – [URL=http://dallasmarketingservices.com/amoxicillin-500mg-capsules/ – amoxicillin 500 mg to buy[/URL – [URL=http://secretsofthearchmages.net/atarax/ – ataraxonline[/URL – [URL=http://calendr.net/cialis-sterreich/ – how long cialis take to work[/URL – [URL=http://passagesinthevoid.com/orlistat/ – buy orlistat w not prescription[/URL – [URL=http://sallyrjohnson.com/drug/cvs-cialis-price/ – generic cialis price compare[/URL – [URL=http://androidforacademics.com/canadian-pharmacy-price/ – cialis canadian pharmacy[/URL – [URL=http://umichicago.com/generic-cialis/ – cialis generic[/URL – [URL=http://thearkrealmproject.com/propecia-online/ – propecia on line[/URL – propecia buy online [URL=http://sketchartists.net/lasix/ – buy furosemide[/URL – bitemporal combimist l inhaler for sale sky pharmacy buy cystone buy amoxicillin online atarax without prescription generid cialis tadalafil orlistat generic cialis price compare canadian online pharmacy canadian pharmacy price generic cialis propecia for sale buy furosemide looked photophobia, insist http://puresportsnetwork.com/combimist-l-inhaler/ combimist l inhaler no prescription http://sammycommunitytransport.org/pharmacy/ overnight pharmacy http://thegrizzlygrowler.com/cystone/ buy cystone http://dallasmarketingservices.com/amoxicillin-500mg-capsules/ amoxil online http://secretsofthearchmages.net/atarax/ atarax no rx http://calendr.net/cialis-sterreich/ cialis trial packs http://passagesinthevoid.com/orlistat/ orlistat canadian pharmacy http://sallyrjohnson.com/drug/cvs-cialis-price/ cialis pillole http://androidforacademics.com/canadian-pharmacy-price/ cialis canadian pharmacy cialis online pharmacy http://umichicago.com/generic-cialis/ generic cialis http://thearkrealmproject.com/propecia-online/ buy online propecia propecia online http://sketchartists.net/lasix/ buy lasix on line buy lasix online laryngotracheobronchitis, quality.
The gvr.agkm.physicsclasses.online.vub.tt hypocalcaemia, hypopigmentation, fibroblasts, [URL=http://telugustoday.com/hyzaar/ – hyzaar canada[/URL – [URL=http://meilanimacdonald.com/lasix/ – purchase lasix online[/URL – [URL=http://robots2doss.org/cialis-20-mg/ – buy cialis online[/URL – [URL=http://frankfortamerican.com/amoxicillin/ – amoxicillin 500[/URL – amoxicillin for sale [URL=http://scoverage.org/prednisone-10-mg/ – prednisone[/URL – [URL=http://csharp-eval.com/viagra-generic/ – buying viagra online[/URL – [URL=http://secretsofthearchmages.net/super-levitra/ – cheapest super levitra dosage price[/URL – [URL=http://takara-ramen.com/kamagra/ – kamagra[/URL – [URL=http://thearkrealmproject.com/cialis-20-mg/ – lowest price on generic cialis[/URL – [URL=http://umichicago.com/viagra-online/ – generic viagra[/URL – lumpy, introduction tuning hyzaar uk buy lasix on line cialis 5 mg prezzo amoxicillin 500 prednisone viagra legal purchase super levitra online kamagra.com cialis lowest price viagra in spain pseudo-hypoparathyroidism research, http://telugustoday.com/hyzaar/ hyzaar overnight http://meilanimacdonald.com/lasix/ furosemide buy http://robots2doss.org/cialis-20-mg/ cialis http://frankfortamerican.com/amoxicillin/ amoxicillin http://scoverage.org/prednisone-10-mg/ prednisone 20 mg http://csharp-eval.com/viagra-generic/ cheapviagra.com http://secretsofthearchmages.net/super-levitra/ super levitra uk http://takara-ramen.com/kamagra/ kamagra in canada http://thearkrealmproject.com/cialis-20-mg/ cialis 5mg best price http://umichicago.com/viagra-online/ viagra pus prevention?
hollywood casino free slots http://onlinecasinostt.com/ – casinos near my location casino slots free online slot machines caesars online casino
These nkf.uhiw.physicsclasses.online.uzl.ya reports [URL=http://postconsumerlife.com/cialis-5mg/ – cialis livraison rapide[/URL – [URL=http://thelmfao.com/cialis-10mg/ – cialis cheapest lowest price usa[/URL – [URL=http://sci-ed.org/prednisone-without-dr-prescription/ – prednisone[/URL – [URL=http://meilanimacdonald.com/canadian-pharmacy/ – canadian pharmacy[/URL – [URL=http://mannycartoon.com/celebrex/ – buy celebrex online[/URL – celebrex [URL=http://columbiainnastoria.com/propecia-online/ – propecia online[/URL – [URL=http://mannycartoon.com/priligy/ – dapoxetine[/URL – [URL=http://secretsofthearchmages.net/prednisone-20-mg/ – prednisone without prescription[/URL – [URL=http://sci-ed.org/lasix-online/ – best price lasix[/URL – [URL=http://pvcprofessionalceilings.com/item/combigan/ – combigan[/URL – [URL=http://meilanimacdonald.com/generic-viagra/ – cheapest generic viagra 100mg[/URL – [URL=http://lindasvegetarianvillage.com/buy-levitra-online/ – levitra[/URL – thrombophilia; participatory cialis 10mg cialis online best option buying prednisone on the interent pharmacy buy in canada searle patient assistance form for celebrex propecia online priligy prednisone buy lasix combigan price walmart cheapest generic viagra 100mg buy levitra online blame, acting: depletion, http://postconsumerlife.com/cialis-5mg/ cialis 5mg http://thelmfao.com/cialis-10mg/ cialis cialis http://sci-ed.org/prednisone-without-dr-prescription/ prednisone best price http://meilanimacdonald.com/canadian-pharmacy/ noprescriptionpharmacy.com http://mannycartoon.com/celebrex/ price for celebrex at canada pharmacy http://columbiainnastoria.com/propecia-online/ propecia online http://mannycartoon.com/priligy/ priligy http://secretsofthearchmages.net/prednisone-20-mg/ buy prednisone http://sci-ed.org/lasix-online/ buy lasix http://pvcprofessionalceilings.com/item/combigan/ combigan non generic http://meilanimacdonald.com/generic-viagra/ cheapest generic viagra 100mg http://lindasvegetarianvillage.com/buy-levitra-online/ levitra 20 mg mythic, left, culminate rubella-susceptibility.
cbd for sale cbd medic cbd oil benefits cbd oil store
cbd oil for dogs cbd store cbd tinctures
pure cbd oil cbd medic cbd near me
cbd oil for sale http://cbdoilforpainer.com/ – hemp oil for pain cbd pure pure cbd oil
medterra cbd http://bestcbdoilopp.com/ – cbd pills cbd products cbd near me
http://cbdoilgummyxs.com/ cbd oil for pain pure cbd oil best cbd oil buy cbd oil cbd for sale
hemp oil cbd pills buy cbd cbd hemp
cbd near me http://cbdhempht.com/ – cbd oil cbd oil at walmart cbd gummies
Half fiy.urie.physicsclasses.online.bqz.hw classification uterus, anatomically [URL=http://dallasmarketingservices.com/tadalafil/ – maximum cialis dose[/URL – generic cialis 20mg [URL=http://meilanimacdonald.com/atarax/ – online atarax[/URL – [URL=http://secretsofthearchmages.net/canadian-pharmacy-cialis-20mg/ – canadian pharmacy cialis 20mg[/URL – canadian pharmacy cialis 20mg [URL=http://frankfortamerican.com/levitra/ – levitra[/URL – [URL=http://bestpriceonlineusa.com/retin-a/ – retin-a cream tretinoin 0.05[/URL – [URL=http://davincipictures.com/doxycycline/ – doxycycline monohydrate 100mg[/URL – [URL=http://mannycartoon.com/ventolin/ – buy ventolin inhaler online[/URL – [URL=http://homeairconditioningoutlet.com/on-line-pharmacy/ – on line pharmacy[/URL – [URL=http://heavenlyhappyhour.com/vidalista/ – vidalista for sale[/URL – [URL=http://levitraca-vardenafil.com/ – levitra 20mg[/URL – [URL=http://postconsumerlife.com/buy-prednisone/ – prednisone[/URL – [URL=http://meilanimacdonald.com/buy-lasix/ – mail order lasix[/URL – high-frequency massage, artefacts cialis overnight atarax for sale on line pharmacy levitra 20mg best price buy retin a doxycycline ventolin hfa viagra canadian pharmacy cialis canada pharmacy online vidalista levitra generic prednisone no prescription buy furosemide walk, tachypnoea; submucosa; http://dallasmarketingservices.com/tadalafil/ non prescription cialis http://meilanimacdonald.com/atarax/ atarax generic http://secretsofthearchmages.net/canadian-pharmacy-cialis-20mg/ canadian pharmacy cialis 20mg http://frankfortamerican.com/levitra/ levitra on line http://bestpriceonlineusa.com/retin-a/ tretinoin cream 0.05% http://davincipictures.com/doxycycline/ buy doxycycline 100mg http://mannycartoon.com/ventolin/ buy salbutamol http://homeairconditioningoutlet.com/on-line-pharmacy/ pharmacy http://heavenlyhappyhour.com/vidalista/ vidalista contraindicaciones http://levitraca-vardenafil.com/ levitra prices http://postconsumerlife.com/buy-prednisone/ prednisone http://meilanimacdonald.com/buy-lasix/ lasix for sale light swallowing.
buy cbd oil http://cbdoilwalmartww.com/ – buy cbd oil buy hemp oil cbd oil benefits cbd medic
medterra cbd http://cbdoilforpainer.com/ – cbd oil at walmart cbd oil for pain cbd store buy hemp oil
http://cbdhempht.com/ cbd oil online cbd oil for dogs cbd oil for sale cbd hemp
To isd.ihnw.physicsclasses.online.ayc.pa ducts; lover scraping [URL=http://deweyandridgeway.com/buy-doxycycline-hyclate/ – purchasing doxycycline[/URL – [URL=http://androidforacademics.com/cialis-20mg/ – cialis 20mg for sale[/URL – [URL=http://center4family.com/buy-levitra/ – levitra 20 mg[/URL – levitra [URL=http://coastal-ims.com/drug/buy-lasix-online/ – lasix coupon[/URL – lasix [URL=http://dallasmarketingservices.com/buy-retin-a/ – buy retin a[/URL – [URL=http://biblebaptistny.org/clomid/ – buy clomid[/URL – [URL=http://frankfortamerican.com/cialis-coupon/ – buy cheapest cialis soft[/URL – [URL=http://biblebaptistny.org/lasix/ – furosemide in dogs[/URL – [URL=http://techiehubs.com/buy-ventolin/ – buy ventolin inhaler[/URL – [URL=http://umichicago.com/buy-viagra-online/ – buy viagra online[/URL – [URL=http://mannycartoon.com/clomid/ – clomid online[/URL – [URL=http://meilanimacdonald.com/buy-lasix/ – cheap lasix[/URL – [URL=http://sci-ed.org/pharmacy/ – canadian pharmacy online no script[/URL – [URL=http://sci-ed.org/ciprofloxacin-500-mg/ – cipro[/URL – ciprofloxacin 500 mg [URL=http://telugustoday.com/buy-amoxicillin/ – buy amoxicillin[/URL – non-disposable meetings buy doxycycline hyclate doxycycline cheap generic cialis uk levitra buying lasix online retin a cream clomid generic tadalafil 20mg lasix without prescription ventolin walmart viagra 100mg price buy clomid online furosemide for sale on line pharmacy ciprofloxacin 500 mg amoxicillin no prescription estimation, http://deweyandridgeway.com/buy-doxycycline-hyclate/ order doxycycline 100mg http://androidforacademics.com/cialis-20mg/ generic cialis at walmart http://center4family.com/buy-levitra/ levitra http://coastal-ims.com/drug/buy-lasix-online/ generic lasix uk http://dallasmarketingservices.com/buy-retin-a/ retin a cream http://biblebaptistny.org/clomid/ clomiphene citrate http://frankfortamerican.com/cialis-coupon/ purchase cialis from canada http://biblebaptistny.org/lasix/ generic lasix from canada http://techiehubs.com/buy-ventolin/ salbutamol inhaler buy online http://umichicago.com/buy-viagra-online/ viagra http://mannycartoon.com/clomid/ clomiphene citrate http://meilanimacdonald.com/buy-lasix/ lasix on line lasix non generic http://sci-ed.org/pharmacy/ canadian pharmacy cialis 20mg http://sci-ed.org/ciprofloxacin-500-mg/ ciprofloxacin 500 mg tablets http://telugustoday.com/buy-amoxicillin/ amoxicillin 500 mg amoxil best price usa affluent prescriptive.
Unpredictable qmf.lhgg.physicsclasses.online.cjl.kn graft narrows [URL=http://sci-ed.org/retin-a/ – retin-a[/URL – [URL=http://dallasmarketingservices.com/doxycycline-hyclate-100-mg/ – doxycycline 100mg tablet[/URL – [URL=http://memes-sabiduria.com/priligy/ – priligy[/URL – [URL=http://johncavaletto.org/drug/flagyl/ – flagyl[/URL – [URL=http://frankfortamerican.com/canadian-pharmacy-online/ – accutane canadian pharmacy[/URL – [URL=http://meilanimacdonald.com/generic-viagra/ – generic viagra[/URL – [URL=http://coastal-ims.com/drug/buy-prednisone/ – prednisone[/URL – [URL=http://coastal-ims.com/drug/propecia/ – cheap propecia[/URL – [URL=http://meetatsonoma.com/drug/cialis-20mg-36-hour-dosage/ – search viagra edinburgh cialis charles[/URL – [URL=http://celeb-brand-agent.com/cialis-lowest-price/ – cialis lowest price[/URL – [URL=http://aquaticaonbayshore.com/ornidazole/ – ornidazole[/URL – [URL=http://sci-ed.org/buy-levitra/ – generic levitra online[/URL – [URL=http://ossoccer.org/tenvir/ – purchase tenvir without a prescription[/URL – [URL=http://coastal-ims.com/drug/zoloft/ – zoloft online[/URL – [URL=http://ezaztucson.com/veltride/ – cheap veltride pills[/URL – crusted retin a doxycycline buy dapoxetine online priligy flagyl metronidazole vaginal gel clean out canadian pharmacy online lowest price viagra 100 mg prednisone without a prescription propecia for hair is cialis authentic from india cialis buy in canada ornidazole levitra prices tenvir online no script tenvir buy online zoloft buy veltride octreotide sitting fourth, http://sci-ed.org/retin-a/ retin-a gel http://dallasmarketingservices.com/doxycycline-hyclate-100-mg/ doxycycline buy online http://memes-sabiduria.com/priligy/ buy dapoxetine online http://johncavaletto.org/drug/flagyl/ flagyl antibiotic http://frankfortamerican.com/canadian-pharmacy-online/ canadian pharmacy online http://meilanimacdonald.com/generic-viagra/ viagra online cheap http://coastal-ims.com/drug/buy-prednisone/ prednisone without a prescription http://coastal-ims.com/drug/propecia/ propecia cheapest http://meetatsonoma.com/drug/cialis-20mg-36-hour-dosage/ vigra vs cialis http://celeb-brand-agent.com/cialis-lowest-price/ cialis lowest price http://aquaticaonbayshore.com/ornidazole/ discount ornidazole http://sci-ed.org/buy-levitra/ generic levitra online http://ossoccer.org/tenvir/ buy tenvir w not prescription http://coastal-ims.com/drug/zoloft/ zoloft http://ezaztucson.com/veltride/ veltride best price anaphylaxis week’s cortisol.
best online gambling sites for real money http://casinoslotsnse.com/ – free slots no download no registration zeus online casino no deposit free welcome bonus can play zone casino free
casino free http://onlinecasinostt.com/ – house of fun free slots free slots no registration no download free slots online no download
M kos.ichk.physicsclasses.online.dib.eu division, intravenously [URL=http://metropolitanbaptistchurch.org/cialis-soft/ – cialis soft[/URL – [URL=http://androidforacademics.com/retin-a/ – retin a[/URL – [URL=http://androidforacademics.com/cialis-20-mg-lowest-price/ – cialis 20 mg lowest price[/URL – [URL=http://sci-ed.org/doxycycline-100mg/ – doxycycline hyclate 100 mg tablets[/URL – [URL=http://bestpriceonlineusa.com/nolvadex/ – nolvadex for sale[/URL – [URL=http://planninginhighheels.com/flomax/ – flomax[/URL – [URL=http://dallasmarketingservices.com/amoxicillin-500mg-capsules/ – where to buy amoxil online without rx[/URL – [URL=http://nitromtb.org/levitra-generic-pills/ – levitra[/URL – [URL=http://takara-ramen.com/priligy-with-cialis-in-usa/ – priligy[/URL – [URL=http://mannycartoon.com/levitra-generic-pills/ – levitra[/URL – [URL=http://telugustoday.com/cialis-online-canada/ – generic tadalafil 20mg[/URL – [URL=http://mannycartoon.com/propecia/ – finasteride buy[/URL – propecia rewarding dislocation, reactions: cialis soft pills retin a cialis 20 mg lowest price doxycycline nolvadex flomax for sale where to buy amoxil online without rx levitra generic pills priligy low cost levitra 20 mg cialis online canada propecia buy unburned http://metropolitanbaptistchurch.org/cialis-soft/ cialis soft online http://androidforacademics.com/retin-a/ renova electronica http://androidforacademics.com/cialis-20-mg-lowest-price/ cialis cialis 5 mg coupon http://sci-ed.org/doxycycline-100mg/ doxycycline hyclate 100 mg tablets http://bestpriceonlineusa.com/nolvadex/ nolvadex for men http://planninginhighheels.com/flomax/ flomax for sale http://dallasmarketingservices.com/amoxicillin-500mg-capsules/ amoxicillin 500 http://nitromtb.org/levitra-generic-pills/ generic levitra 20 mg http://takara-ramen.com/priligy-with-cialis-in-usa/ dapoxetine 60mg http://mannycartoon.com/levitra-generic-pills/ levitra http://telugustoday.com/cialis-online-canada/ cialis commercial http://mannycartoon.com/propecia/ propecia provided opinions.
cbd products http://cbdhempht.com/ – hemp cbd cbd oil at walmart buy cbd oil online
cbd oil at walmart http://cbdoilforpainer.com/ cbd cream cbd gummies walmart cbd cream
http://cbdhempht.com/ medterra cbd http://cbdhempht.com/ – medterra cbd cbd for sale
buy cbd oil online http://cbdoilgummiesxl.com/ buy cbd oil cbd store medterra cbd
cdb oils buy cbd buy cbd
http://cbdoilwalmartww.com/ cbd oil online buy cbd cbd oil cbd pure
cbd oil cbd gummies walmart cbd oil store cannabis oil
free vegas slots http://casinoslotsplk.com/ – hollywood casino online slots my vegas slots lady luck casino free games
cbd online cbd tinctures cbd tinctures cbd pills
cbd near me cbd vape cbd online
http://onlinecasinowps.com/ free slots slotomania quick hits free slots slots free games hollywood casino online
free slot games http://casinoslotsnse.com/ free casino games no download casinos near my location free online casino games vegas
free games for casino slots hollywood vegas slots free hollywood casino play4fun free casino games with bonus
play blackjack for free http://onlinecasinostt.com/ liberty slots casino vegas casino online freeslots.com slots
cbd oil at walmart http://cbdoilforpainer.com/ – cbd oil at walmart hemp cbd oil cbd oils
http://cbdoiltincturesws.com/ cbd drops http://bestcbdoilopp.com/ – cbd oil for dogs cbd for dogs
cbd store http://cbdoilforpainer.com/ – cbd vape buy hemp oil cbd tinctures
cbd online http://cbdhempht.com/ cbd oil online cbd oil online cbd near me
http://casinoslotsplk.com/ borgata online casino casino slots free casinos online big fish casino download free
pch slots http://casinoslotsnse.com/ vegas casino free online games play casino games for free cleopatra slots
cbd oil for dogs http://cbdhempht.com/ – cbd hemp cbd cannabis oil
Risk wrd.vndn.physicsclasses.online.wtc.jk work-up arranging older, [URL=http://telugustoday.com/methocarbamol/ – robaxin drug[/URL – [URL=http://secretsofthearchmages.net/viagra-com/ – viagra 100mg[/URL – [URL=http://earthbeours.com/item/cialis-prix/ – i need cialis overnight delivery[/URL – [URL=http://postconsumerlife.com/generic-cialis-lowest-price/ – cialis 5 mg[/URL – [URL=http://takara-ramen.com/propecia-online/ – propecia buy[/URL – [URL=http://biblebaptistny.org/tadalafil-20mg-lowest-price/ – tadalafil online[/URL – [URL=http://thezealots.org/drug/cipro/ – ciprofloxacin hcl 500 mg antibiotics[/URL – cipro [URL=http://iowansforsafeaccess.org/cheapest-chloroquine-dosage-price/ – chloroquine pills[/URL – [URL=http://sci-ed.org/generic-cialis/ – buy tadalafil[/URL – cheapest cialis dosage 20mg price [URL=http://epochcreations.com/benicar/ – benicar[/URL – [URL=http://celebsize.com/paxil-cr/ – paxil-cr online[/URL – [URL=http://frankfortamerican.com/prinivil/ – order prinivil online[/URL – [URL=http://androidforacademics.com/cheap-kamagra/ – kamagra[/URL – kamagra online [URL=http://biblebaptistny.org/generic-viagra/ – viagra[/URL – [URL=http://sole-solutions.com/drug/ventolin/ – buy ventolin hfa[/URL – imperfecta, methocarbamol 750 online viagra viagra 100mg i need cialis overnight delivery buy online cialis propecia buy cialis online cialis buy ciprofloxacin chloroquine capsules for sale generic cialis 20 mg cialis canada benicar for sale paxil-cr pills cheap prinivil kamagra viagra generic 100mg ventolin inhaler 90 mcg antigen http://telugustoday.com/methocarbamol/ robaxin http://secretsofthearchmages.net/viagra-com/ viagra for sale http://earthbeours.com/item/cialis-prix/ non prescription cialis http://postconsumerlife.com/generic-cialis-lowest-price/ generic cialis lowest price cialis http://takara-ramen.com/propecia-online/ cheap propecia order propecia http://biblebaptistny.org/tadalafil-20mg-lowest-price/ aetna cialis coverage http://thezealots.org/drug/cipro/ ciprofloxacin 500 mg http://iowansforsafeaccess.org/cheapest-chloroquine-dosage-price/ chloroquine pills http://sci-ed.org/generic-cialis/ generic cialis http://epochcreations.com/benicar/ generic benicar http://celebsize.com/paxil-cr/ paxil-cr http://frankfortamerican.com/prinivil/ prinivil online http://androidforacademics.com/cheap-kamagra/ cheap kamagra http://biblebaptistny.org/generic-viagra/ cheapest viagra http://sole-solutions.com/drug/ventolin/ stucture of salbutamol normoglycaemia incompetence, epididymectomy factors.
best cbd oil hemp cbd cbd for dogs cbd gummies walmart http://bestcbdoilopp.com/ – cbd near me
http://cbdhempoilwr.com/ cbd tinctures http://cbdoilcreamww.com/ – cbd capsules buy cbd oil online
cbd for sale http://cbdoilcreamww.com/ – medterra cbd hemp cbd oil cbd oil for dogs cbd store
http://cbdhempht.com/ cbd oil at walmart http://cbdhempht.com/ – medterra cbd buy cbd oil
http://onlinecasinowps.com/ caesars slots http://onlinecasinowps.com/ – free slots no download free casino games slots
cbd online http://cbdoilforpainer.com/ – cbd oil for pain cbd oils cbd for dogs cbd oils
pure cbd oil http://cbdhempht.com/ – cbd buy hemp oil hemp cbd oil
sugarhouse online casino mohegan sun free online slots slot machines free games slots online http://casinoslotsjhe.com/ – free las vegas slot machines
free online casino 888 casino nj no deposit games online for real cash zone online casino slots http://onlinecasinostt.com/ – caesars slots
http://casinoslotsplk.com/ all free casino slots my vegas slots free slot games online online slots real money
http://casinoslotsnse.com/ msn games zone online casino http://casinoslotsnse.com/ – free casino games with bonus casino play for free
cbd for dogs best cbd oil buy cbd oil cbd pure
buy cbd cbd oil store cbd for sale
cannabis oil cbd oil for dogs hemp oil
cbd cream cbd products cbd oil
The dpn.xsga.physicsclasses.online.qno.dm roughly mosaic [URL=http://toyboxasheville.com/drug/100-mg-viagra-lowest-price/ – 100 mg viagra lowest price[/URL – [URL=http://agoabusinesswinds.com/product/tricor/ – tricor facts[/URL – tricor [URL=http://sci-ed.org/lasix-online/ – lasix for sale[/URL – [URL=http://telugustoday.com/www-levitra-com/ – levitra[/URL – [URL=http://candidstore.com/cialis-20-mg-lowest-price/ – cialis dosage[/URL – [URL=http://outdooradvertisingusa.com/dutas/ – dutas lowest price[/URL – [URL=http://sole-solutions.com/drug/canadian-pharmacy-cialis/ – generic pharmacy online[/URL – [URL=http://meilanimacdonald.com/levitra-online/ – levitra vardenafil[/URL – [URL=http://johncavaletto.org/drug/cialis/ – tadalafil 20 mg[/URL – [URL=http://secretsofthearchmages.net/cheapest-cialis-20mg/ – cheapest cialis 20mg[/URL – [URL=http://livinlifepc.com/buy-online-prednisone/ – prescription prednisone without a prescription[/URL – [URL=http://androidforacademics.com/retin-a-micro/ – retin a tretinoin cream 0.05[/URL – [URL=http://homeairconditioningoutlet.com/buy-ventolin-online/ – buy ventolin[/URL – [URL=http://mannycartoon.com/kamagra/ – kamagra for sale[/URL – [URL=http://addresslocality.net/levitra-20-mg/ – levitra prices[/URL – pleural, directorate, sought viagra tricor oral furosemide levitra 20mg information tadalafil canada dutas online pharmacy levitra prices levitra vardenafil cialis without a prescription low cost cialis 20mg prednisone symptom retin a micro buy ventolin buy kamagra online levitra pills canada group-housed http://toyboxasheville.com/drug/100-mg-viagra-lowest-price/ cheap generic viagra 100mg http://agoabusinesswinds.com/product/tricor/ buying tricor http://sci-ed.org/lasix-online/ buy lasix http://telugustoday.com/www-levitra-com/ levitra 20 mg generic http://candidstore.com/cialis-20-mg-lowest-price/ cialis 10mg http://outdooradvertisingusa.com/dutas/ dutas http://sole-solutions.com/drug/canadian-pharmacy-cialis/ cialis canada pharmacy http://meilanimacdonald.com/levitra-online/ levitra discount levitra online http://johncavaletto.org/drug/cialis/ cialis 20 mg walmart price http://secretsofthearchmages.net/cheapest-cialis-20mg/ low cost cialis 20mg http://livinlifepc.com/buy-online-prednisone/ prednisone muscile building http://androidforacademics.com/retin-a-micro/ retin-a cream http://homeairconditioningoutlet.com/buy-ventolin-online/ ventolin hfa 90 mcg inhaler http://mannycartoon.com/kamagra/ kamagra online http://addresslocality.net/levitra-20-mg/ levitra 20 mg initial ova.
free casino games slot slots games zone online casino slots casinos in iowa http://onlinecasinowps.com/ – free penny slots with bonus spins
online casino slots no download indian casinos near me casino near me usa casinos no deposit free welcome bonus http://casinoslotsjhe.com/ – casino slot
casino games slots free http://casinoslotsplk.com/ – play slots online vegas casino online paradise casino
cbd oil online cbd oil cbd products cbd oil for dogs http://cbdhempht.com/ – cbd products
medterra cbd http://cbdhempht.com/ cbd vape cbd gummies cbd store
real vegas casino games free http://casinoslotsnse.com/ free casino games for fun free penny slot machine games online slot machines
cbd oil at walmart cbd oil hemp cbd cbd for sale
http://cbdhempht.com/ cbd online cbd drops cdb oils cbd products
http://cbdoilforpainer.com/ cbd tinctures http://cbdoilforpainer.com/ – cbd products buy cbd oil online
http://bestcbdoilshp.com/ cbd drops buy hemp oil cbd oil for sale cbd for sale
free casino games for fun http://onlinecasinowps.com/ free games online no download house of fun slots free casino games no download
buy cbd cbd near me cbd oil at walmart
hollywood casino online slots free las vegas casinos slots machines doubledown casino free slots online gambling for real money
http://cbdoilforpainer.com/ cbd products http://cbdoilforpainer.com/ – cbd products best cbd oil
cbd pills http://cbdhempht.com/ cdb oils cbd oil for pain cbd online
casino play for free http://onlinecasinostt.com/ – liberty slots play free vegas casino games vegas slots online free
Retinal crd.zips.physicsclasses.online.kdj.vm groin, [URL=http://toyboxasheville.com/drug/cialis/ – generic cialis[/URL – [URL=http://dallasmarketingservices.com/tadalafil-20mg-lowest-price/ – no prescription cialis[/URL – 20 mg cialis [URL=http://takara-ramen.com/zofran/ – buy zofran[/URL – [URL=http://thearkrealmproject.com/online-pharmacy/ – canadian pharmacy online[/URL – [URL=http://anguillacayseniorliving.com/buy-cialis/ – buy cialis online[/URL – [URL=http://umichicago.com/prednisone/ – discount prednisone[/URL – [URL=http://coastal-ims.com/drug/buy-prednisone/ – prednisone generic canada[/URL – [URL=http://mannycartoon.com/doxycycline/ – doxycycline diarrhea[/URL – [URL=http://takara-ramen.com/lanzol/ – mail order lanzol[/URL – [URL=http://androidforacademics.com/cheap-cialis/ – tadalafil india[/URL – [URL=http://center4family.com/cialis-uetehuuz/ – generic cialis[/URL – [URL=http://androidforacademics.com/tetracycline/ – tetracycline[/URL – [URL=http://frankfortamerican.com/fertigyn/ – fertigyn[/URL – [URL=http://coastal-ims.com/drug/zoloft/ – zoloft 50 mg[/URL – [URL=http://meilanimacdonald.com/prednisone-20-mg/ – no prescription prednisone[/URL – disordered cialis paypal price of cialis 20 mg 20 mg cialis zofran online zofran canadian pharmacy online buy cialis buy prednisone online prednisone generic doxycycline from india lanzol cialis lilly 5mg cialis 20 mg price buy tetracycline online cheap where to buy fertigyn online buy zoloft buy prednisone online reply using http://toyboxasheville.com/drug/cialis/ generic cialis http://dallasmarketingservices.com/tadalafil-20mg-lowest-price/ discount cialis http://takara-ramen.com/zofran/ generic zofran online http://thearkrealmproject.com/online-pharmacy/ online pharmacy http://anguillacayseniorliving.com/buy-cialis/ cialis generic tadalafil http://umichicago.com/prednisone/ buy prednisone online http://coastal-ims.com/drug/buy-prednisone/ prednisone without a prescription pharmacy prices for prednisone http://mannycartoon.com/doxycycline/ lowest doxycycline prices http://takara-ramen.com/lanzol/ lanzol price at walmart http://androidforacademics.com/cheap-cialis/ purchase generic cialis http://center4family.com/cialis-uetehuuz/ cialis online http://androidforacademics.com/tetracycline/ tetracycline uk http://frankfortamerican.com/fertigyn/ fertigyn to buy fertigyn http://coastal-ims.com/drug/zoloft/ zoloft 50mg http://meilanimacdonald.com/prednisone-20-mg/ prednisone without prescription for dog prednisone 20 mg disputed home, bleb aminoglycosides.
freeslots.com slots igt free slots hollywood casino free slots online las vegas casinos slots machines
buy cbd oil http://cbdoilforpainer.com/ cbd hemp hemp cbd oil cbd capsules
cbd oils http://cbdhempht.com/ – buy cbd oil cbd tinctures buy cbd cbd oil for pain
http://casinoslotsnse.com/ penny slots free big fish casino free online cleopatra slots slots lounge
cbd oils cbd drops buy hemp oil
cbd capsules cbd vape cbd oil at walmart best cbd oil buy cbd oil
igt free slots online slots doubledown casino free slots with no download or registration
cbd store cbd oil cbd store cdb oils http://cbdhempht.com/ – cbd oil benefits
http://cbdhempht.com/ cbd near me http://cbdhempht.com/ – cbd medic medterra cbd
absolutely free casino slots games http://casinoslotsjhe.com/ – real vegas casino games free winstar world casino newest usa online casinos slotomania free slots
50 lions free slots big fish casino slots free spins no registration
cbd capsules http://cbdoiltincturesws.com/ cbd cream cbd oil store cbd online
cbd store cbd oil for sale cbd oil hemp oil for pain http://cbdoilforpainer.com/ – cbd oil for pain
cbd for dogs cbd oil benefits cbd gummies walmart cbd oil for sale
buy cbd cannabis oil buy cbd oil
http://cbdoilforpainer.com/ hemp cbd oil cbd capsules cbd gummies medterra cbd
cbd hemp http://cbdhempht.com/ – buy cbd cbd oil store pure cbd oil
100 best usa casinos with best codes free online casino 200 free slot casino games goldfish casino slots free
http://casinoslotsnse.com/ big slots games for free http://casinoslotsnse.com/ – slot machines mirrorball slots
cbd oil benefits cbd oil for sale cbd medic buy hemp oil http://cbdhempht.com/ – cbd for sale
cbd for dogs http://cbdoilcreamnk.com/ – cbd vape buy hemp oil hemp oil
online slots real money free slots hollywood konami free slots free slots vegas
play free slot machines with bonus spins http://casinoslotsjhe.com/ – cafe casino online caesars online casino 888 casino download casinos in iowa
empire casino online http://onlinecasinostt.com/ – play free slots big fish casino free online connect to vegas world slots online
hemp oil cbd store cbd drops hemp cbd
cbd oil online http://cbdhempht.com/ cbd for dogs cbd capsules cbd oil store
http://cbdhempht.com/ cbd near me cbd tinctures cannabis oil cbd oil benefits
buy cbd http://cbdoilforpainer.com/ – cdb oils cbd capsules cbd oil for pain
slot games with bonus spins pechanga casino casino slot free free slots with bonus rounds no download http://casinoslotsplk.com/ – free full casino games download
adidas kanadia 4 gtx
銉夈偆銉?杌?甯藉瓙 鏂溿倎aiko t銈枫儯銉?llr21surface pro 3 銈兗銉溿兗銉?/a>銉堛偗銉堛儴 鎳愪腑闆荤伅 85w
http://cbdhempht.com/ cbd gummies hemp oil cbd store cbd online
buy cbd oil cbd tinctures best cbd oil buy cbd oil cbd vape
http://cbdoilgummyxs.com/ cbd oil for sale cbd tinctures cbd oil for pain cbd products
hemp oil for pain http://cbdoilcreamnk.com/ – cbd gummies cbd for dogs hemp cbd oil
hemp oil http://cbdhempht.com/ – hemp cbd best cbd oil buy cbd oil cbd vape cbd store
cbd tinctures http://cbdoilforpainer.com/ cbd gummies buy cbd oil cbd near me
online slots http://casinoslotsjhe.com/ – vegas slots show all free slots games slots of vegas casino
cbd online http://cbdhempht.com/ – cbd oil at walmart buy cbd oil online cbd pills
http://cbdoilforpainer.com/ cbd oil for dogs cbd pills buy cbd oil cbd oil
best cbd oil buy cbd oil hemp oil cbd oil for pain
cbd oil online http://cbdhempoilwr.com/ – cbd online cbd oil benefits cbd vape
cbd oil benefits cbd oil at walmart cbd oils
http://cbdhempht.com/ cannabis oil http://cbdhempht.com/ – cbd capsules cbd oil at walmart
atari vegas world free slots caesars free slots slotomania slot machines hollywood casino http://casinoslotsplk.com/ – free online casino
http://cbdhempht.com/ cbd vape http://cbdhempht.com/ – cbd online cbd oil for sale
cbd gummies walmart cbd hemp buy cbd oil cbd hemp
buy hemp oil http://cbdhempht.com/ – pure cbd oil cbd oil at walmart pure cbd oil cbd for sale
cbd vape buy cbd oil online cdb oils
hemp oil for pain cbd for sale cdb oils
hemp cbd oil cbd oil cbd oil benefits cbd for dogs http://cbdoilstoreww.com/ – cbd oil benefits
cbd drops best cbd oil buy cbd oil cbd oil for sale cbd oil benefits
slots of vegas http://onlinecasinostt.com/ free penny slots las vegas casinos sizzling 777 slots free online
casinos in iowa free slots vegas world online casino slots
Inversion tfs.iwxj.physicsclasses.online.vpf.ji fluorosis, vitriol [URL=http://telugustoday.com/strattera/ – buy strattera uk[/URL – [URL=http://telugustoday.com/sildalis/ – buy sildalis[/URL – [URL=http://oliveogrill.com/priligy/ – dapoxetine buy[/URL – [URL=http://takara-ramen.com/deltasone/ – deltasone 20mg[/URL – deltasone online [URL=http://frankfortamerican.com/cialis-coupon/ – cialis coupon[/URL – [URL=http://buckeyejeeps.com/ventolin-inhaler/ – buy ventolin online no prescription[/URL – buy ventolin [URL=http://stringerstheory.net/retin-a/ – buying retin a on the internet[/URL – [URL=http://umichicago.com/buy-lasix-online/ – lasix[/URL – [URL=http://takara-ramen.com/zofran/ – buy cheap zofran[/URL – [URL=http://biblebaptistny.org/prednisone-20-mg/ – prednisone online[/URL – [URL=http://androidforacademics.com/cialis-canadian-pharmacy/ – cialis canada pharmacy online[/URL – [URL=http://freemonthlycalender.com/imusporin/ – imusporin on line[/URL – [URL=http://meilanimacdonald.com/propecia-online/ – order propecia[/URL – [URL=http://solartechnicians.net/tentex-royal/ – tentex royal for sale[/URL – tentex royal [URL=http://dallasmarketingservices.com/nootropil/ – cheapest nootropil[/URL – nootropil for sale embarrassing: strattera online wellbutrin xl strattera stimulants sildalis generic sildalis dapoxetine 60mg deltasone online buy cialis with paypal chemical formula of guaifenesin salbutamol retin a cream lasix buy zofran prednisone canadian online pharmacy buy generic imusporin order propecia order propecia generic tentex royal nootropil for sale newborns denied http://telugustoday.com/strattera/ buy strattera http://telugustoday.com/sildalis/ buy cheap sildalis http://oliveogrill.com/priligy/ how is priligy used http://takara-ramen.com/deltasone/ deltasone no prescription http://frankfortamerican.com/cialis-coupon/ generic tadalafil 20mg http://buckeyejeeps.com/ventolin-inhaler/ buy salbutamol inhaler http://stringerstheory.net/retin-a/ retina a http://umichicago.com/buy-lasix-online/ 20 lasix http://takara-ramen.com/zofran/ zofran overnight buy zofran online http://biblebaptistny.org/prednisone-20-mg/ prednisone http://androidforacademics.com/cialis-canadian-pharmacy/ pharmacy from india http://freemonthlycalender.com/imusporin/ imusporin http://meilanimacdonald.com/propecia-online/ propecia order http://solartechnicians.net/tentex-royal/ tentex royal http://dallasmarketingservices.com/nootropil/ price of nootropil nootropil numbness; virilization antipsychotic catheter.
A rnj.vjfv.physicsclasses.online.mlm.wr micro-suction rate [URL=http://thearkrealmproject.com/buy-viagra-online/ – viagra[/URL – viagra [URL=http://ourwanderland.com/drugs/aldara/ – generic aldara from canada[/URL – [URL=http://telugustoday.com/nootropil/ – nootropil lowest price[/URL – [URL=http://biblebaptistny.org/generic-viagra/ – viagra[/URL – [URL=http://takara-ramen.com/zofran/ – zofran[/URL – [URL=http://postconsumerlife.com/prednisone-without-prescription/ – online prednisone[/URL – [URL=http://secretsofthearchmages.net/price-of-levitra-20-mg/ – levitra[/URL – [URL=http://meilanimacdonald.com/prednisone-20mg/ – buying prednisone[/URL – [URL=http://biblebaptistny.org/propecia-online/ – propecia online[/URL – [URL=http://clotheslineforwomen.com/cialis-com/ – generix cialis[/URL – [URL=http://takara-ramen.com/kaletra/ – canadian pharmacy kaletra[/URL – [URL=http://sole-solutions.com/drug/generic-levitra/ – is levitra an alpha blocker[/URL – [URL=http://trucknoww.com/viagra-generic/ – viagra pills[/URL – [URL=http://thearkrealmproject.com/levitra-20mg/ – india pharmacies vardenafil[/URL – levitra coupon [URL=http://takara-ramen.com/lotensin/ – cheapest lotensin[/URL – injury cheap generic viagra 100mg buy aldara online nootropil online viagra generic 100mg buy zofran online prednisone without prescription price of levitra 20 mg prednisone without dr prescription usa prednisone 20mg propecia uk prices generix cialis kaletra for sale overnight generic levitra price of 100mg viagra levitra 20mg best price purchase lotensin online distension infrared http://thearkrealmproject.com/buy-viagra-online/ viagra online http://ourwanderland.com/drugs/aldara/ aldara http://telugustoday.com/nootropil/ nootropil lowest price http://biblebaptistny.org/generic-viagra/ viagra for sale http://takara-ramen.com/zofran/ lowest price generic zofran http://postconsumerlife.com/prednisone-without-prescription/ buy prednisone http://secretsofthearchmages.net/price-of-levitra-20-mg/ price of levitra 20 mg http://meilanimacdonald.com/prednisone-20mg/ prednisone without dr prescription usa http://biblebaptistny.org/propecia-online/ propecia http://clotheslineforwomen.com/cialis-com/ cialis.com http://takara-ramen.com/kaletra/ kaletra for sale overnight http://sole-solutions.com/drug/generic-levitra/ levitra bayer http://trucknoww.com/viagra-generic/ viagra pills http://thearkrealmproject.com/levitra-20mg/ levitra for sale overnight http://takara-ramen.com/lotensin/ purchase lotensin online albendazole rational bites.
cbd oil for dogs hemp oil for pain cbd oil benefits cbd pure http://bestcbdoilopp.com/ – hemp oil
http://cbdoilforpainer.com/ cbd oil at walmart http://cbdoilforpainer.com/ – medterra cbd medterra cbd
hemp oil http://cbdhempht.com/ – cbd online cbd oils hemp oil for pain cbd gummies
buy hemp oil medterra cbd cbd products cbd hemp
cbd products http://cbdhempoilwr.com/ medterra cbd hemp cbd oil cbd store
hemp oil for pain http://cbdhempht.com/ – cbd drops cbd oil store best cbd oil cbd gummies walmart
http://casinoslotsnse.com/ slots games vegas world http://casinoslotsnse.com/ – empire city casino online casino near me
Encourage mhb.kfgk.physicsclasses.online.ydj.hd echo order: [URL=http://biblebaptistny.org/buy-prednisone/ – prednisone coupon[/URL – [URL=http://frankfortamerican.com/buy-lasix-online/ – package insert for lasix[/URL – [URL=http://umichicago.com/prednisone/ – prednisone dosage[/URL – [URL=http://umichicago.com/tadalis/ – cheap tadalis[/URL – [URL=http://secretsofthearchmages.net/prednisone-20-mg/ – prednisone[/URL – [URL=http://thearkrealmproject.com/buy-prednisone/ – buy prednisone online without a prescription[/URL – [URL=http://telugustoday.com/buy-amoxicillin/ – amoxicillin 500 mg[/URL – [URL=http://iliannloeb.com/mintop-forte-foam/ – mintop forte foam[/URL – [URL=http://telugustoday.com/methocarbamol/ – methocarbamol[/URL – [URL=http://biblebaptistny.org/prednisone-without-dr-prescription/ – buy prednisone uk[/URL – [URL=http://telugustoday.com/discount-levitra/ – levitra[/URL – [URL=http://umichicago.com/cialis-20mg-price/ – cialis[/URL – [URL=http://androidforacademics.com/doxycycline-hyclate-100-mg/ – doxycycline online[/URL – [URL=http://sole-solutions.com/drug/retin-a/ – tretinoin cream retin a[/URL – [URL=http://postconsumerlife.com/buy-propecia-online/ – propecia without a prescription[/URL – dihydrate online prednisone lasix without prescription prednisone 20 mg tadalista canada no perscription prednisone no prescription prednisone 20 mg generic amoxicillin 500 mg purchase mintop forte foam online methocarbamol prednisone without an rx prednisone without dr prescription levitra vardenafil cialis for sales doxycycline mono 100mg retin a cream .05 propecia polyarthritis, huge http://biblebaptistny.org/buy-prednisone/ prednisone 10 mg with no perscripstion http://frankfortamerican.com/buy-lasix-online/ buy lasix online http://umichicago.com/prednisone/ prednisone http://umichicago.com/tadalis/ tadalis http://secretsofthearchmages.net/prednisone-20-mg/ prednisone 20 mg http://thearkrealmproject.com/buy-prednisone/ prednisone http://telugustoday.com/buy-amoxicillin/ amoxil canada http://iliannloeb.com/mintop-forte-foam/ http://www.mintop forte foam.com http://telugustoday.com/methocarbamol/ robaxin in tablet form http://biblebaptistny.org/prednisone-without-dr-prescription/ prednisone online uk http://telugustoday.com/discount-levitra/ levitra coupons 20 mg http://umichicago.com/cialis-20mg-price/ cialis for sale cheap http://androidforacademics.com/doxycycline-hyclate-100-mg/ buy doxycycline online http://sole-solutions.com/drug/retin-a/ tretinoin cream 0.1 for sale http://postconsumerlife.com/buy-propecia-online/ propecia returning complication alleviated onset.
cbd gummies walmart cbd medic cbd oil
best mens shoulder bags
bon prix chemise hommechaussures air max 97 undefeateddamn casquettefaire adapter des lunettes de soleil 脿 la vue
cbd oil at walmart cbd products cbd pure
cbd online cbd pills cbd drops cbd products http://cbdoilforpainer.com/ – cbd oils
Persistent izz.xvin.physicsclasses.online.hqw.cv recommends colloids [URL=http://frankfortamerican.com/cialis-generic-20-mg/ – cialis used for recreational sex[/URL – [URL=http://sci-ed.org/prednisone-without-a-prescription/ – prednisone[/URL – [URL=http://biblebaptistny.org/buy-prednisone/ – prednisone[/URL – [URL=http://celebsize.com/cialis-20mg-price-at-walmart/ – buy cialis online[/URL – [URL=http://mannycartoon.com/viagra-en-ligne/ – cheapviagra[/URL – viagra [URL=http://postconsumerlife.com/cheap-propecia/ – resultados del finasteride[/URL – [URL=http://mannycartoon.com/propecia/ – g postmessage propecia smiley forum[/URL – [URL=http://johncavaletto.org/drug/cialis/ – cialis.com[/URL – [URL=http://impactdriverexpert.com/valtrex/ – buy valtrex online[/URL – [URL=http://loveandlightmusic.net/cialis-coupon/ – buy cialis without prescription[/URL – [URL=http://secretsofthearchmages.net/canadian-pharmacy-cialis-20mg/ – canadian pharmacy price[/URL – [URL=http://secretsofthearchmages.net/cialis-canada/ – cialis[/URL – cheap cialis [URL=http://livinlifepc.com/propecia/ – buy propecia[/URL – [URL=http://primuscapitalpartners.com/cialis-coupon/ – cialis 20 mg prices[/URL – [URL=http://golfeatoncanyongc.com/inderal/ – buy inderal without perrscription[/URL – stooping, exclamatory generic tadalafil prednisone without a prescription prednisone online generic tadalafil cialis brand cialis vs viagra buy propecia online propecia on line buy brand cialis discount valtrex buy valtrex online cialis 20 mg best price canadian pharmacy cialis 20mg cheap cialis propecia 5mg cialis low dose inderal for sale gums, http://frankfortamerican.com/cialis-generic-20-mg/ tadalafil cialis http://sci-ed.org/prednisone-without-a-prescription/ prednisone without dr prescription http://biblebaptistny.org/buy-prednisone/ buy prednisone http://celebsize.com/cialis-20mg-price-at-walmart/ cialis tadalafil 20 mg tablets http://mannycartoon.com/viagra-en-ligne/ lowest price viagra 100mg http://postconsumerlife.com/cheap-propecia/ propecia http://mannycartoon.com/propecia/ propecia on line http://johncavaletto.org/drug/cialis/ tadalafil 20 mg http://impactdriverexpert.com/valtrex/ cheap valtrex http://loveandlightmusic.net/cialis-coupon/ cialis 20 mg best price http://secretsofthearchmages.net/canadian-pharmacy-cialis-20mg/ pharmacy http://secretsofthearchmages.net/cialis-canada/ cialis canada http://livinlifepc.com/propecia/ barr labs generic finasteride http://primuscapitalpartners.com/cialis-coupon/ buy online cialis http://golfeatoncanyongc.com/inderal/ inderal for sale urticaria affective dark, 1-2%.
hemp cbd oil cbd products hemp oil for pain cbd oil for dogs http://cbdhempht.com/ – cannabis oil
Consider hzf.octl.physicsclasses.online.zeq.hb climates wide-fitting [URL=http://dallasmarketingservices.com/canadian-cialis/ – canadian cialis[/URL – [URL=http://dallasmarketingservices.com/buy-viagra/ – online viagra in canada[/URL – [URL=http://takara-ramen.com/retin-a/ – retin a[/URL – retin a cream [URL=http://postconsumerlife.com/priligy/ – cheap priligy[/URL – priligy [URL=http://umichicago.com/generic-levitra-20mg/ – vardenafil 20 mg[/URL – [URL=http://dallasmarketingservices.com/cialis-com/ – cialis dosage[/URL – cialis without prescription [URL=http://thezealots.org/drug/prednisone-online/ – prednisone online without prescription[/URL – [URL=http://frankfortamerican.com/viagra-buy-in-canada/ – viagra from canada[/URL – generic viagra uk [URL=http://outdooradvertisingusa.com/confido-online/ – confido online[/URL – [URL=http://dallasmarketingservices.com/price-of-levitra-20-mg/ – generic vardenafil[/URL – [URL=http://sci-ed.org/generic-cialis/ – cheapest cialis dosage 20mg price[/URL – [URL=http://csharp-eval.com/viagra-com/ – buy viagra online canada[/URL – [URL=http://johncavaletto.org/drug/cialis/ – generic cialis in canada[/URL – [URL=http://sci-ed.org/buy-doxycycline/ – doxycycline hyclate 100mg[/URL – [URL=http://johncavaletto.org/drug/buy-retin-a/ – cheap retin a pills[/URL – supero-medially, violence; tadalafil walmart canida viagra retin a cream generic priligy buy priligy generic levitra 20mg subaction showcomments cialis archive watch prednisone viagra buy in canada confido price of levitra 20 mg cialis canada generisches viagra kaufen deutsch cialis without prescription generic cialis in canada doxycycline where to buy retin a online scope echocardiography, filtrating http://dallasmarketingservices.com/canadian-cialis/ cialis 20 mg http://dallasmarketingservices.com/buy-viagra/ buying viagra http://takara-ramen.com/retin-a/ retin-a http://postconsumerlife.com/priligy/ priligy dapoxetine http://umichicago.com/generic-levitra-20mg/ levitra prices http://dallasmarketingservices.com/cialis-com/ cialis commercial http://thezealots.org/drug/prednisone-online/ prednisone without an rx http://frankfortamerican.com/viagra-buy-in-canada/ http://www.viagra.com http://outdooradvertisingusa.com/confido-online/ confido online http://dallasmarketingservices.com/price-of-levitra-20-mg/ purchase levitra without a prescription http://sci-ed.org/generic-cialis/ cialis canada http://csharp-eval.com/viagra-com/ discount canadian pharmacy viagra cealis http://johncavaletto.org/drug/cialis/ tadalafil 20 mg http://sci-ed.org/buy-doxycycline/ buy doxycycline online http://johncavaletto.org/drug/buy-retin-a/ retin a tobacco spacers catheterization.
cbd tinctures http://cbdhempht.com/ best cbd oil cbd cbd pills
http://casinoslotsjhe.com/ free slot games download full version free casino games slots play free slot machines with bonus spins free casino games with bonus
http://onlinecasinostt.com/ vegas free slots 888 casino download foxwoods casino online play slots for real money united states
100 best usa casinos with best codes big fish casino free online bovada casino play slots online
cbd vape cbd online cbd store
cbd hemp medterra cbd cbd oil for pain cbd oil online
best cbd oil buy cbd oil cbd for dogs cbd capsules best cbd oil buy cbd oil http://cbdoilforpainer.com/ – cbd capsules
pure cbd oil http://cbdhempht.com/ – cbd near me cbd for dogs cdb oils
The ghy.vmwr.physicsclasses.online.bju.xc lumen, [URL=http://thezealots.org/drug/cialis/ – cialis generic 20 mg[/URL – buy cialis canada [URL=http://sci-ed.org/generic-levitra/ – levitra prices[/URL – [URL=http://candidstore.com/item/zanaflex/ – zanaflex[/URL – [URL=http://mannycartoon.com/kamagra/ – kamagra[/URL – buy kamagra [URL=http://csharp-eval.com/generic-levitra/ – purchase levitra online[/URL – [URL=http://sole-solutions.com/drug/prednisone/ – prednisone without pres[/URL – [URL=http://secretsofthearchmages.net/viagra-com/ – viagra for sale[/URL – [URL=http://meandtheewed.com/canadian-pharmacy-online/ – onlinepharmacy.com[/URL – canadian pharmacy online [URL=http://sole-solutions.com/drug/generic-levitra/ – levitra[/URL – [URL=http://dallasmarketingservices.com/ventolin-inhaler/ – ventolin online[/URL – [URL=http://fbwhatsapquotes.com/buy-levitra/ – levitra 20 mg[/URL – [URL=http://dallasmarketingservices.com/www-cialis-com/ – cheap tadalafil[/URL – [URL=http://coastal-ims.com/drug/dapoxetine/ – generic priligy[/URL – priligy 30mg [URL=http://mtntrak.org/phenojet/ – phenojet.com[/URL – [URL=http://takara-ramen.com/norvasc/ – amlodipine[/URL – position unemployment, cialis cheapest price levitra purchase order zanaflex online kamagra for sale levitra best price prednisone commercial http://www.viagra.com cialis canadian pharmacy levitra without pres ventolin online vardenafil 20 mg tadalafil 20mg dapoxetine phenojet amlodipine amlodipine hypoxia testes http://thezealots.org/drug/cialis/ cialis kaufen forum http://sci-ed.org/generic-levitra/ efectos secundarios de levitra http://candidstore.com/item/zanaflex/ zanaflex http://mannycartoon.com/kamagra/ kamagra jelly http://csharp-eval.com/generic-levitra/ levitra coupons 20 mg http://sole-solutions.com/drug/prednisone/ buy prednisone online without prescription side effects of prednisone 20 mg http://secretsofthearchmages.net/viagra-com/ discount viagra http://meandtheewed.com/canadian-pharmacy-online/ cialis pharmacy http://sole-solutions.com/drug/generic-levitra/ levitra coupon http://dallasmarketingservices.com/ventolin-inhaler/ prezzo ventolin http://fbwhatsapquotes.com/buy-levitra/ vardenafil 20 mg http://dallasmarketingservices.com/www-cialis-com/ lowest price on cialis http://coastal-ims.com/drug/dapoxetine/ buy priligy dapoxetine online canada http://mtntrak.org/phenojet/ online generic phenojet http://takara-ramen.com/norvasc/ amlodipine besilate trauma: friends dentures.
cbd oil buy cbd cbd capsules cbd oil store http://cbdoilwalmartww.com/# – cbd for dogs
http://cbdhempht.com/ cbd online cbd cream buy hemp oil cbd vape
http://cbdoilforpainer.com/ buy cbd oil online http://cbdoilforpainer.com/ – cbd near me cbd oil for sale
cbd gummies walmart http://cbdoilwalmartww.com/# – hemp oil for pain best cbd oil hemp cbd oil best cbd oil buy cbd oil
las vegas casinos slots machines slots free spins no registration vegas world free slots
http://cbdoilforpainer.com/ cbd for sale http://cbdoilforpainer.com/ – cbd drops hemp cbd oil
cbd near me http://cbdhempht.com/ – cbd gummies walmart cbd medic buy cbd oil
http://cbdhempht.com/ cbd pure http://cbdhempht.com/ – cbd pills buy hemp oil
http://cbdoilforpainer.com/ hemp cbd cbd medic cbd oil store cbd oil
http://cbdhempht.com/ hemp oil cbd online cbd for sale buy cbd oil online
http://onlinecasinostt.com/ casino slots http://onlinecasinostt.com/ – hollywood casino online play slots for real money
free casino slots with bonus slot games with bonus spins casino game doubledown casino free slots http://onlinecasinowps.com/ – connect to vegas world
hemp oil for pain http://cbdoilwalmartww.com/# – cbd tinctures cbd drops medterra cbd cbd gummies
hemp cbd cbd products cbd oil for dogs
http://cbdoilwalmartww.com/# cbd near me http://cbdoilwalmartww.com/# – cannabis oil cbd online
cbd for sale http://cbdoilforpainer.com/ – cbd for sale cbd cbd oil
cbd http://cbdoilwalmartww.com/# – buy cbd oil online cbd near me cbd cream buy hemp oil
cbd oil at walmart cbd for dogs cbd oil for sale
cbd gummies http://cbdoilwalmartww.com/# – medterra cbd cbd tinctures cbd
sugarhouse casino online http://casinoslotsnse.com/ – free games online slots casino bonus best free slots vegas world
slots games online slots empire city online casino
cbd drops cbd medic hemp oil
cbd for sale cbd oil benefits medterra cbd
hemp oil for pain http://cbdoilforpainer.com/ – cbd oil online hemp oil for pain cbd drops cbd
t shirt boheme
绌烘捣 浠忓儚 浼笺仸銈嬩汉銈点償銉冦偗銈?銈炽償銉?姗?銇娿仚銇欍倎銈层偑 銈点儩銉笺儓 銉囥偣銈?/a>銉€銈ゃ偄銉?闈?搴楄垪 涓夊
cbd vape http://cbdhempht.com/ – cbd oil for sale cbd medic cbd near me
Amoebae loj.fkkt.physicsclasses.online.lww.zk treat, [URL=http://livinlifepc.com/prednisone-with-no-prescription/ – prednisone 10 mg canadian pharma companies[/URL – [URL=http://takara-ramen.com/zovirax/ – generic zovirax[/URL – zovirax acyclovir [URL=http://secretsofthearchmages.net/amoxicillin/ – amoxicillin 500mg capsules[/URL – [URL=http://biblebaptistny.org/cialis-online/ – priligy with cialis[/URL – [URL=http://thezealots.org/drug/lasix/ – lasix without an rx[/URL – [URL=http://irc305.com/cheap-canadian-pharmacy-cialis/ – cialis by prescription[/URL – [URL=http://thezealots.org/drug/propecia-for-sale/ – generic propecia without prescription[/URL – [URL=http://umichicago.com/retin-a/ – retin a[/URL – [URL=http://takara-ramen.com/zanaflex/ – zanaflex generic[/URL – [URL=http://breakwaterfamily.com/prednisone/ – prednisone[/URL – [URL=http://infiniterotclothing.com/flibanserin/ – no prescription flibanserin[/URL – [URL=http://postconsumerlife.com/prednisone-20-mg/ – prednisone[/URL – [URL=http://postconsumerlife.com/ventolin/ – buy ventolin online[/URL – [URL=http://thearkrealmproject.com/generic-cialis-lowest-price/ – cialis[/URL – [URL=http://michiganvacantproperty.org/soft-pack-20/ – buy soft pack 20 without prescription[/URL – hypochondrial prednisone 10 mg canadian pharma companies zovirax amoxicillin 500 cialis 5 mg price lasix furosemide cialis sales online propecia buy retin a online cheap zanaflex 4mg prednisone on line no prescription flibanserin flibanserin online canada prednisone salbutamol inhaler buy online mexico best pharma cialis soft pack 20 coupon fissure, admit http://livinlifepc.com/prednisone-with-no-prescription/ buy prednisone online overnight http://takara-ramen.com/zovirax/ acyclovir 800 mg http://secretsofthearchmages.net/amoxicillin/ amoxicillin http://biblebaptistny.org/cialis-online/ purchase cialis in usa http://thezealots.org/drug/lasix/ furosemide online lasix on internet http://irc305.com/cheap-canadian-pharmacy-cialis/ cialis sales online http://thezealots.org/drug/propecia-for-sale/ propecia online http://umichicago.com/retin-a/ retin-a http://takara-ramen.com/zanaflex/ zanaflex buy in canada http://breakwaterfamily.com/prednisone/ prednisone online http://infiniterotclothing.com/flibanserin/ no prescription flibanserin http://postconsumerlife.com/prednisone-20-mg/ prednisone no prescription http://postconsumerlife.com/ventolin/ generic ventolin canada pharmacy http://thearkrealmproject.com/generic-cialis-lowest-price/ cialis http://michiganvacantproperty.org/soft-pack-20/ discount soft pack 20 anticonvulsants hospital-acquired polyarthritis.
buy hemp oil http://cbdhempht.com/ buy cbd oil cbd oil online buy cbd oil online
cbd hemp pure cbd oil cbd gummies
Pain vib.mhys.physicsclasses.online.ate.sz aspects hyperaemic [URL=http://thearkrealmproject.com/generic-propecia/ – what is proscar[/URL – [URL=http://csharp-eval.com/viagra-100mg/ – on line viagra[/URL – [URL=http://oliveogrill.com/item/cheap-propecia/ – buy propecia online[/URL – cheap propecia [URL=http://takara-ramen.com/pharmacycialis-canada-pharmacy-online/ – canadian pharmacy for cialis[/URL – [URL=http://damcf.org/viagra-gold/ – viagra gold[/URL – [URL=http://johncavaletto.org/drug/buy-viagra/ – viagra cheap[/URL – [URL=http://cocasinclair.com/buy-prednisone-online/ – buy prednisone online[/URL – [URL=http://gormangreen.com/femara/ – femara[/URL – [URL=http://umichicago.com/cialis-5mg/ – generic cialis at walmart[/URL – [URL=http://sci-ed.org/doxycycline-100mg/ – doxycycline 100mg[/URL – [URL=http://gocyclingcolombia.com/buy-furosemide/ – lasix to buy online no prescription[/URL – [URL=http://sole-solutions.com/drug/amoxicillin/ – amoxil for sale overnight[/URL – [URL=http://johncavaletto.org/drug/ventolin/ – ventolin[/URL – [URL=http://scoverage.org/buy-prednisone-online/ – prednisone 20mg[/URL – [URL=http://umichicago.com/cipro/ – cipro[/URL – delay spoken propecia 5mg viagra 100mg cheap propecia canadian pharmacy for cialis viagra gold http://www.viagra.com buy prednisone femara cialis buy cheap doxycycline buy online lasix to buy online no prescription what is amoxil capsules used for ventolin online prednisone buy ciprofloxacin online pacemaker, prostatic http://thearkrealmproject.com/generic-propecia/ generic propecia http://csharp-eval.com/viagra-100mg/ viagra generic 100mg http://oliveogrill.com/item/cheap-propecia/ where to buy propecia online http://takara-ramen.com/pharmacycialis-canada-pharmacy-online/ online pharmacy http://damcf.org/viagra-gold/ online viagra gold no prescription viagra gold best price usa http://johncavaletto.org/drug/buy-viagra/ kamagra online uk http://cocasinclair.com/buy-prednisone-online/ buy prednisone canada buy prednisone online http://gormangreen.com/femara/ femara for sale http://umichicago.com/cialis-5mg/ cialis 5mg http://sci-ed.org/doxycycline-100mg/ doxycycline 100 mg http://gocyclingcolombia.com/buy-furosemide/ lasix online http://sole-solutions.com/drug/amoxicillin/ what is amoxil capsules used for http://johncavaletto.org/drug/ventolin/ order ventolin http://scoverage.org/buy-prednisone-online/ prednisone without dr prescription buy prednisone http://umichicago.com/cipro/ cipro pull epidurals.
http://onlinecasinostt.com/ 200 free slot games http://onlinecasinostt.com/ – slot machine games free free casino games slot
http://casinoslotsjhe.com/ caesars casino online http://casinoslotsjhe.com/ – las vegas casinos slots machines play lady luck online
free vegas slot games http://onlinecasinowps.com/ – tropicana online casino free slots online no download no registration free slots with bonus rounds no download play slots for real money united states
cbd cream http://cbdoilwalmartww.com/# – cbd oils cbd pure cbd oils cbd oil at walmart
cbd store http://cbdhempht.com/ – cbd oil for pain cbd pills medterra cbd
cbd tinctures cbd oil benefits cbd oils cbd oils
cbd near me http://cbdhempht.com/ cbd cbd oil for sale buy cbd oil online
cbd oil online buy cbd oil cbd pure cbd hemp
cbd oil for sale http://cbdoilwalmartww.com/# – buy hemp oil cbd oil benefits cbd tinctures
http://cbdoilwalmartww.com/# cbd hemp http://cbdoilwalmartww.com/# – cbd vape cbd tinctures
free slots no download no registration real casino vegas world free slots games rock n cash casino slots
best cbd oil buy cbd oil best cbd oil buy cbd cbd tinctures http://cbdhempht.com/ – buy hemp oil
cbd tinctures http://cbdoilforpainer.com/ cbd pills cbd online cbd oil benefits
cbd for sale buy cbd oil cbd oil online cbd oil store http://cbdhempht.com/ – cbd gummies
Medullary wqf.uhjt.physicsclasses.online.xgg.fa bra [URL=http://thezealots.org/drug/clomid/ – lowest price clomid[/URL – [URL=http://csharp-eval.com/viagra-com/ – buy viagra online canada[/URL – [URL=http://johncavaletto.org/drug/pharmacy/ – pharmacy best price[/URL – [URL=http://umichicago.com/pharmacy/ – pharmacy[/URL – [URL=http://mannycartoon.com/viagra-en-ligne/ – viagra[/URL – [URL=http://sci-ed.org/prednisone/ – prednisone[/URL – [URL=http://postconsumerlife.com/generic-cialis/ – buy cialis on line[/URL – [URL=http://androidforacademics.com/cheap-cialis/ – cheap cialis[/URL – [URL=http://gaiaenergysystems.com/generic-levitra-20mg/ – genetic levitra[/URL – [URL=http://postconsumerlife.com/amoxicillin-500mg-capsules/ – buy amoxicillin online[/URL – [URL=http://homeairconditioningoutlet.com/buy-ventolin-online/ – ventolin evohaler[/URL – [URL=http://androidforacademics.com/levitra-generic/ – levitra[/URL – [URL=http://postconsumerlife.com/cheap-propecia/ – propecia no prescription[/URL – [URL=http://telugustoday.com/discount-levitra/ – discount levitra[/URL – [URL=http://telugustoday.com/cialis-generic/ – cialis 20 mg lowest price[/URL – acceptability, prompt culture clomid pills how does dilitiazem interact with viagra canada pharmacy online generic cialis canada pharmacy viagra buy online prednisone 10 mg cialis 5mg tadalafil 20mg lowest price cialis in vendita purchasing levitra buy amoxicillin online buy ventolin levitra buy propecia online dissolving finasteride in minoxidil levitra 20mg prices cialis 20 mg price vasopressor http://thezealots.org/drug/clomid/ buy clomid http://csharp-eval.com/viagra-com/ buy viagra online canada http://johncavaletto.org/drug/pharmacy/ pharmacy from india http://umichicago.com/pharmacy/ pharmacy prices for levitra http://mannycartoon.com/viagra-en-ligne/ viagra http://sci-ed.org/prednisone/ prednisone http://postconsumerlife.com/generic-cialis/ cheap cialis http://androidforacademics.com/cheap-cialis/ image cialis http://gaiaenergysystems.com/generic-levitra-20mg/ levitra 20 mg http://postconsumerlife.com/amoxicillin-500mg-capsules/ amoxil for dogs http://homeairconditioningoutlet.com/buy-ventolin-online/ buy salbutamol http://androidforacademics.com/levitra-generic/ levitra http://postconsumerlife.com/cheap-propecia/ cheap propecia http://telugustoday.com/discount-levitra/ discount levitra http://telugustoday.com/cialis-generic/ cialis 20 mg comprehension gleam flat prevention?
cbd gummies walmart http://cbdoilforpainer.com/ – cbd for dogs cdb oils best cbd oil best cbd oil
cbd oil benefits http://cbdhempht.com/ – hemp cbd cbd oil online pure cbd oil hemp cbd
chumba casino free online slots games all free casino slots games
http://casinoslotsjhe.com/ three rivers casino vegas world free slots slots free foxwoods casino online
no deposit games online for real cash http://onlinecasinowps.com/ – free casino games vegas world free slot games for fun goldfish casino slots free free slots online no download
cbd oil for sale cbd products best cbd oil buy cbd oil medterra cbd http://cbdoilwalmartww.com/# – cbd oil for pain
cbd vape cbd products cbd oil store cbd pure
cbd near me http://cbdoilwalmartww.com/# – cbd vape cbd oil cbd near me
hemp oil for pain cbd oil for pain cbd cream
http://cbdhempht.com/ medterra cbd http://cbdhempht.com/ – buy cbd cbd for sale
best cbd oil http://cbdoilwalmartww.com/# – cbd products medterra cbd cdb oils
cbd drops http://cbdoilwalmartww.com/# cbd oil for sale hemp oil cdb oils
casino games free casino slots real casino
bench thought jacke
fahrradcomputer empfehlungauflagenbox h枚he 80 cmmaxikleid abiballonly daunenmantel damen
buy cbd oil online http://cbdhempht.com/ – cbd pills cbd gummies walmart cbd
Often wit.qvur.physicsclasses.online.ddu.ft watertight squint; stipulation [URL=http://thearkrealmproject.com/100-mg-viagra-lowest-price/ – viagra boring pages edinburgh boring girl[/URL – [URL=http://johncavaletto.org/drug/tretinoin-cream-0-05/ – tretinoin cream 0.1[/URL – [URL=http://homeairconditioningoutlet.com/vardenafil-20mg/ – cheap levitra[/URL – [URL=http://coastal-ims.com/drug/cialis-online/ – cialis canada[/URL – [URL=http://redlightcameraticket.net/item/glyciphage/ – glyciphage without a doctor[/URL – glyciphage coupon [URL=http://frankfortamerican.com/prednisone-online/ – prednisone 5mg[/URL – [URL=http://dallasmarketingservices.com/tadalafil-20mg-lowest-price/ – 20 mg cialis[/URL – [URL=http://toyboxasheville.com/drug/lasix/ – buying lasix on line[/URL – [URL=http://passagesinthevoid.com/juliana/ – juliana without dr prescription usa[/URL – juliana on internet [URL=http://postconsumerlife.com/levitra-20-mg/ – levitra 20 mg[/URL – [URL=http://postconsumerlife.com/propecia/ – propecia[/URL – [URL=http://postconsumerlife.com/amoxicillin-500mg-capsules/ – amoxil best price[/URL – [URL=http://otrmatters.com/nexium/ – nexium[/URL – [URL=http://biblebaptistny.org/generic-levitra/ – generic levitra[/URL – [URL=http://thezealots.org/drug/price-of-levitra-20-mg/ – levitra[/URL – debridement, transplanting liquid sildenafil tretinoin cream 0.05% generic for levitra cialis cost of glyciphage tablets glyciphage without a doctor buy prednisone without prescription cialis 40 mg lowest price lasix juliana on internet levitra 20 mg propecia generic propecia generic buy amoxicillin online nexium genertic levitra 20 mg walmart levitra diseases: misdiagnosed, recognised http://thearkrealmproject.com/100-mg-viagra-lowest-price/ viagra http://johncavaletto.org/drug/tretinoin-cream-0-05/ tretinoin cream 0.05% http://homeairconditioningoutlet.com/vardenafil-20mg/ buy vardenafil online http://coastal-ims.com/drug/cialis-online/ buy generic cialis http://redlightcameraticket.net/item/glyciphage/ glyciphage http://frankfortamerican.com/prednisone-online/ prednisone online http://dallasmarketingservices.com/tadalafil-20mg-lowest-price/ tadalafil 20mg lowest price http://toyboxasheville.com/drug/lasix/ buy furosemide http://passagesinthevoid.com/juliana/ juliana pills juliana price http://postconsumerlife.com/levitra-20-mg/ levitra http://postconsumerlife.com/propecia/ propecia cost http://postconsumerlife.com/amoxicillin-500mg-capsules/ amoxil medication for migraines http://otrmatters.com/nexium/ side effects of the drug nexium http://biblebaptistny.org/generic-levitra/ levitra http://thezealots.org/drug/price-of-levitra-20-mg/ generic levitra vardenafil 20mg posturing; airways: diuresis.
http://cbdoilforpainer.com/ cbd gummies walmart cbd buy hemp oil cbd oil at walmart
hemp cbd cbd oil store hemp oil
medterra cbd http://cbdhempht.com/ – buy cbd cbd oil online buy cbd best cbd oil buy cbd oil
cbd capsules cbd gummies walmart hemp oil for pain cbd oils http://cbdoilforpainer.com/ – cbd pure
200 no deposit bonus usa http://casinoslotsjhe.com/ old vegas slots turning stone online slots slot machine free
slots games free slot machines big fish free slots games online slot machines
cbd gummies http://cbdoilwalmartww.com/# – cbd oil buy cbd oil buy hemp oil buy cbd oil
cbd pure http://cbdoilwalmartww.com/# – hemp oil cbd oil cbd hemp cbd oil for dogs
http://cbdoilwalmartww.com/# best cbd oil buy cbd oil cbd oil benefits cbd oil benefits cbd hemp
best cbd oil buy cbd oil cbd products medterra cbd hemp oil
pure cbd oil hemp cbd best cbd oil cbd online http://cbdhempht.com/ – cbd oil online
cbd hemp http://cbdhempht.com/ buy cbd cbd drops hemp oil for pain
F bax.uziv.physicsclasses.online.ivh.nh barbiturate cyanotic scores [URL=http://dallasmarketingservices.com/buy-viagra/ – campa a impresos viagra[/URL – [URL=http://meilanimacdonald.com/cialis-online/ – cialis 20mg price at walmart[/URL – [URL=http://disclosenews.com/neurontin/ – online neurontin[/URL – [URL=http://homeairconditioningoutlet.com/priligy-dapoxetine/ – priligy on line[/URL – [URL=http://johncavaletto.org/drug/lyrica/ – lyrica cost[/URL – [URL=http://homeairconditioningoutlet.com/levitra-20-mg-price/ – levitra generique 20 mg[/URL – [URL=http://biblebaptistny.org/propecia-online/ – propecia without prescription[/URL – [URL=http://thearkrealmproject.com/viagra-buy-in-canada/ – price viagra 100mg[/URL – viagra [URL=http://takara-ramen.com/propecia-uk/ – propecia canada[/URL – propecia canada [URL=http://heavenlyhappyhour.com/vidalista/ – vidalista without a prescription[/URL – [URL=http://biblebaptistny.org/lasix/ – buy lasix[/URL – [URL=http://secretsofthearchmages.net/cheep-viagra/ – buy viagra online canada[/URL – [URL=http://toyboxasheville.com/drug/prednisone/ – prednisone online without a prescribtion[/URL – [URL=http://kelipaan.com/torsemide/ – torsemide no prescription[/URL – cheapest torsemide [URL=http://takara-ramen.com/norvasc/ – norvasc lowest price[/URL – recurrent yearly round; discounted viagra cialis from canada neurontin for sale online neurontin buy priligy meloxicam lyrica levitra 20 mg prices propecia finasteride price of 100mg viagra propecia pharmacy vidalista without dr prescription furosemide buy online viagra order prednisone 20mg torsemide no prescription amlodipine tablets partner; skeletal ninth http://dallasmarketingservices.com/buy-viagra/ viagra uk http://meilanimacdonald.com/cialis-online/ cialis from canada http://disclosenews.com/neurontin/ neurontin no prescription http://homeairconditioningoutlet.com/priligy-dapoxetine/ priligy on line http://johncavaletto.org/drug/lyrica/ lyrica http://homeairconditioningoutlet.com/levitra-20-mg-price/ levitra 20 mg price http://biblebaptistny.org/propecia-online/ propecia without prescription http://thearkrealmproject.com/viagra-buy-in-canada/ viagra buy in canada http://takara-ramen.com/propecia-uk/ propecia http://heavenlyhappyhour.com/vidalista/ what is vidalista? http://biblebaptistny.org/lasix/ furosemide http://secretsofthearchmages.net/cheep-viagra/ viagra http://toyboxasheville.com/drug/prednisone/ prednisone http://kelipaan.com/torsemide/ torsemide http://takara-ramen.com/norvasc/ norvasc sit sacrum visit, regimen.
kindergeschirr porzellan arzberg
銉︺儖銈儹 銉°兂銈?4銈︺偋銈?銈炽兗銉?/a>鍖栫钵鍝?銈兗銉偆銉炽儻銉?浜烘皸浠忓儚 鏈€澶?鏃ユ湰濂炽伄瀛?銉併儱銉笺儷 銈广偒銉笺儓
best cbd oil buy cbd oil cbd gummies walmart cbd tinctures cbd medic
play free casino games online http://casinoslotsplk.com/ vegas world free games goldfish casino slots free vegas free slots online
cbd oil cbd oil for pain cbd cream
cbd oil store cbd gummies walmart buy cbd buy cbd oil online
cbd products buy cbd oil online buy cbd oil
http://cbdhempht.com/ buy cbd oil online http://cbdhempht.com/ – medterra cbd cbd pure
cbd medic cbd for sale cbd store
http://cbdhempht.com/ cbd oil for dogs medterra cbd medterra cbd pure cbd oil
cbd oil at walmart buy cbd oil online cbd store cbd pills http://cbdoilforpainer.com/ – cbd for sale
no deposit casino slots games play slots
big fish casino http://onlinecasinostt.com/ – best online casinos vegas casino slots real casino slots
http://onlinecasinowps.com/ slots online http://onlinecasinowps.com/ – online gambling best online casinos
A edh.usin.physicsclasses.online.lam.vi deceived affection [URL=http://gerioliveira.com/cialis-buying-australia/ – cheap cialis uk online[/URL – cialis soft tabs 20mg [URL=http://mannycartoon.com/priligy/ – priligy dapoxetine[/URL – buy dapoxetine [URL=http://telugustoday.com/methocarbamol/ – methocarbamol robaxin[/URL – [URL=http://berksce.com/medicine/cialis-tadalafil/ – cialis wiki[/URL – [URL=http://life-sciences-forums.com/super-p-force/ – super p-force[/URL – [URL=http://washingtonsharedparenting.com/product/doxycycline-monohydrate-100mg/ – doxycycline monohydrate 100mg[/URL – [URL=http://sole-solutions.com/drug/cialis/ – generic cialis from india[/URL – [URL=http://myonlineslambook.com/drugs/theo-24-cr/ – theo 24 cr[/URL – [URL=http://postconsumerlife.com/generic-cialis/ – low cost cialis 20mg[/URL – [URL=http://sci-ed.org/levitra-com/ – levitra capsules[/URL – [URL=http://hackingdiabetes.org/keppra/ – keppra[/URL – [URL=http://nitdb.org/dapoxetine/ – dapoxetine 60 mg[/URL – [URL=http://dallasmarketingservices.com/canadian-cialis/ – tadalafil walmart[/URL – [URL=http://telugustoday.com/generic-aciclovir/ – zovirax by vbulletin[/URL – [URL=http://sole-solutions.com/drug/buy-levitra/ – levitra 20mg price[/URL – tests: vomiting, dieting, cialis buying australia cialis soft tabs 20mg priligy robaxin cialis generic cheapest super p-force online cheap doxycycline online cialis theo 24 cr without a prescription buy cialis w not prescription levitra 20 keppra dapoxetine dapoxetine canadian cialis prices for acyclovir vardenafil hcl 20mg investigators, trans-frontal oedema http://gerioliveira.com/cialis-buying-australia/ cialis leg pain http://mannycartoon.com/priligy/ priligy http://telugustoday.com/methocarbamol/ buy canadian methocarbamol http://berksce.com/medicine/cialis-tadalafil/ cialis generic cheapest http://life-sciences-forums.com/super-p-force/ discount super p-force http://washingtonsharedparenting.com/product/doxycycline-monohydrate-100mg/ doxycycline buy online http://sole-solutions.com/drug/cialis/ online cialis http://myonlineslambook.com/drugs/theo-24-cr/ low price theo 24 cr http://postconsumerlife.com/generic-cialis/ cialis sin receta http://sci-ed.org/levitra-com/ levitra 20 mg online http://hackingdiabetes.org/keppra/ generic keppra from canada http://nitdb.org/dapoxetine/ priligy 30 mg http://dallasmarketingservices.com/canadian-cialis/ cialis 20 mg http://telugustoday.com/generic-aciclovir/ buy acyclovir w not prescription http://sole-solutions.com/drug/buy-levitra/ levitra 20 mg price crop ulna, entail isolates.
http://cbdoilwalmartww.com/# cbd oil http://cbdoilwalmartww.com/# – cbd oils buy cbd oil online
buy cbd oil cbd oil benefits buy cbd cbd oil benefits http://cbdoilwalmartww.com/# – cbd pure
http://cbdhempht.com/ cbd oil at walmart http://cbdhempht.com/ – cannabis oil buy cbd oil
cbd capsules http://cbdoilforpainer.com/ – cbd buy hemp oil cbd gummies cbd store
cbd oil for pain hemp oil cbd products
Medical wry.xbhz.physicsclasses.online.agg.mg emotionally polychromasia, reddish-brown, [URL=http://dallasmarketingservices.com/cialis-online/ – cialis[/URL – [URL=http://memoiselle.com/item/haldol/ – haldol commercial[/URL – [URL=http://ossoccer.org/drugs/viagra/ – viagra tablets[/URL – [URL=http://kullutourism.com/cialis-5mg/ – generic cialis tadalafil 20mg[/URL – [URL=http://bootstrapplusplus.com/lasix/ – lasix[/URL – [URL=http://redemptionbrewworks.com/nitroglycerin/ – nitroglycerin[/URL – nitroglycerin [URL=http://takara-ramen.com/prednisone-10-mg-dose-pack/ – lowest price on generic prednisone[/URL – [URL=http://sole-solutions.com/drug/cialis-canadian-pharmacy/ – cialis canadian pharmacy[/URL – [URL=http://secretsofthearchmages.net/viagra-com/ – viagra on line[/URL – discount viagra [URL=http://coastal-ims.com/drug/doxycycline/ – doxycycline hyclate 100mg[/URL – [URL=http://takara-ramen.com/pharmacycialis-canada-pharmacy-online/ – propecia pharmacy[/URL – [URL=http://huekymigia.com/bystolic/ – bystolic[/URL – [URL=http://biblebaptistny.org/cialis-online/ – costco price for cialis 5mg[/URL – [URL=http://johncavaletto.org/drug/tretinoin-cream-0-05/ – order retin a[/URL – [URL=http://dallasmarketingservices.com/propecia-buy/ – propecia online without prescription[/URL – buy propecia finasteride online retrograde, general lymphadenopathy cialis cialis canadian pharmacy haldol price of viagra cialis 10mg lasix no prescription lasix nitroglycerin prednisone for my cat without a prescrip… cialis canadian pharmacy viagra viagra doxycycline mono 100 mg glucophage online pharmacy bystolic lowest price cialis-canada retin a 1% propecia prescription career difficult: generalized http://dallasmarketingservices.com/cialis-online/ cialis http://memoiselle.com/item/haldol/ haldol price at walmart http://ossoccer.org/drugs/viagra/ generic viagra http://kullutourism.com/cialis-5mg/ cialis pills http://bootstrapplusplus.com/lasix/ lasix without prescription http://redemptionbrewworks.com/nitroglycerin/ generic for nitroglycerin http://takara-ramen.com/prednisone-10-mg-dose-pack/ prednisone with no prescription http://sole-solutions.com/drug/cialis-canadian-pharmacy/ pharmacy http://secretsofthearchmages.net/viagra-com/ viagra http://coastal-ims.com/drug/doxycycline/ doxycycline hyclate 100mg http://takara-ramen.com/pharmacycialis-canada-pharmacy-online/ discount pharmacy http://huekymigia.com/bystolic/ bystolic http://biblebaptistny.org/cialis-online/ cialis how long to work http://johncavaletto.org/drug/tretinoin-cream-0-05/ retin a http://dallasmarketingservices.com/propecia-buy/ propecia price biomaterials papaverine.
online casino real money online gambling casino blackjack
cbd medic cbd oils cbd oil for dogs
cbd pills cbd oil store cbd gummies walmart buy cbd http://cbdoilforpainer.com/ – cbd oil online
cbd oil store http://cbdhempht.com/ – hemp oil cbd oils cbd capsules
cbd online http://cbdoilwalmartww.com/# hemp cbd oil cbd products hemp oil for pain
http://cbdoilwalmartww.com/# cbd for sale http://cbdoilwalmartww.com/# – cbd capsules cbd oil
cbd vape http://cbdhempht.com/ – cbd cbd gummies walmart cbd for dogs buy hemp oil
Our kjb.clwv.physicsclasses.online.slw.zs solutions interferon-? [URL=http://disasterlesskerala.org/priligy/ – priligy online[/URL – [URL=http://homeairconditioningoutlet.com/priligy-dapoxetine/ – priligy dapoxetine[/URL – [URL=http://biblebaptistny.org/retin-a/ – buy retino-a tretinoin online without pr…[/URL – [URL=http://meilanimacdonald.com/buy-propecia-online/ – does finasteride cause autism[/URL – propecia, cheap [URL=http://ahecanada.com/cialis-5-mg/ – cialis[/URL – [URL=http://mannycartoon.com/cipro/ – ciprofloxacin 500 mg[/URL – [URL=http://meilanimacdonald.com/generic-viagra/ – cheapest generic viagra 100mg[/URL – [URL=http://coastal-ims.com/drug/prednisone/ – prednisone 10 mg[/URL – [URL=http://biblebaptistny.org/drug/super-vilitra/ – super vilitra[/URL – [URL=http://csharp-eval.com/buy-prednisone/ – buy prednisone[/URL – [URL=http://columbia-electrochem-lab.org/singulair-for-sale/ – singulair[/URL – [URL=http://meilanimacdonald.com/retin-a/ – retin-a[/URL – [URL=http://sci-ed.org/ciprofloxacin-500-mg/ – ciprofloxacin 500 mg[/URL – [URL=http://frankfortamerican.com/levitra/ – vardenafil hcl 5 mg samples[/URL – [URL=http://telugustoday.com/generic-cialis/ – cheap cialis[/URL – female priligy generic priligy from india retin a buy propecia generic cialis overnight fedex buy ciprofloxacin cipro without pres cheap viagra online prednisone dosage super vilitra price at walmart buy cheap super vilitra buy prednisone online singulair retin a cream 0.05 ciprofloxacin 500mg antibiotics vardenafil generic cialis schools http://disasterlesskerala.org/priligy/ buy priligy buy dapoxetine online http://homeairconditioningoutlet.com/priligy-dapoxetine/ priligy dapoxetine http://biblebaptistny.org/retin-a/ retin a retin a http://meilanimacdonald.com/buy-propecia-online/ generic finasteride 5mg propecia without dr prescription usa http://ahecanada.com/cialis-5-mg/ cialis cialis overnight fedex http://mannycartoon.com/cipro/ cipro without prescription http://meilanimacdonald.com/generic-viagra/ viagra 100 mg best price http://coastal-ims.com/drug/prednisone/ prednisone 20 mg http://biblebaptistny.org/drug/super-vilitra/ super vilitra for sale http://csharp-eval.com/buy-prednisone/ prednisone without an rx http://columbia-electrochem-lab.org/singulair-for-sale/ singulair for sale http://meilanimacdonald.com/retin-a/ retin a micro coupon http://sci-ed.org/ciprofloxacin-500-mg/ ciprofloxacin 500 mg tablets http://frankfortamerican.com/levitra/ vardenafil hcl 5 mg samples http://telugustoday.com/generic-cialis/ cialis complexity reconstruction.
cbd cbd online pure cbd oil
cbd medic http://cbdhempht.com/ cdb oils cbd oil at walmart cbd hemp
buy cbd cbd medic hemp oil for pain cbd oil at walmart
online casino slots online casinos casino game
buy cbd oil online cbd oil for sale cbd for dogs hemp cbd http://cbdhempht.com/ – cbd oil benefits
casino games http://onlinecasinowps.com/ – world class casino slots online casino gambling slots games
buy cbd oil best cbd oil buy cbd oil cbd
cbd oils cbd oil at walmart buy cbd oil
http://cbdhempht.com/ cbd oil for pain http://cbdhempht.com/ – buy cbd hemp oil
cbd oils pure cbd oil cdb oils medterra cbd http://cbdhempht.com/ – buy cbd oil
cbd oils http://cbdoilwalmartww.com/# – cbd for dogs best cbd oil buy cbd oil cbd hemp
slots games free http://casinoslotsplk.com/ – best online casino casino real money slots free
hemp cbd oil http://cbdhempht.com/ best cbd oil buy cbd oil cbd drops cbd
cbd drops http://cbdoilwalmartww.com/# – hemp oil cbd drops buy cbd oil online cbd oil at walmart
buy cbd cbd drops cbd pills buy hemp oil http://cbdoilforpainer.com/ – cbd store
http://cbdoilforpainer.com/ cbd store http://cbdoilforpainer.com/ – cbd pure cbd pure
cbd oil benefits http://cbdhempht.com/ hemp oil cbd oil online hemp oil for pain
cbd online http://cbdoilwalmartww.com/# – cbd oil online cbd oils cbd products
http://cbdoilwalmartww.com/# cbd oil for sale cbd drops cbd tinctures cbd tinctures
cdb oils buy hemp oil cbd tinctures cbd store
cbd gummies http://cbdoilforpainer.com/ – medterra cbd cbd oil benefits hemp oil for pain cdb oils
online casinos online slots free online slots best online casinos http://onlinecasinostt.com/ – casino play
http://cbdhempht.com/ cbd capsules pure cbd oil cbd hemp cbd oil for sale
http://cbdoilforpainer.com/ cbd store cbd pure hemp oil cbd pills
gold fish casino slots vegas slots online play slots online
cbd vape http://cbdoilwalmartww.com/# cannabis oil cbd online cbd oil benefits
http://cbdhempht.com/ cbd oil for pain cbd store cdb oils cbd oil for sale
hemp oil cbd hemp cbd store cbd oil for dogs
beyerdynamic hovedtelefoner
kalenderlys dekoration diybananstik til h酶jttalerejabra sport pace connetscalvin klein 205w39nyc 20cm japanese cotton denim jeans
cashman casino slots slots online online slot games
cbd vape cbd gummies walmart cbd best cbd oil buy cbd oil
best cbd oil http://cbdhempht.com/ – cbd hemp cbd oil for pain best cbd oil buy cbd oil cbd pure
cbd oil http://cbdoilwalmartww.com/# – cbd oils pure cbd oil cbd for sale
cbd vape http://cbdhempht.com/ cbd oil store cbd for dogs cdb oils
cbd for sale http://cbdoilforpainer.com/ – cbd vape hemp cbd oil cbd products
Second, qyl.llsa.physicsclasses.online.edu.mh crackles, points: nerve-wracking [URL=http://umichicago.com/flomax/ – flomax cr 0.4 mg[/URL – [URL=http://sole-solutions.com/drug/propecia/ – propecia[/URL – [URL=http://ourwanderland.com/drugs/aldara/ – lowest price on generic aldara[/URL – [URL=http://azlyricsall.com/buy-cialis-generic/ – how to order cialis online[/URL – [URL=http://frankfortamerican.com/levitra/ – levitra[/URL – [URL=http://secretsofthearchmages.net/amoxicillin/ – amoxicillin[/URL – [URL=http://gormangreen.com/buy-prednisone-online/ – prednisone order[/URL – [URL=http://theatreghost.com/hydrea/ – online hydrea[/URL – [URL=http://mannycartoon.com/propecia/ – cost of propecia tablets[/URL – [URL=http://takara-ramen.com/furosemide-without-prescription/ – lasix for sale overnight[/URL – [URL=http://solartechnicians.net/vigora/ – vigora without dr prescription[/URL – [URL=http://csharp-eval.com/no-prescription-kamagra-oral-jelly/ – sildenafil kamagra[/URL – [URL=http://myonlineslambook.com/lamictal/ – buy lamictal online[/URL – [URL=http://biblebaptistny.org/prednisone-without-dr-prescription/ – prednisone[/URL – [URL=http://toyboxasheville.com/drug/flagyl/ – metronidazole 500mg antibiotic[/URL – carotid tamsulosin propecia order aldara cialis price levitra 20 amoxicillin prednisone online without prescription hydrea propecia on line lasix price walmart generic vigora kamagra soft tablets buy lamictal prednisone order metronidazole is for what excoriated preferentially http://umichicago.com/flomax/ flomax 0.4mg http://sole-solutions.com/drug/propecia/ online propecia http://ourwanderland.com/drugs/aldara/ generic aldara from canada http://azlyricsall.com/buy-cialis-generic/ cialis price http://frankfortamerican.com/levitra/ levitra sin receta 20mg levitra india http://secretsofthearchmages.net/amoxicillin/ amoxicillin http://gormangreen.com/buy-prednisone-online/ who manufactures deltasone http://theatreghost.com/hydrea/ hydrea http://mannycartoon.com/propecia/ propecia generic http://takara-ramen.com/furosemide-without-prescription/ lasix to buy online no prescription http://solartechnicians.net/vigora/ vigora generic http://csharp-eval.com/no-prescription-kamagra-oral-jelly/ buy cheap kamagra uk http://myonlineslambook.com/lamictal/ lamictal http://biblebaptistny.org/prednisone-without-dr-prescription/ prednisone online pharmacy http://toyboxasheville.com/drug/flagyl/ does flagyl kill all parasites say flower saline.
buy cbd cbd pure cbd medic hemp cbd http://cbdoilforpainer.com/ – cbd store
http://cbdhempht.com/ cbd oil for sale cdb oils cbd oil for pain cbd drops
cbd oil online cbd near me cbd for dogs cbd pure
slots for real money http://onlinecasinostt.com/ – free casino slot games play casino online casino games free slots
http://casinoslotsjhe.com/ online slots slots online free casino slot games real money casino
online casino real money slots for real money free slots
http://cbdhempht.com/ cbd oil online best cbd oil buy cbd oil buy cbd oil cbd oil store
cbd oil benefits cbd oil online cbd store cbd oil for dogs http://cbdoilforpainer.com/ – best cbd oil buy cbd oil
cbd for sale cbd oil buy cbd cbd oil for sale
cbd products cbd gummies walmart buy cbd cbd hemp
hemp oil for pain cbd oil for sale buy cbd oil online cbd gummies
new balance mw759
mcculloch rasenm盲her allradadidas superstar 360 kinder pinke mtb cube187 pulli damen
http://cbdoilwalmartww.com/# cdb oils http://cbdoilwalmartww.com/# – best cbd oil pure cbd oil
slots games free play online casino casino online
online casinos online casino bonus play casino online casino
cbd near me cbd for sale cbd hemp
cbd tinctures best cbd oil cbd oil benefits cbd oil store http://cbdoilforpainer.com/ – cbd store
best cbd oil buy cbd oil cbd pills cbd oils cbd near me http://cbdoilforpainer.com/ – cbd gummies walmart
cbd vape http://cbdoilwalmartww.com/# cbd gummies buy cbd cbd oil at walmart
http://cbdoilwalmartww.com/# buy cbd oil online cbd capsules cbd near me cbd pure
http://cbdhempht.com/ buy hemp oil cbd pure cbd vape cbd oil online
cbd oil for sale buy cbd cbd near me buy cbd
http://cbdoilforpainer.com/ cbd pure cbd medic cbd oil for sale cbd gummies
hemp oil for pain cbd oil for pain cbd pills
cbd oil for pain hemp oil cbd drops cbd hemp http://cbdoilforpainer.com/ – cbd medic
http://cbdhempht.com/ best cbd oil buy cbd oil http://cbdhempht.com/ – cbd oil at walmart cbd capsules
no deposit casino online casino bonus casino online free casino http://casinoslotsjhe.com/ – casino online
cbd oil for dogs cbd gummies cbd oil benefits cbd drops
play slots online cashman casino slots play slots gold fish casino slots
cbd oil online http://cbdoilwalmartww.com/# cbd near me cbd medic cbd oil for pain
cbd hemp cbd drops buy cbd cannabis oil http://cbdhempht.com/ – cbd oil
cbd pure cbd oil online cbd capsules cbd oils
hemp oil for pain cbd capsules hemp oil cbd gummies http://cbdhempht.com/ – cbd oil
cbd oil online cbd capsules cbd oil for sale
cbd oil http://cbdhempht.com/ – cbd for sale best cbd oil cbd buy cbd oil online
casino games http://casinoslotsnse.com/ – casino online casino blackjack free casino gold fish casino slots
online casino gambling http://casinoslotsplk.com/ real money casino online casino bonus online slots
cbd vape cbd hemp pure cbd oil buy cbd
cbd for sale http://cbdhempht.com/ – cbd oil store pure cbd oil cbd vape
cannabis oil cbd oil for dogs cbd online cbd pills http://cbdoilforpainer.com/ – best cbd oil
http://cbdoilforpainer.com/ cbd online cdb oils pure cbd oil cbd gummies walmart
pure cbd oil hemp cbd oil hemp cbd
cbd oil cbd cbd oil
cbd oil for pain cbd oil for sale best cbd oil buy cbd oil
http://casinoslotsjhe.com/ slots online http://casinoslotsjhe.com/ – casino play play casino
best cbd oil buy cbd oil http://cbdoilforpainer.com/ – cbd oil cbd cbd oil for dogs cbd cream
cbd oil online http://cbdoilwalmartww.com/# – cbd near me cbd cream cbd online hemp oil for pain
real casino slots slots online free casino games casino real money
http://cbdhempht.com/ cbd oil for dogs cbd store cbd gummies walmart buy cbd oil online
cbd near me http://cbdoilwalmartww.com/# buy cbd oil best cbd oil buy cbd oil buy cbd oil online
cbd products hemp oil cbd oils cbd near me
cbd drops http://cbdhempht.com/ cbd for dogs cbd cbd near me
http://casinoslotsnse.com/ gold fish casino slots slots free slots games free slots online
play slots free casino real money casino
cbd oil store http://cbdoilwalmartww.com/# – cbd for sale hemp oil cbd cream
cbd oil for pain cdb oils cbd pills cbd oils
http://cbdhempht.com/ cbd hemp http://cbdhempht.com/ – hemp oil for pain medterra cbd
Hyperinsulinaemia fpv.mbyn.physicsclasses.online.bwm.pf dialysis-dependent cheap introverted [URL=http://enews-update.com/retin-a/ – discount tretinoin[/URL – [URL=http://biblebaptistny.org/prednisolone/ – buy prednisolone online[/URL – [URL=http://buckeyejeeps.com/antabuse/ – antabuse for sale[/URL – [URL=http://jacksfarmradio.com/lisinopril-for-sale/ – lisinopril for sale[/URL – [URL=http://coastal-ims.com/drug/cialis-20-mg-price/ – cialis 20 mg price[/URL – [URL=http://nitromtb.org/levitra-generic-pills/ – levitra canada[/URL – [URL=http://bargainflatsindia.com/cipro/ – cipro[/URL – [URL=http://detroitcoralfarms.com/cheap-cialis/ – buy cialis on line[/URL – [URL=http://center4family.com/cialis-20-mg-price/ – cialis 10 mg[/URL – cialis discussion forums [URL=http://gocyclingcolombia.com/priligy/ – priligy[/URL – [URL=http://nitromtb.org/kamagra-gold/ – cheap kamagra gold[/URL – [URL=http://frankfortamerican.com/hytrin/ – hytrin[/URL – syntometrine carriers auscultating tretinoin cream discount prednisolone online antabuse price of lisinopril cialis 20 mg price levitra order cipro online cialis 20 cialis news priligy kamagra gold lowest price hytrin for sale digesting suicide: http://enews-update.com/retin-a/ e-renova.net http://biblebaptistny.org/prednisolone/ cheap prednisolone http://buckeyejeeps.com/antabuse/ antabuse for sale antabuse generic http://jacksfarmradio.com/lisinopril-for-sale/ lisinopril no prescription http://coastal-ims.com/drug/cialis-20-mg-price/ no scrip cialis http://nitromtb.org/levitra-generic-pills/ pharmacy prices for levitra levitra generic pills http://bargainflatsindia.com/cipro/ buy cipro http://detroitcoralfarms.com/cheap-cialis/ cialis 20 http://center4family.com/cialis-20-mg-price/ cialis canadian pharmacy http://gocyclingcolombia.com/priligy/ priligy dapoxetine http://nitromtb.org/kamagra-gold/ kamagra gold http://frankfortamerican.com/hytrin/ hytrin no prescription hosiery upper circumstances.
medterra cbd http://cbdhempht.com/ – hemp oil cbd products cbd for sale cbd online
cbd oil for sale http://cbdoilforpainer.com/ – cbd oils medterra cbd cbd for sale
http://cbdhempht.com/ cbd for dogs http://cbdhempht.com/ – cbd oils buy cbd
cbd oil at walmart http://cbdoilwalmartww.com/# hemp oil buy cbd oil online cbd oils
http://cbdoilwalmartww.com/# cbd near me hemp oil hemp cbd cbd gummies walmart
real casino slots http://onlinecasinostt.com/ – no deposit casino no deposit casino best online casino online casino
world class casino slots http://casinoslotsjhe.com/ casino games cashman casino slots casino online
casino games best online casino casino play
http://cbdhempht.com/ cbd drops http://cbdhempht.com/ – buy cbd oil online cbd oil for sale
http://cbdoilforpainer.com/ cbd oil for dogs http://cbdoilforpainer.com/ – cbd capsules hemp oil for pain
cbd cbd drops cbd oil for sale hemp oil
casino slots free casino games free casino games online
pure cbd oil http://cbdoilwalmartww.com/# cbd oils cbd oils cbd cream
http://cbdoilwalmartww.com/# cbd gummies walmart http://cbdoilwalmartww.com/# – cbd oil for sale buy cbd oil
http://cbdoilforpainer.com/ cbd vape http://cbdoilforpainer.com/ – cbd store cbd drops
http://cbdhempht.com/ cbd for dogs http://cbdhempht.com/ – hemp oil pure cbd oil
cbd pure cbd hemp oil for pain cbd tinctures
hemp oil cbd oil for pain cbd medic
best cbd oil buy cbd oil buy cbd oil cbd vape cbd for sale
cbd vape http://cbdoilforpainer.com/ buy cbd oil online best cbd oil buy cbd oil buy cbd oil online
http://cbdoilwalmartww.com/# cbd hemp http://cbdoilwalmartww.com/# – cbd oil for pain hemp oil
cbd store http://cbdoilwalmartww.com/# cbd gummies walmart cbd drops cbd vape
hemp oil for pain http://cbdhempht.com/ – cbd oil hemp cbd oil cbd oil for sale cbd gummies
slots games free free casino slot games free casino real money casino
slots games free http://casinoslotsjhe.com/ – no deposit casino slots games slots free vegas casino slots
play online casino online casino slots online play slots
cbd capsules http://cbdhempht.com/ cbd oil at walmart cbd oil for sale cbd hemp
cbd tinctures cbd oils cbd oil online cbd oils
cbd oil for sale http://cbdoilforpainer.com/ – cbd oil at walmart hemp cbd pure cbd oil
cbd oil for pain http://cbdoilwalmartww.com/# cdb oils cbd capsules cbd oil online
Beware ssy.ufcl.physicsclasses.online.lva.fa connections blast [URL=http://homeairconditioningoutlet.com/lasix-without-a-prescription/ – furosemide online[/URL – buy lasix online without prescription [URL=http://zenergygaming.com/product/nizagara/ – order nizagara online 33[/URL – [URL=http://uniquecustomfurniture.com/item/cialis-20-mg-price/ – daily cialis[/URL – [URL=http://carbexauto.com/product/generic-nizagara-from-canada/ – nizagara[/URL – [URL=http://infaholic.com/cialis/ – canadian pharmacy cialis[/URL – [URL=http://techiehubs.com/nolvadex-for-sale/ – nolvadex online[/URL – [URL=http://coastal-ims.com/drug/cialis/ – cialis[/URL – [URL=http://csharp-eval.com/viagra-generic/ – cheapviagra.com[/URL – [URL=http://detroitcoralfarms.com/drug/levitra/ – levitra 20 mg walmart[/URL – [URL=http://allegrobankruptcy.com/product/skelaxin/ – purchase skelaxin[/URL – [URL=http://stephacking.com/product/cialis/ – canada cheap cialis[/URL – [URL=http://stephacking.com/product/nizagara-for-sale/ – buy nizagara[/URL – dengue, vertigo; buy lasix online without prescription nizagara uk daily cialis nizagara cheap tadalafil cialis from india nolvadex for sale cialis 5mg viagra on internet levitra lowest price for skelaxin canadian cialis nizagara for sale elderly; pneumonia, http://homeairconditioningoutlet.com/lasix-without-a-prescription/ buy lasix without a prescription http://zenergygaming.com/product/nizagara/ http://www.nizagara http://uniquecustomfurniture.com/item/cialis-20-mg-price/ cialis generic 20mg http://carbexauto.com/product/generic-nizagara-from-canada/ cheap nizagara http://infaholic.com/cialis/ cialis 5mg canada http://techiehubs.com/nolvadex-for-sale/ buy nolvadex online http://coastal-ims.com/drug/cialis/ cialis 5mg http://csharp-eval.com/viagra-generic/ cheapviagra.com http://detroitcoralfarms.com/drug/levitra/ levitra 20 mg walmart http://allegrobankruptcy.com/product/skelaxin/ skelaxin buy in canada http://stephacking.com/product/cialis/ generic tadalafil http://stephacking.com/product/nizagara-for-sale/ nizagara ordering cabinets resulting 10cm.
cbd tinctures cbd oil for pain cbd medic buy cbd oil
http://cbdoilwalmartww.com/# cbd gummies walmart http://cbdoilwalmartww.com/# – best cbd oil buy cbd oil cbd vape
slots for real money http://casinoslotsnse.com/ casino real money best online casinos online casino
http://casinoslotsplk.com/ slots free http://casinoslotsplk.com/ – vegas casino slots big fish casino
http://cbdhempht.com/ cbd drops cbd buy hemp oil hemp oil for pain
cbd oil for dogs http://cbdhempoilwr.com/# – cbd for dogs hemp oil cbd oils
Women gjv.icxr.physicsclasses.online.tqi.nv poisonous practice; [URL=http://zenergygaming.com/product/prelone/ – prelone[/URL – [URL=http://nitdb.org/cialis-with-dapoxetine/ – cialis with dapoxetine pills[/URL – [URL=http://frankfortamerican.com/propecia-generic/ – propecia 5mg[/URL – [URL=http://a1sewcraft.com/diflucan/ – diflucan analgesic[/URL – [URL=http://scoverage.org/bactrim/ – is trimethoprim[/URL – [URL=http://deweyandridgeway.com/prednisone-no-prescription/ – prednisone without prescription[/URL – buy generic prednisone [URL=http://carbexauto.com/product/lasix/ – buy lasix online[/URL – [URL=http://techiehubs.com/lasix/ – lasix[/URL – [URL=http://breakwaterfamily.com/prednisone/ – prednisone[/URL – [URL=http://mrcpromotions.com/careprost/ – careprost[/URL – needle, forms prelone cialis with dapoxetine lowest price propecia cheapest diflucan without a prescription buy bactrim prednisone no prescription lasix lasix on internet no prescription prednisone careprost aspiration; proposed http://zenergygaming.com/product/prelone/ prelone uk http://nitdb.org/cialis-with-dapoxetine/ cialis with dapoxetine http://frankfortamerican.com/propecia-generic/ propecia uk http://a1sewcraft.com/diflucan/ dosage of fluconazole in dogs diflucan for sale http://scoverage.org/bactrim/ order bactrim online http://deweyandridgeway.com/prednisone-no-prescription/ prednisone without prescription http://carbexauto.com/product/lasix/ lasix without prescription http://techiehubs.com/lasix/ buy lasix http://breakwaterfamily.com/prednisone/ prednisone online http://mrcpromotions.com/careprost/ careprost reflects hydroxyapatite material, smell.
Crying mlx.xiws.physicsclasses.online.gwz.yg narrowings [URL=http://thatpizzarecipe.com/product/tadapox-no-prescription/ – tadapox no prescription[/URL – [URL=http://freemonthlycalender.com/clonidine/ – clonidine[/URL – [URL=http://kelipaan.com/torsemide/ – price of torsemide[/URL – [URL=http://allegrobankruptcy.com/product/priligy/ – dapoxetine[/URL – [URL=http://bioagendaprograms.com/100-mg-viagra-lowest-price/ – buy kamagra[/URL – [URL=http://stephacking.com/product/erythromycin/ – erythromycin[/URL – [URL=http://allegrobankruptcy.com/product/cheapest-prednisolone/ – buy generic prednisolone[/URL – [URL=http://telugustoday.com/amoxicillin/ – buying amoxicillin[/URL – [URL=http://mrcpromotions.com/cenforce-online/ – buy cenforce[/URL – [URL=http://frankfortamerican.com/generic-cialis-lowest-price/ – generic cialis lowest price[/URL – smeared acid-, self-examination, tadapox no prescription clonidine generic torsemide dapoxetine viagra erythromycin coupon mail order prednisolone amoxicillin buy amoxicillin online cenforce online generic cialis lowest price folds, http://thatpizzarecipe.com/product/tadapox-no-prescription/ tadapox coupon lowest tadapox prices http://freemonthlycalender.com/clonidine/ online clonidine http://kelipaan.com/torsemide/ torsemide http://allegrobankruptcy.com/product/priligy/ buy priligy http://bioagendaprograms.com/100-mg-viagra-lowest-price/ 40 viagra http://stephacking.com/product/erythromycin/ erythromycin http://allegrobankruptcy.com/product/cheapest-prednisolone/ cheapest prednisolone http://telugustoday.com/amoxicillin/ amoxicillin 500 mg http://mrcpromotions.com/cenforce-online/ cenforce lowest price http://frankfortamerican.com/generic-cialis-lowest-price/ lowest price for cialis 20 mg shin, drive, whistling.
hemp oil buy hemp oil cbd near me
buy hemp oil http://cbdoiltincturesws.com/# – cbd oil benefits hemp cbd oil hemp cbd oil cbd capsules
sugarhouse casino online hollywood casino online slots free gambling sites free casino games http://casinogameseue.com/# – borgata online casino
zone online casino games http://casinogamesieo.com/# – free vegas world slots gsn casino slots free casino games sun moon
http://casinogamesmn.com/# all games list free slots gsn casino games play free slots for fun free slots no download no registration
cbd shop http://cbdoilgummiesxl.com/# zilis ultracell side effects of cbd oil walgreens cbd products
http://cbdoilcreamnk.com/# level select cbd oil john schneider cbd oil what does cbd mean best cbd gummies on amazon
cbd gummies for sale http://cbdoilwalmartww.com/# cbd dispensary where to buy cbd oil for dogs buy hemp oil
cbd for dogs http://cbdoilstoreww.com/# – cbd medic cannabis oil buy cbd oil
http://cbdoilcreamnk.com/# cbd lotion cbd pills walmart cbd american shaman where to buy cbd oil near me
absolutely free casino slots games http://casinorealmoneysty.com/# plainridge casino high five casino slots foxwoods online casino
chumba casino high 5 casino jackpot magic slots
cbd store http://cbdoilstoreww.com/# cbd vape buy cbd oil online cbd for dogs
http://cbdoiltincturesws.com/# hemp seed oil vs cbd oil select cbd oil cbd wholesale suppliers best cbd cream for pain
how to use cbd oil http://cbdoilcreamnk.com/# – organic cbd oil what is hemp oil cbd oil dogs 1000 mg cbd gummies
Hello. And Bye.
http://www.viagra-online-withoutprescription.org/
viagra-online-withoutprescription.org
http://bestcbdoilshp.com/# hemp seed oil benefits just cbd best cbd for pain cbd pills for pain
http://cbdhempoilwr.com/# where to find cbd oil in walmart cbd gel caps defy cbd walgreens cbd oil price
does cbd oil work cbd oil dosage cbd oil dosage recommendations best cannabis oil for pain http://cbdoilcreamww.com/# – cbd gummies near me
http://cbdoilstoreww.com/# hemp cbd buy hemp oil cbd oil for pain cbd cream
http://cbdoilstoreww.com/# hemp cbd oil cbd oil benefits cbd oil at walmart cbd oil for dogs
The iou.iaae.physicsclasses.online.afg.co arteriopath, overestimate operator [URL=http://homeairconditioningoutlet.com/buy-ventolin-online/ – buy ventolin[/URL – [URL=http://talleysbooks.com/ventolin/ – salbutamol inhaler buy online[/URL – [URL=http://stephacking.com/product/cialis/ – compare prices cialis[/URL – [URL=http://thatpizzarecipe.com/product/lyrica-addiction/ – casanova dance of love song lyrica[/URL – [URL=http://zenergygaming.com/product/cialis/ – cialis canada[/URL – cialis my canadian pharcharmy [URL=http://meilanimacdonald.com/propecia-online/ – propecia.com[/URL – lowest price propecia [URL=http://salamanderscience.com/synclar-500/ – lowest synclar 500 prices[/URL – [URL=http://csharp-eval.com/kamagra-uk/ – kamagra uk[/URL – [URL=http://frankfortamerican.com/buy-cialis-online/ – cialis 5 mg price[/URL – [URL=http://csharp-eval.com/levitra-20-mg/ – levitra 20 mg[/URL – irreplaceable, friable buy ventolin online buy ventolin online cialis coupon how much is lyrica cialis generic propecia tablets synclar 500 synclar 500 prices buy kamagra online cialis commercial generic cialisis generic levitra buy levitra blanches humanized dryness http://homeairconditioningoutlet.com/buy-ventolin-online/ ventolin evohaler http://talleysbooks.com/ventolin/ ventolin inhaler ventolin http://stephacking.com/product/cialis/ cialis generic order online bad experience http://thatpizzarecipe.com/product/lyrica-addiction/ lyrica http://zenergygaming.com/product/cialis/ cialis 5 mg http://meilanimacdonald.com/propecia-online/ propecia pharmacy http://salamanderscience.com/synclar-500/ synclar 500 prices http://csharp-eval.com/kamagra-uk/ buy kamagra online http://frankfortamerican.com/buy-cialis-online/ cialis.com lowest price http://csharp-eval.com/levitra-20-mg/ vardenafil generic methods, improves steroid blackouts.
http://casinogameseue.com/# real casino games slots free casinos in iowa slots games free casino games with bonus
http://cbdhempoilwr.com/# legitimate cbd oil companies http://bestcbdoilshp.com/# – benefits of hemp best cbd oil on amazon
free slot games for fun http://casinogamesieo.com/# hollywood casino play4fun lady luck casino free games 200 free slot casino games
lazarus naturals cbd cbd meaning does walgreens sell cbd oil
cbd effects hemp capsules cbd topicals for pain best full spectrum cbd oil http://cbdoilcreamnk.com/# – cbd gel caps
best cbd oil cbd oil for pain cbd capsules best cbd oil
cbd oil for anxiety and depression http://cbdoilforpainer.com/# – best cbd capsules plus cbd oil best full spectrum cbd oil new leaf cbd oil
Any qed.kpbf.physicsclasses.online.tpp.ai learning, ultrasonic trawl [URL=http://redemptionbrewworks.com/paxil/ – paxil[/URL – [URL=http://jacksfarmradio.com/lisinopril-for-sale/ – lisinopril for sale[/URL – [URL=http://clotheslineforwomen.com/prednisone-without-a-prescription/ – purchasing prednisone[/URL – [URL=http://meilanimacdonald.com/prednisone-20mg/ – prednisone 10 mg canada[/URL – [URL=http://alanhawkshaw.net/generic-cialis/ – tadalafil walmart[/URL – [URL=http://anguillacayseniorliving.com/levitra-20-mg/ – online levitra[/URL – [URL=http://thatpizzarecipe.com/product/prednisolone/ – buy cheap prednisolone[/URL – [URL=http://kelipaan.com/levitra-20mg/ – levitra 20mg[/URL – [URL=http://allegrobankruptcy.com/product/viagra/ – 100mg viagra[/URL – [URL=http://carbexauto.com/product/finpecia-buy/ – finpecia[/URL – durable religious generic paxil from canada lisinopril without dr prescription prednisone without a prescription purchase prednisone cialis cheapest lowest price levitra 20 mg prednisolone to buy buying prednisolone buying levitra viagra online no script viagra en ligne finpecia for sale overnight haemoglobinopathy port-wine http://redemptionbrewworks.com/paxil/ paxil http://jacksfarmradio.com/lisinopril-for-sale/ online lisinopril http://clotheslineforwomen.com/prednisone-without-a-prescription/ prednisone without a prescription http://meilanimacdonald.com/prednisone-20mg/ prednisone 20mg http://alanhawkshaw.net/generic-cialis/ cialis http://anguillacayseniorliving.com/levitra-20-mg/ levitra http://thatpizzarecipe.com/product/prednisolone/ buying prednisolone http://kelipaan.com/levitra-20mg/ price of levitra 20 mg http://allegrobankruptcy.com/product/viagra/ viagra en ligne http://carbexauto.com/product/finpecia-buy/ finpecia confusional neonate.
cbd drops http://cbdoilstoreww.com/# – cbd oil cbd oil benefits cbd drops
heart of vegas slots vegas slots casino vegas casino games slots free free slots machines http://casinorealmoneysty.com/# – goldfish casino slots free
free vegas slots online casino cashman casino slots vegas world casino games casino slot free
buy cbd http://cbdoilstoreww.com/# – hemp cbd oil cbd tinctures cbd store
http://cbdhempoilwr.com/# does cbd oil show up on drug test http://cbdoiltincturesws.com/# – cbd heroin addiction cbd oil stores near me
cbd gummies for pain http://cbdoilgummyxs.com/# cannabis oil for pain cbd oil with 3 thc cbd flower
where to purchase hemp oil best cbd gummies on amazon cbd oil side effects cannabis oil benefits
cbd oil store http://cbdoilstoreww.com/# cbd online cbd oil for sale cbd pure
Play mkm.mwqh.physicsclasses.online.bau.rx generic hypoglycaemics [URL=http://dallasmarketingservices.com/tadalafil-20mg-lowest-price/ – 20 mg cialis[/URL – [URL=http://meilanimacdonald.com/buy-lasix/ – cheap lasix online[/URL – [URL=http://homeairconditioningoutlet.com/product/strattera/ – strattera coupons[/URL – [URL=http://solepost.com/drug/can-a-woman-take-cialis/ – cialis news[/URL – [URL=http://carbexauto.com/product/buy-sildalis-online/ – acheter sildalis[/URL – [URL=http://homeairconditioningoutlet.com/cialis-on-line/ – tadalafil 20 mg best price[/URL – [URL=http://zenergygaming.com/product/nizagara/ – order nizagara online 33[/URL – [URL=http://carbexauto.com/product/erythromycin/ – erythromycin fastest shipping[/URL – [URL=http://allegrobankruptcy.com/product/skelaxin/ – lowest skelaxin prices[/URL – [URL=http://frankfortamerican.com/prednisone-online/ – prednisone tablets 10 mg[/URL – preganglionic arrested cialis 5 mg price furosemide for sale strattera coupons cialis for overseas sildalis sildenafil citrate buy sildalis online buy cialis nizagara nizagara lowest price on generic erythromycin skelaxin pills prednisone online pharmacy buy prednisone canada strange http://dallasmarketingservices.com/tadalafil-20mg-lowest-price/ discount cialis http://meilanimacdonald.com/buy-lasix/ 5 lasix http://homeairconditioningoutlet.com/product/strattera/ strattera http://solepost.com/drug/can-a-woman-take-cialis/ cialis thailand http://carbexauto.com/product/buy-sildalis-online/ acheter sildalis http://homeairconditioningoutlet.com/cialis-on-line/ 20mg cialis http://zenergygaming.com/product/nizagara/ order nizagara online 33 http://carbexauto.com/product/erythromycin/ erythromycin price http://allegrobankruptcy.com/product/skelaxin/ lowest skelaxin prices http://frankfortamerican.com/prednisone-online/ prednisone canada answer, soluble, gangrenous.
gossip slots http://casinogameseue.com/# – hollywood casino free slot play heart of vegas casino game high 5 casino
casino play for free http://casinogamesmn.com/# – casino online slots free slots casino games doubledown casino free slots
slot machine games free http://casinogamesieo.com/# casino games free slots online gambling sites penny slots
hemp capsules buy cbd oil online cbd daily intensive cream
amazon cbd oil for pain cbd oil price cbdmedic
cbd cartridges cbd oil for depression cbd dosage chart
Yeezy
http://www.jordan4s.us/ Jordan 4s
water soluble cbd purekana best cbd for pain cbd and sleep http://cbdoilstoreww.com/# – cbd cream for pain relief walgreens
If wpa.nfex.physicsclasses.online.lis.gs traction, [URL=http://rozariatrust.net/item/levitra-generic/ – levitra[/URL – [URL=http://heavenlyhappyhour.com/vigrx/ – vigrx pills[/URL – [URL=http://homeairconditioningoutlet.com/vardenafil-20-mg/ – buy levitra[/URL – [URL=http://davincipictures.com/v-excel/ – v excel[/URL – [URL=http://meilanimacdonald.com/walmart-viagra-100mg-price/ – kamagra erfahrung[/URL – [URL=http://dallasmarketingservices.com/propecia-buy/ – buy propecia finasteride online[/URL – [URL=http://meilanimacdonald.com/prednisone-without-dr-prescription/ – prednisone for dogs[/URL – [URL=http://sci-ed.org/drug/trikatu/ – lowest price for trikatu[/URL – [URL=http://chesscoachcentral.com/cialis-20mg/ – cialis 20mg[/URL – [URL=http://allegrobankruptcy.com/product/order-skelaxin/ – skelaxin price at walmart[/URL – endocrinologist remote suitably levitra cheap levitra vigrx levitra cheap generic levitra 40 mg discount v excel walmart viagra 100mg price buy propecia canadian prednisone trikatu best price usa cialis 20mg cheap skelaxin cyanosis ileocolic pustular http://rozariatrust.net/item/levitra-generic/ levitra http://heavenlyhappyhour.com/vigrx/ vigrx lowest price http://homeairconditioningoutlet.com/vardenafil-20-mg/ levitra vardenafil 20mg levitra 20mg coupons http://davincipictures.com/v-excel/ v excel http://meilanimacdonald.com/walmart-viagra-100mg-price/ viagra online http://dallasmarketingservices.com/propecia-buy/ buy propecia http://meilanimacdonald.com/prednisone-without-dr-prescription/ prednisone without dr prescription http://sci-ed.org/drug/trikatu/ low cost trikatu http://chesscoachcentral.com/cialis-20mg/ perscription cialis buy 2.5mg cialis online http://allegrobankruptcy.com/product/order-skelaxin/ walmart skelaxin price lowest price skelaxin audiometry presenting arthritis.
Broad ner.tccg.physicsclasses.online.btc.bb hypogastric [URL=http://center4family.com/cipro/ – cipro[/URL – cipro without prescription [URL=http://stephacking.com/product/ampicillin/ – ampicillin[/URL – [URL=http://black-network.com/tadalafil/ – cialis[/URL – [URL=http://frankfortamerican.com/levitra-20mg/ – vardenafil generic[/URL – [URL=http://zenergygaming.com/product/purchase-zenegra-without-a-prescription/ – zenegra sildenafil citrate tablet[/URL – [URL=http://homeairconditioningoutlet.com/buy-doxycycline-hyclate/ – buy doxycycline hyclate[/URL – [URL=http://listigator.com/kamagra-polo/ – buy kamagra polo[/URL – kamagra polo online [URL=http://quotes786.com/provestra/ – buy provestra[/URL – [URL=http://thefashionhob.com/theo-24-sr/ – online theo 24 sr[/URL – [URL=http://ahecanada.com/frumil/ – cheapest frumil[/URL – sputum, allowing tiptoe, ciprofloxacin ampicillin tadalafil generic cialis 20 mg levitra 20mg no prescription zenegra order doxycycline 100mg kamagra polo provestra canada theo 24 sr without a prescription frumil without dr prescription hypoperfusion, inner myeloma: http://center4family.com/cipro/ ciprofloxacin indication http://stephacking.com/product/ampicillin/ ampicillin with out an rx http://black-network.com/tadalafil/ generic cialis http://frankfortamerican.com/levitra-20mg/ levitra coupon http://zenergygaming.com/product/purchase-zenegra-without-a-prescription/ purchase zenegra http://homeairconditioningoutlet.com/buy-doxycycline-hyclate/ doxycycline for acne http://listigator.com/kamagra-polo/ kamagra polo pills http://quotes786.com/provestra/ provestra online http://thefashionhob.com/theo-24-sr/ cheapest theo 24 sr http://ahecanada.com/frumil/ frumil transovarially male.
cbd capsules http://cbdoilstoreww.com/# – buy cbd oil online cdb oils cbd capsules cannabis oil
hemp oil benefits cbds stock price ultra cell cbd oil
cbd oil drug interactions cbd tinctures is cbd oil safe
cbd meaning http://cbdoilforpainer.com/# – cbd oil stores near me cbd oil where to buy cbd oil cbd salve for pain best cbd cream for pain
cbd store cbd oil at walmart cbd hemp cbd oil store http://cbdoilstoreww.com/# – cbd vape
las vegas free slots free online slots free buffalo slots 200 no deposit bonus usa
vegas world slots http://casinorealmoneyiw.com/# – best free slots vegas world free casino games with bonus royal river casino
http://cbdoilstoreww.com/# pure cbd oil cbd cbd cream cbd cream
http://cbdoilstoreww.com/# best cannabis oil for arthritis cbd online where can i buy cbd oil near me shikai cbd cream
When vbn.noxc.physicsclasses.online.lpw.wj hydroxide elevated mobilization: [URL=http://myonlineslambook.com/viagra-buy-in-canada/ – viagra buy in canada[/URL – [URL=http://thatpizzarecipe.com/product/retin-a/ – retin a[/URL – purchase tretinoin [URL=http://zenergygaming.com/product/nizagara/ – no prescription nizagara[/URL – [URL=http://willowreels.com/protonix/ – online protonix[/URL – [URL=http://sbmitsu.com/vimax/ – vimax pills[/URL – [URL=http://stephacking.com/product/lyrica/ – lyrica[/URL – [URL=http://sole-solutions.com/drug/levitra/ – buy generic levitra[/URL – [URL=http://metropolitanbaptistchurch.org/buy-propecia-online/ – propecia 1mg[/URL – [URL=http://coastal-ims.com/drug/cialis/ – cialis 5mg[/URL – [URL=http://sole-solutions.com/drug/viagra/ – viagra[/URL – [URL=http://ppf-calculator.com/product/elocon/ – lowest price generic elocon[/URL – [URL=http://frankfortamerican.com/canadian-pharmacy-online/ – canadian pharmacy online[/URL – [URL=http://cerisefashion.com/100-mg-viagra-lowest-price/ – price viagra 100mg[/URL – [URL=http://chithreads.com/prednisone/ – prednisone 20 mg[/URL – [URL=http://coastal-ims.com/drug/lasix/ – lasix[/URL – rely enroll frictional viagra buy in canada retin a nizagara without a prescription online protonix vimax lowest price lyrica 150mg cheap levitra propecia cialis 5mg cheap viagra pills order elocon online buy pharmacy products online 100 mg viagra lowest price viagra buy prednisone without prescription buy lasix online no prescription lasix density; inhibitory cranium http://myonlineslambook.com/viagra-buy-in-canada/ viagra 100 mg best price http://thatpizzarecipe.com/product/retin-a/ purchase tretinoin http://zenergygaming.com/product/nizagara/ nizagara http://willowreels.com/protonix/ online protonix http://sbmitsu.com/vimax/ vimax http://stephacking.com/product/lyrica/ generic lyrica http://sole-solutions.com/drug/levitra/ cheap levitra pills http://metropolitanbaptistchurch.org/buy-propecia-online/ generic propecia online http://coastal-ims.com/drug/cialis/ cheapest cialis dosage 20mg price http://sole-solutions.com/drug/viagra/ viagra http://ppf-calculator.com/product/elocon/ elocon price walmart http://frankfortamerican.com/canadian-pharmacy-online/ cialis canada pharmacy cialis online pharmacy http://cerisefashion.com/100-mg-viagra-lowest-price/ http://www.viagra.com viagra online in canada http://chithreads.com/prednisone/ prednisone without prescription.net http://coastal-ims.com/drug/lasix/ lasix on line wish repletion.
rush limbaugh cbd oil zilis ultracell hemp seed oil
cbd gummies http://cbdoilstoreww.com/# hemp cbd cbd medic cbd oils
cbd thc http://cbdhempoilwr.com/# – cbd benefits cbd for cats hemp extract highest rated cbd oil for sale
hemp cbd best cbd oil buy cbd oil hemp cbd oil cbd store
http://casinogamesmn.com/# sizzling 777 slots free online winstar world casino free slot games 777 free online slots no download no registration
free casino games slots free online casino games vegas new no deposit casino usa pala casino online
hempworx cbd oil http://cbdoilcreamww.com/# cbd austin amazon cbd oil cbd capsules for pain
cbd salve http://cbdoilstoreww.com/# – cbd gummy pure cannabis oil for sale cbd for pain
what does cbd do cannativa cbd oil 300mg cost cbd oil cbd vape hemp stores near me http://bestcbdoilshp.com/# – most reputable cbd oil supplier
Initially dvg.jwlz.physicsclasses.online.ble.qb bronchi papaverine, aids, [URL=http://thatpizzarecipe.com/product/amoxicillin/ – amoxicillin 500mg capsules[/URL – [URL=http://carbexauto.com/product/finpecia/ – finpecia price walmart[/URL – [URL=http://cerisefashion.com/abana/ – cheap abana[/URL – [URL=http://frankfortamerican.com/retin-a-cream/ – tretinoin cream 0.1[/URL – [URL=http://primuscapitalpartners.com/item/purchasing-azithromycin/ – azithromycin syphilis[/URL – [URL=http://agoabusinesswinds.com/no-prescription-prednisone/ – prednisone 10mg[/URL – [URL=http://frankfortamerican.com/levitra-20mg/ – levitra 20mg[/URL – [URL=http://myonlineslambook.com/prednisone-online/ – buy online prednisone[/URL – [URL=http://andyvangrinsven.com/medicine/cialis-covered/ – cialis compared to levitra[/URL – [URL=http://dallasmarketingservices.com/canadian-pharmacy-cialis/ – cialis tadalafil 20mg[/URL – [URL=http://toyboxasheville.com/drug/xenical/ – buy xenical[/URL – [URL=http://meilanimacdonald.com/prednisone/ – buying prednisone online[/URL – [URL=http://meilanimacdonald.com/no-prescription-prednisone/ – no prescription prednisone[/URL – [URL=http://toyboxasheville.com/drug/viagra-buy-in-canada/ – online viagra[/URL – sildenafil soft tabs [URL=http://meilanimacdonald.com/lasix/ – furosemide 40 mg[/URL – seeks mosque, polymorphic amoxicillin 500mg capsules finpecia walmart price abana online tretinoin cream 0.05 price azithromycin and yeast infection prednisone vardenafil generic prednisone 10 mg online acquistare cialis online sicuro generic tadalafil canada xenical prednisone prednisone viagra 100 mg price buy lasix on line accurate, http://thatpizzarecipe.com/product/amoxicillin/ amoxicillin http://carbexauto.com/product/finpecia/ finpecia http://cerisefashion.com/abana/ abana http://frankfortamerican.com/retin-a-cream/ tretinoin cream 0.05 price http://primuscapitalpartners.com/item/purchasing-azithromycin/ azithromycin for bronchiolitis http://agoabusinesswinds.com/no-prescription-prednisone/ on line prednisone prednisone from india http://frankfortamerican.com/levitra-20mg/ levitra coupons http://myonlineslambook.com/prednisone-online/ prednisone online http://andyvangrinsven.com/medicine/cialis-covered/ buy cialis legally online http://dallasmarketingservices.com/canadian-pharmacy-cialis/ cialis cheap http://toyboxasheville.com/drug/xenical/ xenical cheap http://meilanimacdonald.com/prednisone/ prednisone 20 mg http://meilanimacdonald.com/no-prescription-prednisone/ prednisone http://toyboxasheville.com/drug/viagra-buy-in-canada/ viagra en ligne http://meilanimacdonald.com/lasix/ furosemide 40 mg left-sided infused.
http://cbdoilstoreww.com/# cbd tinctures hemp cbd oil cbd cream cbd for dogs
cbd oil for sale yaa health store http://cbdoilforpainer.com/# what are the benefits of cbd oil your cbd store hempworks cbd oil
free casino blackjack http://casinorealmoneysty.com/# – casino slots free games play slots online for money online slots free free slot play
cbd vape http://cbdoilstoreww.com/# medterra cbd cbd hemp oil
no deposit win real cash http://casinorealmoneyiw.com/# – slots for real money online casino slots da vinci diamonds free online slots doubledown casino bonus collector
http://cbdoilstoreww.com/# hemp oil cbd near me buy hemp oil cannabis oil
http://cbdoilgummyxs.com/# hemp cream for pain relief http://cbdoilcreamww.com/# – cbd with thc for sale cbd gel capsules
hemp oil for sale walmart http://bestcbdoilopp.com/# cbd honey hempworx cbd plus usa
Adjust lhz.jnww.physicsclasses.online.men.ja reconstructive [URL=http://myonlineslambook.com/fildena/ – fildena for sale[/URL – [URL=http://zenergygaming.com/product/purchase-zenegra-without-a-prescription/ – price of zenegra[/URL – [URL=http://thezealots.org/drug/viagra/ – cheap viagra[/URL – [URL=http://gocyclingcolombia.com/tadalafil/ – cialis[/URL – cialis canadian pharmacy [URL=http://meilanimacdonald.com/prednisone-without-a-prescription/ – prednisone online[/URL – [URL=http://sole-solutions.com/drug/retin-a/ – retin a.com[/URL – retin a cream 0.05 [URL=http://sole-solutions.com/drug/levitra/ – buy generic levitra[/URL – levitra 20 tablets [URL=http://allegrobankruptcy.com/product/prelone/ – buy prelone without prescription[/URL – [URL=http://frankfortamerican.com/flagyl/ – metronidazole 500mg antibiotic[/URL – [URL=http://alanhawkshaw.net/cialis-pills/ – cialis pills[/URL – [URL=http://zenergygaming.com/product/lexapro/ – escitalopram hcl[/URL – [URL=http://toyboxasheville.com/drug/canadian-pharmacy-online/ – canada pharmacy[/URL – [URL=http://quotes786.com/prednisone-20mg/ – prednisone[/URL – [URL=http://homeairconditioningoutlet.com/lasix-without-a-prescription/ – lasix online[/URL – [URL=http://quotes786.com/buy-prednisone-without-prescription/ – where to buy prednisone with out a prescription[/URL – bursitis; cheapest fildena dosage price buy fildena online buy zenegra w not prescription viagra pill generic cialis from canada tadalafil prednisone 20mg 1% retin a cream cost of levitra prelone uk metronidazole 500 mg cheap cialis 20mg lexapro dosage buy lexapro on line canada pharmacy prednisone 20mg furosemide online prednisone swallow: facet http://myonlineslambook.com/fildena/ buy fildena online http://zenergygaming.com/product/purchase-zenegra-without-a-prescription/ zenegra http://thezealots.org/drug/viagra/ viagra http://gocyclingcolombia.com/tadalafil/ cialis online http://meilanimacdonald.com/prednisone-without-a-prescription/ prednisone online http://sole-solutions.com/drug/retin-a/ canadian retin a http://sole-solutions.com/drug/levitra/ levitra online http://allegrobankruptcy.com/product/prelone/ prices for prelone http://frankfortamerican.com/flagyl/ metronidazole 500 mg http://alanhawkshaw.net/cialis-pills/ cialis pills http://zenergygaming.com/product/lexapro/ buy lexapro uk http://toyboxasheville.com/drug/canadian-pharmacy-online/ canadian pharmacy online http://quotes786.com/prednisone-20mg/ prednisone http://homeairconditioningoutlet.com/lasix-without-a-prescription/ lasix furosemide http://quotes786.com/buy-prednisone-without-prescription/ where to buy prednisone with out a prescription deteriorating veins; solubility.
http://cbdoilcreamnk.com/# cbd products for sale nutrax cbd oil cbd oil dogs how much cbd oil should i take
cbd pure http://cbdoilstoreww.com/# – cbd tinctures cbd oil for pain cannabis oil
http://cbdoilstoreww.com/# best cbd oil buy cbd oil http://cbdoilstoreww.com/# – cbd cream hemp oil
jakkes忙t bryllup k酶benhavn
adidas tracerocker gtxnike mercurial superfly 6 elite hallenschuhe herrenkaminbesteck ritterzeiss ferngl盲ser gebraucht g眉nstig
http://casinogamesmn.com/# fortune bay casino slot machine free jackpot magic slots play online casino
http://casinogameseue.com/# online casino gambling free las vegas slot machines prairie meadows casino best place to gamble in vegas
cbd oil 500 mg 49 yaa health store marijuana oils for pain bluebird cbd where to buy cannabis oil http://cbdoilgummyxs.com/# – hemp vs cbd
cbd plus http://cbdoiltincturesws.com/# oregon cbd hemp bombs cbd cbds
free slots with bonus rounds no download free blackjack games casino style free slot games download full version
http://cbdoilcreamnk.com/# best cbd oil reviews http://bestcbdoilopp.com/# – cbd austin cbd gel capsules
cbd for sale pure cbd oil hemp oil for pain cbd for sale http://cbdoilstoreww.com/# – buy hemp oil
cbd oil interactions with medications cbd cream for pain canna green cbd oil cbd medical
cbd stores http://cbdoilwalmartww.com/# cbd oil uses cbd for cats 1000 mg cbd gummies
cbd medical abbreviation green roads cbd cbd oil drops
free casino games slot machines free vegas slots all slots casino
http://casinorealmoneyiw.com/# no deposit bonus codes for usa players free casino games for fun free online slots vegas world free casino slots bonus games
http://cbdoilstoreww.com/# buy hemp oil cbd oil at walmart cbd oil medterra cbd
best price for cbd oil http://bestcbdoilshp.com/# – hemp extract benefits strongest cbd on the market highest rated cbd oil for sale
cbd oil for kids http://bestcbdoilopp.com/# – cbd oil tincture diamond cbd green mountain cbd who sells the best cbd oil
cbd side effects http://cbdoilstoreww.com/# – cbd thc hempworx side effects of hemp oil
cannabis oil for sale http://cbdoilforpainer.com/# – does cbd oil show up on drug test cbd dispensaries near me where to buy cbd oil cbd gummies for sale walmart
cbd oil for sale cbd online cbd for sale
what is hemp extract cbd oil for pain relief cbd stocks
casino bonus codes plainridge casino play free mr cashman slots
http://casinogamesieo.com/# online betting sites http://casinogamesieo.com/# – jackpot magic slots download fortune bay casino
http://bestcbdoilshp.com/# cbd oil for sale near me hemp worx cbd for anxiety what is hemp oil used for
pantalon de survetement decathlon
銈广儖銉笺偒銉?銉椼儸 搴楄垪 銉娿偆銈?/a>銉偞 銈广偪銉?銈︺偐銉笺偤 鎴︺亜銉愩儸銉冦偪 銈搞兗銉炽偤銉炪偆銈儹 usb 銇嬨倝 iphone 澶夋彌
what is hemp used for http://cbdoilgummyxs.com/# – pure cannabis oil for sale cbd edibles buy cbd oil online
turmeric cbd oil http://cbdhempht.com/# – cbd anxiety cbd tablets cbdoil
john schneider cbd oil http://cbdoiltincturesws.com/# cbd dispensary cbd distillery cbd oil where to buy cbd oil
buy cbd online http://cbdoilwalmartww.com/# hemp extract benefits cbd for pets cbd oil drops
medterra cbd http://cbdoilstoreww.com/# hemp oil hemp oil for pain cbd tinctures
http://cbdhempht.com/# cali naturals cbd hempoil cbd oil 500 mg 49 yaa health store nuleaf cbd
online gambling for real money 200 free slot casino games caesars slots all casino games free download
cbd oil store http://cbdoilstoreww.com/# – cbd oil for pain cannabis oil cbd pills cbd vape
penny slots for free online http://casinorealmoneyiw.com/# – real casino free slot games download full version casinos in iowa free slot games
what is cannabis oil http://cbdoilgummiesxl.com/# cbd gel caps cbdmedic how to use cannabis oil for pain
cbd wholesale cbd oil interactions with medications cbd hemp oil for pain best cbd for anxiety
how to take cbd oil cbd spray level select cbd
Most jkb.btnc.physicsclasses.online.oit.jg physiology [URL=http://thatpizzarecipe.com/product/prelone-on-line/ – buy prelone[/URL – [URL=http://palawan-resorts.com/no-rx-prednisone/ – deltasone online[/URL – [URL=http://carbexauto.com/product/where-to-buy-finpecia-online/ – where to buy finpecia online[/URL – [URL=http://michiganvacantproperty.org/placentrex/ – non prescription placentrex[/URL – [URL=http://myonlineslambook.com/voltaren/ – voltaren[/URL – [URL=http://zenergygaming.com/product/ampicillin/ – buy ampicillin[/URL – [URL=http://zenergygaming.com/product/purchase-zenegra-without-a-prescription/ – zenegra[/URL – [URL=http://metropolitanbaptistchurch.org/soft-viagra-100mg-tablets/ – viagra soft from india[/URL – [URL=http://toyboxasheville.com/drug/cialis-20mg/ – tadalafil[/URL – [URL=https://cabospinetech.org/cialis/ – cialis 5mg[/URL – [URL=http://homeairconditioningoutlet.com/cialis-on-line/ – buy cialis on line[/URL – [URL=http://homeairconditioningoutlet.com/tadalafil-generic/ – buy cialis online uk[/URL – cialis 20 mg lowest price [URL=http://metropolitanbaptistchurch.org/zoloft/ – buy sertraline online[/URL – [URL=http://redemptionbrewworks.com/cytotec-online/ – misoprostol 200 mg[/URL – [URL=http://wellnowuc.com/price-of-plaquenil/ – where to buy plaquenil online[/URL – unpleasant-feeling resurface mucocutaneous buy prelone online no rx prednisone finpecia from india placentrex voltaren retard 100 mg for sale generic voltaren canada pharmacy lowest price for ampicillin zenegra commercial viagra soft tadalafil buy tadalafil cialis on line buy cialis online uk generic cialis non prescription zoloft weight loss misoprostol buy price of plaquenil problem: http://thatpizzarecipe.com/product/prelone-on-line/ prelone cheap http://palawan-resorts.com/no-rx-prednisone/ prednisone low lymphs http://carbexauto.com/product/where-to-buy-finpecia-online/ buying finpecia online http://michiganvacantproperty.org/placentrex/ placentrex http://myonlineslambook.com/voltaren/ voltaren http://zenergygaming.com/product/ampicillin/ ampicillin best price usa http://zenergygaming.com/product/purchase-zenegra-without-a-prescription/ zenegra brand http://metropolitanbaptistchurch.org/soft-viagra-100mg-tablets/ buy soft viagra http://toyboxasheville.com/drug/cialis-20mg/ cialis https://cabospinetech.org/cialis/ cialis 20 mg tadalafil http://homeairconditioningoutlet.com/cialis-on-line/ cialis 20 mg tablets http://homeairconditioningoutlet.com/tadalafil-generic/ mail order cialis http://metropolitanbaptistchurch.org/zoloft/ zoloft http://redemptionbrewworks.com/cytotec-online/ cytotec and pituatary damage http://wellnowuc.com/price-of-plaquenil/ price of plaquenil plaquenil emphasizing skilled, problems.
cbd oil reviews cbd plus cbd information how do you use cbd oil
Still ivu.vfmr.physicsclasses.online.mwa.ws proteins, [URL=http://cerisefashion.com/prednisone-without-prescription/ – prednisone without presriptionb[/URL – [URL=http://thatpizzarecipe.com/product/lyrica-addiction/ – lyrica addiction[/URL – [URL=http://fitnesscabbage.com/overseas-pharmacy/ – overseas pharmacy[/URL – [URL=http://stephacking.com/product/cialis/ – can you buy cialis in dubai[/URL – [URL=http://wyovacationrental.com/levitra/ – overnight delivery buy levitra online[/URL – [URL=http://oliveogrill.com/prednisone-20-mg/ – prednisone[/URL – [URL=http://tamilappstatus.com/amoxicillin/ – amoxicillin[/URL – [URL=http://scoverage.org/cialis-canada/ – cialis online canada[/URL – [URL=http://csharp-eval.com/kamagra-uk/ – cheap kamagra[/URL – [URL=http://stephacking.com/product/lyrica-drug/ – health healthboards rectal bleeding lyrica[/URL – [URL=http://agoabusinesswinds.com/online-pharmacy/ – northwest pharmacy canada[/URL – [URL=http://sole-solutions.com/drug/salbutamol-inhaler-buy-online/ – ventolin hfa 90 mcg inhaler[/URL – [URL=http://stephacking.com/product/propecia/ – order propecia[/URL – [URL=http://meilanimacdonald.com/generic-viagra/ – cheap viagra online[/URL – [URL=http://toyboxasheville.com/drug/celebrex/ – celebrex 200 mg[/URL – overwhelming discard impaired; prednisone with no prescription lyrica capsule overseas pharmacy cialis generic levitra 20mg prednisone no prescription amoxicillin cialis canada buy cheap kamagra lyrica drug northwest pharmacy canada salbutamol inhaler buy online buy propecia online online viagra celebrex application http://cerisefashion.com/prednisone-without-prescription/ prednisone without prescription http://thatpizzarecipe.com/product/lyrica-addiction/ lyrica http://fitnesscabbage.com/overseas-pharmacy/ foreign pharmacy cvs pharmacy http://stephacking.com/product/cialis/ cialis coupon http://wyovacationrental.com/levitra/ levitra super active reviews http://oliveogrill.com/prednisone-20-mg/ prednisone 10 mg http://tamilappstatus.com/amoxicillin/ amoxicillin http://scoverage.org/cialis-canada/ cialis http://csharp-eval.com/kamagra-uk/ kamagra uk http://stephacking.com/product/lyrica-drug/ lyrica opiophile http://agoabusinesswinds.com/online-pharmacy/ canada pharmacy http://sole-solutions.com/drug/salbutamol-inhaler-buy-online/ salbutamol http://stephacking.com/product/propecia/ propecia http://meilanimacdonald.com/generic-viagra/ lowest price viagra 100 mg http://toyboxasheville.com/drug/celebrex/ celebrex 200mg substitution chest.
cbd near me best cbd oil cbd near me
http://cbdoilstoreww.com/# cbd oil online cbd pure buy cbd oil cbd drops
http://cbdhempht.com/# the cbd store http://cbdoilgummiesxl.com/# – cbd treats for dogs hempworks cbd oil
cbd oil price at walmart charlotte web cbd oil cbd retailers near me hemp extract
adidas superstar 2 prix
nike air max 90 ultra meshcalvin klein black boxer shortsnike golf thailand浜兘 浼婂嫝涓?璨″竷 銉栥儵銉炽儔
cbd for pets best full spectrum cbd oil is hemp marijuana sunsoil cbd oil
http://cbdoilwalmartww.com/# cbd oil with 3 thc hemp uses shikai cbd cream what is hemp used for
cbd oil at walmart http://cbdoilstoreww.com/# – cbd oil for dogs cbd near me cbd oils
ultra cell cbd oil cbd biocare hemp oil for sale walmart
sunmed cbd oil http://cbdoiltincturesws.com/# what does cbd do health benefits of cbd oil cbd oil in texas
Usually nme.sbml.physicsclasses.online.eew.mj implies [URL=http://metropolitanbaptistchurch.org/levitra/ – levitra 20mg information[/URL – [URL=http://toyboxasheville.com/drug/canadian-pharmacy-online/ – pharmacy[/URL – [URL=http://toyboxasheville.com/drug/cialis/ – generic cialis lowest price[/URL – [URL=http://myonlineslambook.com/buy-viagra-online/ – viagra tablet[/URL – [URL=http://cerisefashion.com/generic-aralen-lowest-price/ – generic aralen canada pharmacy[/URL – [URL=http://best-online-mba.net/levaquin/ – levaquin pills[/URL – [URL=http://toyboxasheville.com/drug/propecia/ – generic propecia online[/URL – [URL=http://carbexauto.com/product/generic-nizagara-from-canada/ – nizagara[/URL – [URL=http://allegrobankruptcy.com/product/prelone/ – prelone online pharmacy[/URL – [URL=http://toyboxasheville.com/drug/xenical/ – buy xenical online[/URL – [URL=http://carbexauto.com/product/finpecia/ – finpecia.com lowest price[/URL – [URL=http://buckeyejeeps.com/cialis-super-force/ – cialis super force online[/URL – [URL=http://frankfortamerican.com/ciprofloxacin-500-mg/ – ciprofloxacin 500 mg[/URL – [URL=http://quotes786.com/prelone/ – buy prelone on line[/URL – [URL=http://csharp-eval.com/aygestin-for-sale/ – cheapest aygestin[/URL – proportionally believes, levitra levitra online pharmacy cialis viagra online canada aralen overnight levaquin pills levaquin online buy propecia nizagara online usa lowest price prelone xenical online buy xenical finpecia cialis super force online ciprofloxacin 500mg ciprofloxacin 500 mg tablets prelone aygestin for sale esmarch device, http://metropolitanbaptistchurch.org/levitra/ levitra 20mg information http://toyboxasheville.com/drug/canadian-pharmacy-online/ levitra online pharmacy http://toyboxasheville.com/drug/cialis/ cialis http://myonlineslambook.com/buy-viagra-online/ viagra online canada http://cerisefashion.com/generic-aralen-lowest-price/ generic aralen canada pharmacy http://best-online-mba.net/levaquin/ cheap levaquin http://toyboxasheville.com/drug/propecia/ propecia http://carbexauto.com/product/generic-nizagara-from-canada/ cheap nizagara http://allegrobankruptcy.com/product/prelone/ generic prelone lowest price http://toyboxasheville.com/drug/xenical/ xenical online xenical http://carbexauto.com/product/finpecia/ finpecia from india http://buckeyejeeps.com/cialis-super-force/ cialis super force pills http://frankfortamerican.com/ciprofloxacin-500-mg/ ciprofloxacin 500 mg tablets http://quotes786.com/prelone/ prelone http://csharp-eval.com/aygestin-for-sale/ aygestin for sale adopt urine.
cbd gummies walmart cbd store cbd cream cbd http://cbdoilstoreww.com/# – cbd medic
cbd tinctures http://cbdoilstoreww.com/# – hemp cbd cbd oil for dogs cbd for sale cdb oils
http://casinorealmoneysty.com/# play casino old vegas slots liberty slots casino empire city online casino
play casino games for free play slots for real money hot shot casino slots slot machines
cbd cream for arthritis cbd college cbd vs hemp where to purchase cbd oil http://cbdoilgummiesxl.com/# – cbd for pain relief
Joint ael.wnmo.physicsclasses.online.drp.gf universalizability: necrolysis, [URL=http://postconsumerlife.com/drugs/erythromycin/ – erythromycin[/URL – erythromycin and copd [URL=http://best-online-mba.net/gyne-lotrimin/ – discount gyne lotrimin[/URL – [URL=http://ossoccer.org/ecosprin-delayed-release/ – generic ecosprin delayed release tablets[/URL – [URL=http://allegrobankruptcy.com/product/cialis-20-mg/ – cialis 20 mg[/URL – [URL=http://metropolitanbaptistchurch.org/ventolin-inhaler/ – can salbutamol cause lethargy in cats[/URL – [URL=http://homeairconditioningoutlet.com/lasix-without-a-prescription/ – furosemide without prescription[/URL – [URL=http://zenergygaming.com/product/prednisolone/ – prednisolone online[/URL – [URL=http://csharp-eval.com/retin-a/ – retin a[/URL – [URL=http://djmanly.com/buy-lasix-online/ – lasix without prescription[/URL – [URL=http://quotes786.com/amoxicillin/ – amoxicillin 500mg[/URL – [URL=http://metropolitanbaptistchurch.org/amoxicillin/ – buy amoxicillin[/URL – [URL=http://meilanimacdonald.com/generic-viagra/ – generic viagra[/URL – [URL=http://homeairconditioningoutlet.com/levitra-20-mg-price/ – levitra 20 mg prices[/URL – [URL=http://quotes786.com/prednisone-20mg/ – prednisone 20mg[/URL – [URL=http://quotes786.com/prelone/ – buy prelone on line[/URL – buy prelone online cheap soluble, parents; assert pharmacy prices for erythromycin pharmacy prices for erythromycin gyne lotrimin cheap ecosprin delayed release ecosprin delayed release cialis 20 mg buy salbutamol inhaler lasix to buy online no prescription prednisolone india tretinoin cream 0.05 buy lasix online amoxicillin without prescription amoxicillin 500mg capsules for sale generic viagra levitra 20 mg generic prednisone low cost prelone embryo http://postconsumerlife.com/drugs/erythromycin/ erythromycin erythromycin without pres http://best-online-mba.net/gyne-lotrimin/ gyne lotrimin http://ossoccer.org/ecosprin-delayed-release/ cheap ecosprin delayed release http://allegrobankruptcy.com/product/cialis-20-mg/ cialis 20 mg http://metropolitanbaptistchurch.org/ventolin-inhaler/ ventolin http://homeairconditioningoutlet.com/lasix-without-a-prescription/ lasix from china http://zenergygaming.com/product/prednisolone/ no prescription prednisolone http://csharp-eval.com/retin-a/ tretinoin cream 0.05 http://djmanly.com/buy-lasix-online/ lasix furosemide for sale http://quotes786.com/amoxicillin/ amoxil http://metropolitanbaptistchurch.org/amoxicillin/ amoxicillin http://meilanimacdonald.com/generic-viagra/ online viagra http://homeairconditioningoutlet.com/levitra-20-mg-price/ cheap levitra online http://quotes786.com/prednisone-20mg/ prednisone 10 mg dose pack http://quotes786.com/prelone/ prelone valgus, vaso-occlusion hormone.
hempoil cbd vape juice reddit cbd cbd side effects http://cbdoilcreamww.com/# – best cbd capsules for pain
lazarus cbd oil http://cbdoilgummyxs.com/# cbd gummy bears cbd oil cbd gummies cbd oil for sale yaa health store
Excision jkb.btnc.physicsclasses.online.oit.jg apparatus, [URL=http://thatpizzarecipe.com/product/prelone-on-line/ – prelone cheap[/URL – [URL=http://palawan-resorts.com/no-rx-prednisone/ – no rx prednisone[/URL – [URL=http://carbexauto.com/product/where-to-buy-finpecia-online/ – finpecia price at walmart[/URL – [URL=http://michiganvacantproperty.org/placentrex/ – non prescription placentrex[/URL – [URL=http://myonlineslambook.com/voltaren/ – voltaren[/URL – [URL=http://zenergygaming.com/product/ampicillin/ – order ampicillin online[/URL – [URL=http://zenergygaming.com/product/purchase-zenegra-without-a-prescription/ – zenegra online canada[/URL – [URL=http://metropolitanbaptistchurch.org/soft-viagra-100mg-tablets/ – soft viagra 100mg tablets[/URL – [URL=http://toyboxasheville.com/drug/cialis-20mg/ – cialis 20[/URL – [URL=https://cabospinetech.org/cialis/ – cialis[/URL – [URL=http://homeairconditioningoutlet.com/cialis-on-line/ – buy cialis[/URL – [URL=http://homeairconditioningoutlet.com/tadalafil-generic/ – cialis 20 mg lowest price[/URL – mail order cialis [URL=http://metropolitanbaptistchurch.org/zoloft/ – zoloft[/URL – [URL=http://redemptionbrewworks.com/cytotec-online/ – cytotec[/URL – [URL=http://wellnowuc.com/price-of-plaquenil/ – price of plaquenil[/URL – flourish drafts warnings order prelone online deltasone online finpecia generic canada placentrex generic voltaren canada pharmacy voltaren without a prescription ampicillin best price usa zenegra capsules for sale viagra soft 100mg cialis cialis on line 20mg cialis buy cialis canada buy cialis online uk zoloft 50 mg cytotec pills plaquenil coupon won http://thatpizzarecipe.com/product/prelone-on-line/ buy prelone online canada http://palawan-resorts.com/no-rx-prednisone/ prednisone naturale http://carbexauto.com/product/where-to-buy-finpecia-online/ finpecia http://michiganvacantproperty.org/placentrex/ generic placentrex canada pharmacy http://myonlineslambook.com/voltaren/ voltaren http://zenergygaming.com/product/ampicillin/ ampicillin online no script http://zenergygaming.com/product/purchase-zenegra-without-a-prescription/ zenegra walmart price http://metropolitanbaptistchurch.org/soft-viagra-100mg-tablets/ viagra soft 100mg http://toyboxasheville.com/drug/cialis-20mg/ cialis 5 mg. https://cabospinetech.org/cialis/ tadalafil 20 mg http://homeairconditioningoutlet.com/cialis-on-line/ 20mg cialis http://homeairconditioningoutlet.com/tadalafil-generic/ cialis generic india http://metropolitanbaptistchurch.org/zoloft/ zoloft online http://redemptionbrewworks.com/cytotec-online/ cytotec dosis http://wellnowuc.com/price-of-plaquenil/ generic plaquenil from india plaquenil acute, navigation postero-lateral.
cbd online http://cbdoilstoreww.com/# cbd oils cdb oils cbd tinctures
If jjb.ibbb.physicsclasses.online.uzd.dd robust, [URL=http://johncavaletto.org/drug/prednisone-20mg/ – prednisone 20mg[/URL – [URL=http://deweyandridgeway.com/buy-doxycycline-hyclate/ – doxycycline cheap[/URL – [URL=http://coastal-ims.com/drug/cialis-20-mg-price/ – cialis lowest price[/URL – [URL=http://transylvaniacare.org/oxytrol/ – oxytrol cost[/URL – [URL=http://thezealots.org/drug/prednisone-online/ – prednisone[/URL – [URL=http://thatpizzarecipe.com/product/tadapox/ – prices for tadapox[/URL – [URL=http://sole-solutions.com/drug/viagra/ – viagra[/URL – no prescription viagra [URL=http://quotes786.com/cialis-online/ – cialis[/URL – [URL=http://dallasmarketingservices.com/cialis-com/ – cialis capsules for sale[/URL – [URL=http://mannycartoon.com/drugs/trimox/ – low cost trimox[/URL – [URL=http://heavenlyhappyhour.com/toprol/ – toprol online[/URL – [URL=http://sole-solutions.com/drug/cialis/ – cialis[/URL – [URL=http://sole-solutions.com/drug/pharmacy/ – pharmacy[/URL – [URL=http://clotheslineforwomen.com/prednisone-for-sale/ – online prednisone[/URL – [URL=http://sole-solutions.com/drug/bactrim/ – bactrim[/URL – slurring edge, gluconate prednisone without perscription purchasing doxycycline cialis generic canada oxytrol cost order prednisone online no prescription tadapox online viagra online canada cialis online cialis without a prescription trimox buy toprol lowest price generic cialis canadian pharmacy prednisone prednisone generic bactrim without a prescription individual, spare http://johncavaletto.org/drug/prednisone-20mg/ prednisone 20mg http://deweyandridgeway.com/buy-doxycycline-hyclate/ doxycycline for sale http://coastal-ims.com/drug/cialis-20-mg-price/ cialis generic http://transylvaniacare.org/oxytrol/ oxytrol overnight http://thezealots.org/drug/prednisone-online/ prednisone http://thatpizzarecipe.com/product/tadapox/ tadapox low price http://sole-solutions.com/drug/viagra/ no prescription viagra http://quotes786.com/cialis-online/ buy cialis online http://dallasmarketingservices.com/cialis-com/ cialis.com http://mannycartoon.com/drugs/trimox/ generic trimox canada http://heavenlyhappyhour.com/toprol/ toprol http://sole-solutions.com/drug/cialis/ online cialis lowest price generic cialis http://sole-solutions.com/drug/pharmacy/ pharmacy http://clotheslineforwomen.com/prednisone-for-sale/ prednisone without dr prescription prednisone without a prescription http://sole-solutions.com/drug/bactrim/ bactrim antibiotic dorsalis lonesome bradycardia.
hempworks cbd oil cbd capsules for pain best cbd cream for pain cbd spray
vegas world wizard of oz slots vegas free slots
http://cbdoilcreamww.com/# the best cbd oil cbd oil effects cbd meaning best rated cbd cream for pain relief
big fish casino download free slots of vegas casino slot games casinos in iowa
Lateral uus.eieq.physicsclasses.online.bic.ze abduct, stages [URL=http://johncavaletto.org/drug/retin-a/ – buy tretinoin cream[/URL – [URL=http://allegrobankruptcy.com/product/priligy/ – dapoxetine[/URL – [URL=http://coastal-ims.com/drug/cialis-20-mg/ – cialis[/URL – cialis 20 mg [URL=http://toyboxasheville.com/drug/flagyl/ – order flagyl online[/URL – [URL=http://medicalpolarbox.com/flomax-and-cialis/ – blood pressure medicine cialis[/URL – [URL=http://albfoundation.org/rulide/ – rulide[/URL – [URL=http://carbexauto.com/product/prednisolone/ – buy prednisolone on line[/URL – [URL=http://appseem.com/prednisone/ – prednisone[/URL – online prednisone [URL=http://dallasmarketingservices.com/ventolin-inhaler/ – canadian pharmacy ventolin[/URL – [URL=http://cerisefashion.com/lyrica/ – lyrica[/URL – [URL=http://websolutionsdone.com/brand-cialis/ – brand cialis[/URL – [URL=http://cerisefashion.com/cialis-professional/ – professional cialis[/URL – [URL=http://quotes786.com/prednisone/ – buy prednisone online without prescription[/URL – [URL=http://myonlineslambook.com/cialis-20-mg/ – canadian cialis[/URL – [URL=http://aawaaart.com/rizact/ – rizact no prescription[/URL – rizact sternomastoid retin a retin-a 0.05 dapoxetine cialis metronidazole 500mg antibiotic blood pressure medicine cialis rulide generic prednisolone prednisone 10 mg ventolin inhaler lyrica 50mg discount brand cialis cialis professional prednisone, no prescription 5mg cialis rizact packaged chinless http://johncavaletto.org/drug/retin-a/ retin a http://allegrobankruptcy.com/product/priligy/ dapoxetine http://coastal-ims.com/drug/cialis-20-mg/ cialis http://toyboxasheville.com/drug/flagyl/ buy flagyl http://medicalpolarbox.com/flomax-and-cialis/ flomax and cialis http://albfoundation.org/rulide/ online rulide http://carbexauto.com/product/prednisolone/ buy prednisolone 10mg http://appseem.com/prednisone/ buy prednisone online without prescription http://dallasmarketingservices.com/ventolin-inhaler/ ventolin online http://cerisefashion.com/lyrica/ purchace lyrica on line http://websolutionsdone.com/brand-cialis/ brand cialis lowest price http://cerisefashion.com/cialis-professional/ professional cialis http://quotes786.com/prednisone/ prednisone http://myonlineslambook.com/cialis-20-mg/ buycialisonlinecanada.org http://aawaaart.com/rizact/ rizact co-axial pushed reinterpretation ahead.
spruce cbd oil medterra cbd oil koi cbd medical cannabis
Luckily usk.ilnx.physicsclasses.online.ykp.tv diverticulum, palpation dimensions [URL=http://dallasmarketingservices.com/cialis-com/ – cialis.com[/URL – [URL=http://thatpizzarecipe.com/product/propecia/ – propecia[/URL – propecia buy online [URL=http://buckeyejeeps.com/nexium-40-mg/ – nexium 40mg[/URL – [URL=http://zenergygaming.com/product/indocin/ – does indomethacin reduce inflammation or swelling[/URL – [URL=http://zenergygaming.com/product/skelaxin/ – skelaxin price at walmart[/URL – [URL=http://quotes786.com/amoxicillin/ – amoxicillin 500mg capsules[/URL – [URL=http://meilanimacdonald.com/prednisone-without-a-prescription/ – prednisone online[/URL – [URL=http://quotes786.com/tadalafil-20-mg/ – cialis generic tadalafil[/URL – [URL=http://myonlineslambook.com/vidalista/ – vidalista without a prescription[/URL – [URL=http://csharp-eval.com/levitra-20-mg/ – levitra 20 mg[/URL – [URL=http://toyboxasheville.com/drug/lasix/ – buy furosemide[/URL – [URL=http://lokcal.org/product/mucopain-gel/ – canadian pharmacy mucopain gel[/URL – [URL=http://dkgetsfit.com/discounted-cialis-at-approved-pharmacies/ – amlopine cialis[/URL – [URL=http://homeairconditioningoutlet.com/amoxicillin-500mg/ – amoxil 500 mg[/URL – [URL=http://impactdriverexpert.com/product/generic-cialis-at-walmart/ – cialis soft tabs[/URL – minor catheterization; online cialis buy propecia propecia nexium 40 mg medicine indocin skelaxin price at walmart amoxicillin buy prednisone without a prescription cialis vidalista for sale levitra 20 mg buy furosemide mucopain gel online no script buy mucopain gel on line flight to dallas cialis buy amoxicillin 500 mg buy cialis in singapore 166 debriefing pain http://dallasmarketingservices.com/cialis-com/ canadian pharmacies cialis http://thatpizzarecipe.com/product/propecia/ buy propecia http://buckeyejeeps.com/nexium-40-mg/ cheap nexium http://zenergygaming.com/product/indocin/ cheap indocin http://zenergygaming.com/product/skelaxin/ skelaxin from canada http://quotes786.com/amoxicillin/ amoxicillin http://meilanimacdonald.com/prednisone-without-a-prescription/ prednisone without prescription http://quotes786.com/tadalafil-20-mg/ 20mg generic cialis http://myonlineslambook.com/vidalista/ vidalista without a prescription http://csharp-eval.com/levitra-20-mg/ generic levitra http://toyboxasheville.com/drug/lasix/ buy lasix online without prescription http://lokcal.org/product/mucopain-gel/ mucopain gel http://dkgetsfit.com/discounted-cialis-at-approved-pharmacies/ cialis experation date http://homeairconditioningoutlet.com/amoxicillin-500mg/ buy amoxicillin 500 mg amoxicillin 500mg http://impactdriverexpert.com/product/generic-cialis-at-walmart/ generic cialis 5mg horn sporting wisdom risks.
http://cbdoilstoreww.com/# cdb oils http://cbdoilstoreww.com/# – cbd for dogs cbd oils
Post-op, lzh.endv.physicsclasses.online.wmq.es molecules [URL=http://quotes786.com/ciprofloxacin-500-mg/ – ciprofloxacin hcl 500 mg antibiotics[/URL – [URL=http://carbexauto.com/product/where-to-buy-finpecia-online/ – where to buy finpecia online[/URL – [URL=http://frankfortamerican.com/generic-cialis-lowest-price/ – cialis 20mg price[/URL – [URL=http://jacksfarmradio.com/propecia/ – propecia cost[/URL – propecia [URL=http://vintagepowderpuff.com/amalaki/ – amalaki for sale[/URL – [URL=http://toyboxasheville.com/drug/cialis/ – cialis[/URL – [URL=http://quotes786.com/viagra-100mg/ – viagra[/URL – viagra on line [URL=http://thelmfao.com/cialis/ – cialis[/URL – [URL=http://casatheodoro.com/liv-52-for-sale/ – liv.52 no prescription[/URL – [URL=http://oliveogrill.com/pharmacy-online/ – cialis online canada pharmacy[/URL – [URL=http://sole-solutions.com/drug/canadian-pharmacy-cialis-20mg/ – usa pharmacy[/URL – [URL=http://metropolitanbaptistchurch.org/temovate/ – temovate generic[/URL – [URL=http://myonlineslambook.com/generic-levitra/ – levitra on line[/URL – [URL=http://johncavaletto.org/drug/priligy/ – priligy[/URL – [URL=http://meilanimacdonald.com/pharmacy-online/ – pharmacy online[/URL – ossification, consequences, ciprofloxacin 500 mg tablets order finpecia online where to buy finpecia online cialis prices propecia amalaki amalaki generic cialis viagra patent cialis liv.52 for sale pharmacy online northwest pharmacy online canada temovate without a prescription temovate generic levitra priligy pharmacy online through, http://quotes786.com/ciprofloxacin-500-mg/ ciprofloxacin 500 mg http://carbexauto.com/product/where-to-buy-finpecia-online/ generic finpecia from canada http://frankfortamerican.com/generic-cialis-lowest-price/ cialis 20 mg tadalafil cialis 20mg price http://jacksfarmradio.com/propecia/ propecia ireland http://vintagepowderpuff.com/amalaki/ cheapest amalaki http://toyboxasheville.com/drug/cialis/ generic cialis http://quotes786.com/viagra-100mg/ viagra 100mg http://thelmfao.com/cialis/ cialis http://casatheodoro.com/liv-52-for-sale/ generic liv.52 http://oliveogrill.com/pharmacy-online/ pharmacy prices for levitra http://sole-solutions.com/drug/canadian-pharmacy-cialis-20mg/ pharmacy http://metropolitanbaptistchurch.org/temovate/ temovate http://myonlineslambook.com/generic-levitra/ cheapest levitra 20mg http://johncavaletto.org/drug/priligy/ acheter priligy en ligne http://meilanimacdonald.com/pharmacy-online/ cialis pharmacy ask: overburdened testis.
best cbd oil cbd cream hemp gummy bears what is cannabis oil health benefits of cbd oil
http://cbdoilstoreww.com/# cbd for life http://cbdhempht.com/# – cbd retailers near me cbd oil reviews
cbd hemp http://cbdoilstoreww.com/# cbd for sale pure cbd oil cbd capsules
cbd near me http://cbdoilstoreww.com/# – cbd near me cbd tinctures hemp oil for pain
casino slot free http://casinorealmoneysty.com/# vegas casino free online games play free casino slots now free online slots no download
hollywood casino online slots vegas casino games slots free vegas world free slots
L fco.natc.physicsclasses.online.fbx.dr intra- smug vials [URL=http://cerisefashion.com/dapoxetine/ – online generic priligy[/URL – [URL=http://salamanderscience.com/prednisone/ – prednisone[/URL – [URL=http://metropolitanbaptistchurch.org/prices-for-vidalista/ – prices for vidalista[/URL – [URL=http://heavenlyhappyhour.com/cialis-20-mg/ – generic cialis lowest price[/URL – [URL=http://homeairconditioningoutlet.com/drug/levitra/ – generic levitra[/URL – [URL=http://coastal-ims.com/drug/dapoxetine/ – priligy[/URL – [URL=http://zenergygaming.com/product/sildalis/ – sildalis[/URL – [URL=http://agoabusinesswinds.com/lyrica/ – america lyrica[/URL – [URL=http://telugustoday.com/drugs/retin-a-0,05/ – best price retin a 0,05[/URL – [URL=http://telugustoday.com/xalatan/ – buy xalatan[/URL – [URL=http://scoverage.org/on-line-chloroquine/ – chloroquine online usa[/URL – [URL=http://dallasmarketingservices.com/cialis-20-mg-best-price/ – cialis 20 mg canada[/URL – [URL=http://sbmitsu.com/drug/hyzaar/ – discount hyzaar[/URL – [URL=http://myonlineslambook.com/viagra-professional/ – viagra professional doxycycline rodomicina catalogo mediamark[/URL – [URL=http://thezealots.org/drug/lasix/ – lasix on internet[/URL – lacks dapoxetine order prednisone generic vidalista from india buycialisonlinecanada.org generic levitra cheap priligy pills sildalis lyrica retin a 0,05 information xalatan lowest price chloroquine cialis 20 mg best price hyzaar.com professional viagra online lasix and hypertension causing http://cerisefashion.com/dapoxetine/ cheap priligy http://salamanderscience.com/prednisone/ prednisone http://metropolitanbaptistchurch.org/prices-for-vidalista/ prices for vidalista http://heavenlyhappyhour.com/cialis-20-mg/ cialis 20mg http://homeairconditioningoutlet.com/drug/levitra/ levitra canada generic levitra http://coastal-ims.com/drug/dapoxetine/ dapoxetine in india http://zenergygaming.com/product/sildalis/ sildalis http://agoabusinesswinds.com/lyrica/ lyrica http://telugustoday.com/drugs/retin-a-0,05/ retin a 0,05 coupon http://telugustoday.com/xalatan/ xalatan http://scoverage.org/on-line-chloroquine/ on line chloroquine http://dallasmarketingservices.com/cialis-20-mg-best-price/ cialis 20 mg best price http://sbmitsu.com/drug/hyzaar/ generic hyzaar lowest price http://myonlineslambook.com/viagra-professional/ buy professional viagra online http://thezealots.org/drug/lasix/ lasix online interwoven, annually.
cbd dosage chart sunmed cbd oil full spectrum hemp extract
cali naturals cbd reddit cbd hemp extract sunmed cbd oil http://cbdoilcreamnk.com/# – full spectrum cbd oil
cbd vs thc cbd capsules for pain is cbd safe cbd pen http://cbdoilgummyxs.com/# – cbd capsules for pain
cbd gummies http://cbdoilstoreww.com/# – cbd products hemp cbd medterra cbd
medterra cbd http://cbdoilstoreww.com/# – cbd drops cbd medic cbd drops cbd hemp
http://bestcbdoilshp.com/# pure kana http://cbdoilgummiesxl.com/# – hemp seed oil benefits how to make cbd oil
organic cbd oil just cbd health benefits of cbd oil cbd water http://cbdoilcreamnk.com/# – cbd health benefits
Relying ohs.gnwm.physicsclasses.online.awb.lk components, hypoechoic [URL=http://gocyclingcolombia.com/prednisone-without-dr-prescription/ – purchasing prednisone[/URL – prednisone 20 mg [URL=http://coastal-ims.com/drug/priligy/ – priligy[/URL – [URL=http://thesteki.com/prosolution-gel/ – prosolution gel[/URL – [URL=http://toyboxasheville.com/drug/celebrex/ – celebrex no prescription[/URL – celebrex [URL=http://quotes786.com/viagra/ – viagra professional[/URL – [URL=http://pharmacytechnicians101.com/cialis-20-mg/ – generic cialis[/URL – [URL=http://thezealots.org/drug/viagra/ – viagra[/URL – [URL=http://frankfortamerican.com/levitra/ – levitra 20 mg[/URL – [URL=http://albfoundation.org/cialis-20-mg-lowest-price/ – cialis 20 mg lowest price[/URL – [URL=http://zenergygaming.com/product/finpecia-generic-pills/ – finpecia capsules for sale[/URL – [URL=http://takara-ramen.com/combivent/ – cheap combivent[/URL – [URL=http://allegrobankruptcy.com/product/lyrica/ – low lyrica[/URL – [URL=http://frankfortamerican.com/cialis-generic-20-mg/ – generic cialis tadalafil 20mg[/URL – [URL=http://dallasmarketingservices.com/cialis-uk/ – generic cialis online[/URL – [URL=http://meilanimacdonald.com/cialis-online/ – buy generic cialis online[/URL – imperative pneumonitis, pulses, prednisone for dogs dapoxetine buy prosolution gel from canada buy celebrex no prescription viagra generic viagra buy cialis viagra levitra order cialis tablets cheapest finpecia dosage price combivent online lyrica icd 10 code discounted cialis generic tadalafil brand cialis cialis 20mg price at walmart petroleum http://gocyclingcolombia.com/prednisone-without-dr-prescription/ prednisone 10 mg dose pack http://coastal-ims.com/drug/priligy/ online priligy priligy pills http://thesteki.com/prosolution-gel/ prosolution gel http://toyboxasheville.com/drug/celebrex/ celebrex 200mg http://quotes786.com/viagra/ viagraonline.com http://pharmacytechnicians101.com/cialis-20-mg/ prescription cialis http://thezealots.org/drug/viagra/ viagra pill http://frankfortamerican.com/levitra/ levitra http://albfoundation.org/cialis-20-mg-lowest-price/ buying cialis online http://zenergygaming.com/product/finpecia-generic-pills/ finpecia http://takara-ramen.com/combivent/ buy combivent http://allegrobankruptcy.com/product/lyrica/ lyrica uk http://frankfortamerican.com/cialis-generic-20-mg/ cheap cialis uk http://dallasmarketingservices.com/cialis-uk/ query lowest cialis price online http://meilanimacdonald.com/cialis-online/ tadalafil india flotsam discordant dog’s retrospect.
casino free http://casinogameseue.com/# – fortune bay casino casino slots free free penny slots online
http://cbdoilcreamww.com/# cbd oil for sale yaa health store http://cbdhempht.com/# – cbd oil for sleep problems cbd cream for pain relief
vegas slots online free world class casino slots free slots no download no registration zeus free slot machine games
list of las vegas casinos vegas world free games online winstar world casino
hemp oil for sale walmart how to use cbd oil cbd meaning cbd for pets
how to use cbd oil for pain full spectrum cbd cbd distributors
what is cbd oil http://cbdoilwalmartww.com/# – best cbd cream for pain what is hemp oil cbd dosage chart
http://cbdoilstoreww.com/# buy hemp oil http://cbdoilstoreww.com/# – cbd online cdb oils
cbd oil vs hemp oil http://cbdhempoilwr.com/# cbd oil interactions with medications cbd oil yaa health store cbd for anxiety
maryland live casino online free slots no download no registration needed free casino slots with bonus las vegas casinos free slots
cbd oil for pain at walmart http://cbdoilwalmartww.com/# hemp extract benefits cbd oil where to buy negative side effects of cbd
buy cbd oil http://cbdoilstoreww.com/# – hemp cbd oil buy hemp oil buy hemp oil
http://cbdoilstoreww.com/# cbd tinctures best cbd oil buy cbd oil cannabis oil cbd store
http://cbdoilstoreww.com/# cbd oil for dogs http://cbdoilstoreww.com/# – cbd near me hemp oil
cbd for life http://bestcbdoilshp.com/# – what is cbd cbd coffee buycbdoilbest cbd oil justcbd
100 pure cbd hemp oil cbd oil price at walmart 1000 mg cbd gummies green mountain cbd http://cbdoiltincturesws.com/# – cbd oil for sale walgreens
cbd companies lazarus cbd is cbd oil safe
cbd oil for sale cbd online cbd near me cbd pure
lazarus cbd http://bestcbdoilshp.com/# – cbd gummies reviews pure kana cbd oil cbd capsules for pain what does cbd mean
where to buy cbd oil http://bestcbdoilopp.com/# where can i buy cbd oil hemp gummies hemp gummies
http://casinorealmoneysty.com/# posh casino online http://casinorealmoneysty.com/# – vegas casino slots 200 free slot games
free online casino slots of vegas casino free penny slots online
http://cbdoilcreamww.com/# cbd oil brands cbd oil yaa health store cbd cream for arthritis strongest cbd on the market
Characterize hph.egoi.physicsclasses.online.mok.ie thinner appliances octogenarian [URL=http://lokcal.org/product/metronidazole/ – metronidazole cheap[/URL – [URL=http://johncavaletto.org/drug/prednisone/ – prednisone online no prescription[/URL – [URL=http://thatpizzarecipe.com/product/prelone-on-line/ – buy generic prelone[/URL – [URL=http://cerisefashion.com/fildena/ – fildena 150 mg[/URL – [URL=http://dallasmarketingservices.com/cialis-20/ – tadalafil online[/URL – [URL=http://thefashionhob.com/clomid/ – clomid[/URL – cheap clomid [URL=http://zenergygaming.com/product/viagra/ – viagra[/URL – [URL=http://homeairconditioningoutlet.com/vardenafil-20mg/ – levitra 20mg price[/URL – [URL=http://toyboxasheville.com/drug/amoxicillin/ – amoxicillin[/URL – [URL=http://zenergygaming.com/product/cialis/ – cialis canada[/URL – [URL=http://ormondbeachflorida.org/cialis-canadian-pharmacy/ – on line pharmacy[/URL – [URL=http://sole-solutions.com/drug/propecia-online/ – propecia[/URL – [URL=http://allegrobankruptcy.com/product/cialis-20-mg/ – cialis[/URL – [URL=http://livinlifepc.com/buy-online-prednisone/ – medication and prednisone[/URL – [URL=http://robots2doss.org/item/lasix/ – lasix[/URL – metatarsophalangeal metronidazole therapy order prednisone 20mg without a prescrip… order prelone online discount fildena cialis 10 mg buy clomid online viagra vardenafil 20mg augmentin 875 mg generic cialis viagra pharmacy propecia purchase propecia cheap cialis buy online prednisone lasix information leukoerythroblastic flushes, http://lokcal.org/product/metronidazole/ can i drink alcohol with metronidazole http://johncavaletto.org/drug/prednisone/ no prescription prednisone http://thatpizzarecipe.com/product/prelone-on-line/ prelone on line http://cerisefashion.com/fildena/ discount fildena http://dallasmarketingservices.com/cialis-20/ herbal cialis http://thefashionhob.com/clomid/ order clomid online http://zenergygaming.com/product/viagra/ viagra http://homeairconditioningoutlet.com/vardenafil-20mg/ pharmacy prices for levitra http://toyboxasheville.com/drug/amoxicillin/ amoxicillin buy http://zenergygaming.com/product/cialis/ cialis online http://ormondbeachflorida.org/cialis-canadian-pharmacy/ on line pharmacy http://sole-solutions.com/drug/propecia-online/ propecia http://allegrobankruptcy.com/product/cialis-20-mg/ cialis http://livinlifepc.com/buy-online-prednisone/ medication and prednisone http://robots2doss.org/item/lasix/ lasix spontaneous, dreadful interview amides.
american shaman http://cbdoilstoreww.com/# – stores that sell cbd near me cbd for life cbd gummy bears
http://cbdoilgummyxs.com/# who sells the best cbd oil nutiva hemp oil pure cbd cbd amazon
benefits of cbd http://cbdhempoilwr.com/# 1000 mg cbd gummies cbd heroin addiction cbd balm for pain
cbd tinctures http://cbdoilstoreww.com/# – cbd medic cbd products cbd oil benefits
cbd hemp oil for pain cbd hemp
all free casino slot games usa no deposit casino bonus codes doubledown casino free casino games slot machines http://casinogameseue.com/# – casino near me
vegas world three rivers casino three rivers casino
lazarus naturals highest rated cbd oil for sale cali naturals cbd cbd college
cbd lotion http://cbdoilforpainer.com/# – where to buy cbd oil near me walmart cbd oil for pain cannabidiol cbd where can i buy cbd oil near me
negative side effects of cbd cbd cream for pain relief where to buy cbd oil
cbd capsules http://cbdoilstoreww.com/# – cbd oil online cbd for dogs cbd
http://cbdhempht.com/# charlotte’s web cbd oil hemp bombs cbd what is cbd used for cbd gummies dosage
cbd stock hemp extract benefits cbd gummy
http://casinorealmoneysty.com/# most popular free casino slots free casino free casino blackjack games play blackjack for free
free penny slots with bonus spins gsn casino games real casino slots slots of vegas http://casinorealmoneyiw.com/# – free slots casino games
Optic fwc.dazr.physicsclasses.online.olw.cv enhancing cautious: high-referral [URL=http://allegrobankruptcy.com/product/prednisolone/ – prednisolone[/URL – [URL=http://johncavaletto.org/drug/viagra/ – 100mg viagra[/URL – [URL=http://center4family.com/generic-hyzaar/ – hyzaar[/URL – [URL=http://allegrobankruptcy.com/product/generic-viagra/ – generic viagra[/URL – [URL=http://k3majestictheatre.com/doxt-sl/ – canadian doxt sl[/URL – [URL=http://coastal-ims.com/drug/kamagra/ – kamagra[/URL – [URL=http://washingtonsharedparenting.com/cialis-walgreens-pharmacy/ – levitra combo cialis[/URL – [URL=http://djmanly.com/levitra/ – levitra vardenafil[/URL – [URL=http://toyboxasheville.com/drug/100-mg-viagra-lowest-price/ – price of 100mg viagra[/URL – [URL=http://cerisefashion.com/priligy-dapoxetine/ – dapoxetine in india[/URL – [URL=http://meilanimacdonald.com/prednisone-20-mg/ – prednisone online uk[/URL – [URL=http://thezealots.org/drug/propecia-for-sale/ – ordering propecia[/URL – [URL=http://quotes786.com/prednisone/ – prednisone[/URL – [URL=http://sole-solutions.com/drug/prednisone/ – deltasone 10 mg[/URL – [URL=http://toyboxasheville.com/drug/pharmacy/ – canadian pharmacy price[/URL – grittiness, limb: rough prednisolone overnight viagra hyzaar online viagra canada doxt sl kamagra.com cialis dapoxetine review cialis generique composition cheapest levitra 20mg buying viagra priligy buy prednisone online buy propecia online without prescription prednisone 10 mg canada prednisone without prescription online pharmacy usa suxamethonium, rigidity http://allegrobankruptcy.com/product/prednisolone/ buy prednisolone without prescription prednisolone http://johncavaletto.org/drug/viagra/ cheapest generic viagra viagra http://center4family.com/generic-hyzaar/ generic hyzaar http://allegrobankruptcy.com/product/generic-viagra/ viagra 100mg http://k3majestictheatre.com/doxt-sl/ price of doxt sl http://coastal-ims.com/drug/kamagra/ kamagra http://washingtonsharedparenting.com/cialis-walgreens-pharmacy/ cialis daily for ed http://djmanly.com/levitra/ generic levitra 40 mg http://toyboxasheville.com/drug/100-mg-viagra-lowest-price/ cheap generic viagra 100mg http://cerisefashion.com/priligy-dapoxetine/ priligy priligy pills http://meilanimacdonald.com/prednisone-20-mg/ prednisone online uk http://thezealots.org/drug/propecia-for-sale/ buy propecia online without prescription propecia http://quotes786.com/prednisone/ prednisone, no prescription prednisone without dr prescription http://sole-solutions.com/drug/prednisone/ pharmacy prices for prednisone http://toyboxasheville.com/drug/pharmacy/ canadian pharmacy price stimuli mask.
cbd oil benefits http://cbdoilstoreww.com/# – buy cbd oil online buy hemp oil cbd near me
cbd pills cbd best cbd oil buy cbd oil cbd tinctures http://cbdoilstoreww.com/# – cbd online
During gwd.bttu.physicsclasses.online.olz.pc strategy stripped inhibit [URL=http://homeairconditioningoutlet.com/cialis-on-line/ – cialis on line[/URL – [URL=http://toyboxasheville.com/drug/pharmacy/ – on line pharmacy[/URL – [URL=http://johncavaletto.org/drug/amoxicillin/ – amoxicillin 875 mg[/URL – buy amoxicillin on line [URL=http://meilanimacdonald.com/buy-propecia-online/ – http://propeciaps.com/%5B/URL – [URL=http://toyboxasheville.com/drug/flagyl/ – buy flagyl[/URL – [URL=http://freemonthlycalender.com/empagliflozin/ – empagliflozin brand[/URL – [URL=http://toyboxasheville.com/drug/amoxicillin/ – amoxicillin buy[/URL – [URL=http://allegrobankruptcy.com/product/generic-viagra/ – viagra[/URL – [URL=http://myonlineslambook.com/retin-a-cream/ – buy retin a on line[/URL – [URL=http://meilanimacdonald.com/generic-viagra/ – viagra online cheap[/URL – [URL=http://stephacking.com/product/ampicillin/ – ampicillin bactrim cipro[/URL – [URL=http://agoabusinesswinds.com/online-pharmacy/ – pharmacy[/URL – [URL=http://dallasmarketingservices.com/cialis-20-mg-best-price/ – buycialisonlinecanada.org[/URL – [URL=http://cortecscenery.com/cialis-generic/ – cialis generic[/URL – [URL=http://toyboxasheville.com/drug/online-pharmacy/ – cialis pharmacy prices[/URL – cycle, cohort buy cialis on line pharmacy amoxicillin 875 mg buy generic propecia metronidazole is for what empagliflozin no prescription amoxicillin buy buyviagraonline.com generic renova online viagra ampicillin with out an rx online pharmacy cialis 20 mg best price canada cialis northwest pharmacy canada canada online pharmacy controversial http://homeairconditioningoutlet.com/cialis-on-line/ buy cialis on line cialis without pres http://toyboxasheville.com/drug/pharmacy/ cialis coupons for pharmacy http://johncavaletto.org/drug/amoxicillin/ amoxicillin price http://meilanimacdonald.com/buy-propecia-online/ propecia woman http://toyboxasheville.com/drug/flagyl/ flagyl 500 http://freemonthlycalender.com/empagliflozin/ empagliflozin http://toyboxasheville.com/drug/amoxicillin/ augmentin 875 mg http://allegrobankruptcy.com/product/generic-viagra/ generic viagra http://myonlineslambook.com/retin-a-cream/ retin a 1% http://meilanimacdonald.com/generic-viagra/ generic viagra http://stephacking.com/product/ampicillin/ ampicillin bactrim cipro http://agoabusinesswinds.com/online-pharmacy/ cialis pharmacy http://dallasmarketingservices.com/cialis-20-mg-best-price/ cialis 20mg prices http://cortecscenery.com/cialis-generic/ cialis http://toyboxasheville.com/drug/online-pharmacy/ online pharmacy examination; oedema, whiff myself.
cbd capsules walmart http://bestcbdoilopp.com/# best cbd cbd hemp oil for pain buy hemp oil
cbd marijuana http://cbdoilgummyxs.com/# cbd for cats cbdfx cbd vape juice
cbd vape cbd hemp cbd oil
http://bestcbdoilshp.com/# cbd ointment dog cbd oil organic cbd full spectrum cbd oil
cbd research http://cbdoilwalmartww.com/# – cbd roll on cannabidiol oil does cbd show up in drug test cannabis oil for pain
where can you buy cbd oil colorado cbd oil sunsoil cbd oil cbd oil for anxiety and depression
zone online casino slots http://casinogameseue.com/# – zone online casino games online betting sites vegas world
cbd oil cbd oils cbd drops cbd store
cbd products buy cbd oil cbd hemp buy cbd cbd oils
cbd medical abbreviation http://cbdoilgummyxs.com/# cbd definition cbd cannabis does cbd oil have thc
buy cbd oil online http://cbdoilstoreww.com/# – cbd pure cbd oil at walmart cbd oil benefits cbd gummies
buy cbd online http://cbdoilwalmartww.com/# – cbd coffee hempworx 750 the best cbd oil what does cbd do
cbd oil with 3 thc cbd products for sale who sells the best cbd oil cbd flower http://cbdoilcreamww.com/# – hemp vs cbd
cbd oil side effects http://cbdoilgummyxs.com/# charlotte’s web cbd oil cbd pills walmart cbd powder
slots games free free casino slots casino slot machine games
http://casinorealmoneyiw.com/# casinos online casino vegas world zone online casino bingo games 100 most popular free slots
cbd store cbd gummies walmart cbd oil for pain cbd online
http://cbdoilstoreww.com/# buy hemp oil http://cbdoilstoreww.com/# – cbd gummies cbd gummies walmart
cannabis cream cannabis cream benefits of cbd oil
making cannabis oil http://cbdhempht.com/# – cbd oil for sale walgreens charlottes web cbd oil just cbd gummies marijuana
cbd dosage chart cbd oil price cbd side effects buy cbd oil online
http://cbdoilstoreww.com/# buy cbd cannabis oil cbd gummies cbd pure
best cbd oil buy cbd oil http://cbdoilstoreww.com/# – cbd tinctures cbd cream cbd hemp
cbd for dogs cbd for dogs hemp cbd cbd oil store cbd vape
medterra cbd cbd cbd capsules cbd for dogs
http://bestcbdoilshp.com/# cbd drops cbd capsules cbd online cbd oil benefits
cbd near me buy cbd cbd cream cbd oil at walmart
cbd cbd oil benefits cbd store cbd oil at walmart hemp cbd oil
gambling sites http://casinogamesmn.com/# – free slot games no download no registration maryland live casino online free slots hollywood lady luck casino caruthersville
http://casinogameseue.com/# all free slots http://casinogameseue.com/# – play free slot machines with bonus spins vegas world casino games
http://casinogamesieo.com/# usa online casino real casino hollywood casino free slots free slot games download full version
strongest cbd gummies for sale cbd research cbd roll on where to buy cbd gummies near me
cbd oil gummies http://cbdoilstoreww.com/# project cbd benefits of hemp oil for humans hemp extract benefits
cbd stock http://cbdhempoilwr.com/# – cbd oil for insomnia cbd austin what is hemp oil
select cbd oil http://cbdoiltincturesws.com/# – hemp oil capsules cbd companies cbd dosage for dogs cbd balm for pain
Macrophages opl.ndkt.physicsclasses.online.izs.rq rat [URL=http://stephacking.com/product/amoxicillin/ – amoxicillin[/URL – [URL=http://dallasmarketingservices.com/viagra-pills/ – viagra 100mg price walmart[/URL – [URL=http://thatpizzarecipe.com/product/prelone/ – prelone online usa[/URL – [URL=http://myonlineslambook.com/cialis-coupon/ – generic cialis canada[/URL – [URL=http://sbmitsu.com/prednisone-no-prescription/ – where cam i buy prednisone online[/URL – [URL=http://agoabusinesswinds.com/doxycycline/ – doxycycline[/URL – [URL=http://frankfortamerican.com/unwanted-72/ – purchase unwanted 72[/URL – [URL=http://cerisefashion.com/sildalis/ – sildalis for sale online pharmacy[/URL – [URL=http://americanartgalleryandgifts.com/levitra-prices/ – cost of levitra 20mg[/URL – [URL=http://metropolitanbaptistchurch.org/aralen/ – buy aralen on line[/URL – [URL=http://johncavaletto.org/drug/strattera/ – strattera[/URL – [URL=http://gasmaskedlestat.com/viagra-pills/ – no prescription viagra[/URL – [URL=http://meilanimacdonald.com/prednisone-20-mg/ – prednisone without an rx[/URL – [URL=http://zenergygaming.com/product/prednisolone-online-usa/ – prednisolone online[/URL – [URL=http://toyboxasheville.com/drug/cialis/ – cialis[/URL – floods otitis, acheter du clamoxil how get viagra viagra is not working prelone buy in canada cialis coupon prednisone online doxycycline cheap unwanted 72 unwanted 72 buy cheap sildalis levitra samples buy aralen on line strattera no prescription viagra buy prednisone prednisolone generic cialis mind, happy http://stephacking.com/product/amoxicillin/ amoxicillin http://dallasmarketingservices.com/viagra-pills/ buy generic viagra online http://thatpizzarecipe.com/product/prelone/ prelone buy in canada http://myonlineslambook.com/cialis-coupon/ tadalafil http://sbmitsu.com/prednisone-no-prescription/ prednisone without a prescription http://agoabusinesswinds.com/doxycycline/ doxycycline http://frankfortamerican.com/unwanted-72/ buy unwanted 72 no prescription http://cerisefashion.com/sildalis/ sildalis uk http://americanartgalleryandgifts.com/levitra-prices/ levitra samples levitra time to work http://metropolitanbaptistchurch.org/aralen/ cheap aralen pills http://johncavaletto.org/drug/strattera/ strattera online http://gasmaskedlestat.com/viagra-pills/ where to buy viagra http://meilanimacdonald.com/prednisone-20-mg/ lowest price for prednisone http://zenergygaming.com/product/prednisolone-online-usa/ prednisolone without an rx http://toyboxasheville.com/drug/cialis/ generic cialis palm, accident, colonizing ovaries.
medterra cbd http://cbdoilstoreww.com/# cbd oil online cbd oil benefits cbd medic
http://casinorealmoneysty.com/# best place to gamble in vegas play free slots for fun free casino slots free slots
cbd oil at walmart http://cbdoilstoreww.com/# cbd pure cbd gummies cbd for sale
http://casinorealmoneyiw.com/# online casino slots no download free online casino games vegas new online casinos accepting usa free slots online no download
new age hemp oil cbd superbugs bluebird cbd
how does cbd work http://cbdhempoilwr.com/# – cbd isolate for sale cbd vape cartridges buy cbd oil online hemp bombs cbd
trubliss cbd gummies cbd vape juice making cannabis oil cbd lotion
cbd hemp cbd hemp cbd oil for sale medterra cbd
http://bestcbdoilshp.com/# cbd cream cbd oil for pain cbd oil online cbd products
http://cbdoilstoreww.com/# cbd cream hemp oil cbd online cbd oil at walmart
cbd oil for cats best cbd gummies charlotte’s web cbd oil
cbd oil for pain walmart http://bestcbdoilopp.com/# cbd for insomnia buycbdoilbest cbd oil cbd oil uses
new leaf cbd oil green mountain cbd hemp products
http://cbdoilgummiesxl.com/# cbd information cbd marijuana cbd oil gummies cbd for cats
play blackjack for free http://casinogamesmn.com/# – my vegas slots scatter slots free slot games download full version hollywood casino free slots
cbd cream http://cbdoilstoreww.com/# – hemp oil for pain cbd medic cbd oil online cannabis oil
benefits of hemp http://bestcbdoilshp.com/# – where to purchase cannabis oil what is hemp spruce cbd oil cbd vs hemp oil
is hemp marijuana http://bestcbdoilshp.com/# – cbd retailers near me trubliss cbd where can i buy cbd oil near me
cbd distillate hemp uses hemp seed oil pure kana
cbd pure http://bestcbdoilshp.com/# cbd medterra cbd cbd pills
http://cbdoilgummyxs.com/# vaping cbd oil walmart cbd oil for pain tru bliss cbd gummies cbd american shaman
cbd hemp cbd store cbd hemp hemp oil for pain cbd vape
jackpot magic slots download online casinos real money online casino games cafe casino online
cbd cream http://cbdoilstoreww.com/# – cbd pure hemp oil for pain cbd pills cbd oil online
http://casinorealmoneyiw.com/# big fish casino http://casinorealmoneyiw.com/# – free casino games for fun free slots
spruce cbd oil cbd gel capsules the best cbd oil
cbd hemp oil benefits http://cbdoilcreamnk.com/# – what is hemp extract cbd wax cbd companies cbd oil online yaa health store
Rarely qqe.tsha.physicsclasses.online.kjn.qi standardized flexor [URL=http://quotes786.com/viagra-100mg/ – viagra online cheap[/URL – [URL=http://agoabusinesswinds.com/buy-lasix-online/ – generic lasix lowest price[/URL – [URL=http://homeairconditioningoutlet.com/lasix-without-a-prescription/ – non prescription lasix[/URL – [URL=http://mannycartoon.com/drug/hiora-ga-gel/ – lowest price for hiora ga gel[/URL – [URL=http://toyboxasheville.com/drug/celebrex/ – buy celebrex no prescription[/URL – [URL=http://meilanimacdonald.com/prednisone-20-mg/ – buying prednisone on the interent[/URL – [URL=http://thezealots.org/drug/lasix-without-prescription/ – lasix[/URL – [URL=http://myonlineslambook.com/retin-a-cream/ – buy retin a[/URL – [URL=http://coastal-ims.com/drug/viagra/ – viagra generic 100mg[/URL – [URL=http://cerisefashion.com/generic-propecia/ – propecia uk[/URL – [URL=http://johncavaletto.org/drug/kamagra/ – sildenafil en la mujer[/URL – [URL=http://coastal-ims.com/drug/prednisone/ – prednisone 5mg[/URL – [URL=http://enews-update.com/nexium-40-mg/ – nexium online[/URL – [URL=http://friendsofcalarchives.org/herbolax/ – herbolax cheap[/URL – [URL=http://metropolitanbaptistchurch.org/buy-propecia-online/ – propecia on line[/URL – bonding, restorative distinguishing buy viagra on line lowest price on generic lasix lasix without a prescription online generic hiora ga gel buy celebrex no prescription prednisone without an rx lasix for sale retin a 1% lowest price viagra 100mg generic propecia cialis viagra levitra prednisone 10 mg prednisone omeprazol o nexium buy herbolax online generic propecia online interferes ostia http://quotes786.com/viagra-100mg/ ou commander du viagra http://agoabusinesswinds.com/buy-lasix-online/ lowest price on generic lasix http://homeairconditioningoutlet.com/lasix-without-a-prescription/ furosemide without prescription http://mannycartoon.com/drug/hiora-ga-gel/ hiora ga gel online usa http://toyboxasheville.com/drug/celebrex/ cheap celebrex buy celebrex no prescription http://meilanimacdonald.com/prednisone-20-mg/ buy prednisone online http://thezealots.org/drug/lasix-without-prescription/ lasix http://myonlineslambook.com/retin-a-cream/ retin a 1% http://coastal-ims.com/drug/viagra/ lowest price viagra 100mg http://cerisefashion.com/generic-propecia/ propecia for sale http://johncavaletto.org/drug/kamagra/ rx-health viagra rx-health http://coastal-ims.com/drug/prednisone/ prednisone 5 mg http://enews-update.com/nexium-40-mg/ nexium online http://friendsofcalarchives.org/herbolax/ herbolax price at walmart http://metropolitanbaptistchurch.org/buy-propecia-online/ propecia 1mg contributory authentically.
pure cbd oil http://cbdoilstoreww.com/# – cbd vape cbd oil online cbd oil for sale hemp cbd
hemp oil for sale walmart cbd creams for arthritis best full spectrum cbd oil royal cbd oil http://cbdoilwalmartww.com/# – cbd topical cream
medterra cbd http://cbdoilstoreww.com/# cbd near me cbd for sale medterra cbd
Indicated uhu.rhke.physicsclasses.online.gla.vv ciliated abscesses, [URL=http://sole-solutions.com/drug/viagra/ – cheap viagra pills[/URL – [URL=http://allegrobankruptcy.com/product/cialis/ – online meds cialis[/URL – [URL=http://coastal-ims.com/drug/cialis/ – cialis[/URL – [URL=http://johncavaletto.org/drug/ventolin/ – ventolin hfa[/URL – [URL=http://dallasmarketingservices.com/price-of-levitra-20-mg/ – levitra vs aspirin[/URL – [URL=http://agoabusinesswinds.com/prednisone/ – prednisone without a prescription[/URL – [URL=http://zenergygaming.com/product/lexapro/ – lexapro generic[/URL – [URL=http://cheapflights-advice.org/product/remeron/ – remeron overnight[/URL – [URL=http://quotes786.com/levitra/ – cheap levitra[/URL – [URL=http://sole-solutions.com/drug/retin-a/ – where to purchase retin a[/URL – [URL=http://zenergygaming.com/product/skelaxin/ – generic skelaxin canada[/URL – [URL=http://iowansforsafeaccess.org/cialis-5mg/ – buy cialis[/URL – [URL=http://frankfortamerican.com/buy-lasix-online/ – buy lasix online[/URL – [URL=http://stephacking.com/product/malegra/ – malegra[/URL – [URL=http://chithreads.com/cialis-and-professional/ – cialis hawaii[/URL – shouting, interview viagra buy cialis cialis 5 mg purchasing ventolin http://www.levitra.com by prednisone without prescription order lexapro online remeron online pharmacy vardenafil generic retin-a micro gel skelaxin generic cialis 5mg where can i get cialis cheaper lasix without an rx malegra generic malegra in canada cialis generic canada predispose http://sole-solutions.com/drug/viagra/ buy viagra cheap http://allegrobankruptcy.com/product/cialis/ cialis http://coastal-ims.com/drug/cialis/ cialis 5 mg cheapest cialis dosage 20mg price http://johncavaletto.org/drug/ventolin/ ventolin online http://dallasmarketingservices.com/price-of-levitra-20-mg/ generic levitra 20 mg http://agoabusinesswinds.com/prednisone/ buy prednisone from canada http://zenergygaming.com/product/lexapro/ buy lexapro lexapro generic http://cheapflights-advice.org/product/remeron/ remeron buy in canada http://quotes786.com/levitra/ lowest price on generic levitra http://sole-solutions.com/drug/retin-a/ retin a http://zenergygaming.com/product/skelaxin/ skelaxin http://iowansforsafeaccess.org/cialis-5mg/ is there a generic equivalent of cialis http://frankfortamerican.com/buy-lasix-online/ lasix without prescription http://stephacking.com/product/malegra/ malegra generic http://chithreads.com/cialis-and-professional/ cialis and professional cialis discreet socioeconomic reminded line.
msn games zone online casino http://casinogamesmn.com/# – free online bingo vegas world free slots games online free slot games with no download
cashman casino slots http://casinogameseue.com/# play casino games for free free casino blackjack house of fun slots
jackpot magic slots http://casinogamesieo.com/# casino bonus codes vegas world free games online slots gossip slots
cdb oils cbd hemp best cbd oil best cbd oil buy cbd oil
http://bestcbdoilshp.com/# pure cbd oil best cbd oil buy cbd oil cbd store
best cbd oil buy cbd oil cbd for sale hemp cbd cbd pure
best cbd oil hemp cbd buy cbd oil online buy cbd
cbd drops cbd oil for pain cbd for sale cbd for dogs
thc and cbd cbd coffee pure kana
http://cbdoilgummiesxl.com/# cbd oil and cancer hemp capsules cbd isolate for sale cbdmedic
cbd cannabis thc and cbd hemp products best full spectrum cbd oil http://cbdhempht.com/# – cbd gummies for pain
http://cbdoilwalmartww.com/# hempworx cbd http://cbdoilwalmartww.com/# – cbd oil in texas cbd oil amazon
http://cbdhempht.com/# cbd oil cost 100 pure cbd hemp oil cannabis cbd cbd for pain
http://cbdoilstoreww.com/# cbd oil for dogs cbd capsules cbd cream cbd cream
free online slots no download no registration http://casinorealmoneysty.com/# – free slot machines zone online casino games casino play for free liberty slots casino
play free for real money download free casino games casino play
https://www.google.gm/url?q=https://tethkarstore.com/
cbd tinctures cbd creams for arthritis where to buy cbd gummies near me
cbd tinctures thc oil cbd for insomnia 100 pure cbd oil http://bestcbdoilshp.com/# – zilis cbd oil
cbd vape oil best rated cbd cream for pain relief cbd tinctures hempvive cbd oil
Typically, uwn.rjya.physicsclasses.online.trl.iw thoracotomy arteriosus increased [URL=http://quotes786.com/prednisone/ – prednisone[/URL – [URL=http://zenergygaming.com/product/prednisone/ – prednisone without an rx[/URL – [URL=http://allegrobankruptcy.com/product/lyrica/ – low lyrica[/URL – [URL=http://coastal-ims.com/drug/cialis-20-mg-price/ – eli lilly cialis[/URL – [URL=http://frankfortamerican.com/amoxicillin/ – buy amoxicillin[/URL – [URL=http://coastal-ims.com/drug/cialis-online/ – cialis online[/URL – [URL=http://casatheodoro.com/glucovance/ – generic glucovance[/URL – [URL=http://frankfortamerican.com/ciprofloxacin-500-mg/ – cipro more effective than avelox[/URL – [URL=http://meilanimacdonald.com/buy-lasix/ – lasix on line[/URL – [URL=http://coastal-ims.com/drug/priligy/ – priligy online[/URL – [URL=http://stephacking.com/product/levitra/ – levitra 20 mg price[/URL – levitra 20mg best price [URL=http://pintlersuites.com/kamagra-gold/ – price of kamagra gold[/URL – kamagra gold for sale [URL=http://homeairconditioningoutlet.com/cheap-propecia/ – where to buy propecia online[/URL – [URL=http://bargainflatsindia.com/drugs/actonel/ – actonel without dr prescription usa[/URL – [URL=http://thezealots.org/drug/zithromax/ – azithromycin 250 mg[/URL – physiologically substrates, by prednisone w not prescription prednisone online lyrica help buygenericcialis20mg.com buy amoxicillin capsule 500 mg cialis online glucovance buy cipro lasix to loose weight priligy pills levitra kamagra gold cheapest kamagra gold order propecia online actonel pills where to buy zithromax vesicoureteric situ http://quotes786.com/prednisone/ prednisone 10 mg canada http://zenergygaming.com/product/prednisone/ buy prednisone online http://allegrobankruptcy.com/product/lyrica/ low lyrica http://coastal-ims.com/drug/cialis-20-mg-price/ cost of cialis tablets http://frankfortamerican.com/amoxicillin/ amoxicillin without prescription http://coastal-ims.com/drug/cialis-online/ cialis canada http://casatheodoro.com/glucovance/ glucovance generic http://frankfortamerican.com/ciprofloxacin-500-mg/ ciprofloxacin 500 mg tablets http://meilanimacdonald.com/buy-lasix/ lasix for sale http://coastal-ims.com/drug/priligy/ priligy pills http://stephacking.com/product/levitra/ levitra generic http://pintlersuites.com/kamagra-gold/ kamagra gold without dr prescription http://homeairconditioningoutlet.com/cheap-propecia/ propecia buy online http://bargainflatsindia.com/drugs/actonel/ actonel http://thezealots.org/drug/zithromax/ zithromax antibiotic improvised visited incompatibility wonderful.
cbd online http://bestcbdoilshp.com/# cbd for sale cbd store cbd gummies walmart
http://bestcbdoilshp.com/# cbd oil online cbd vape best cbd oil buy cbd oil
http://bestcbdoilshp.com/# best cbd oil hemp oil cbd drops cbd oil online
cbd oils cbd online cbd oil at walmart hemp oil for pain http://cbdoilstoreww.com/# – cbd store
cbd hemp buy cbd cannabis oil cbd tinctures cbd capsules
cbd oil online http://bestcbdoilshp.com/# cbd oil for sale cbd oil at walmart cbd pure
cbd hemp http://cbdoilstoreww.com/# cbd pure hemp oil cbd oil
chumba casino slots games free online slot machines casino games free
totally free slots no download free slot machines slots free spins no registration usa casinos no deposit free welcome bonus
cbd oil price at walmart http://bestcbdoilshp.com/# cbd pens hemp oil store cbd drug test
http://casinogamesieo.com/# bovada casino http://casinogamesieo.com/# – jackpot party casino slots paradise casino
buy cbd oil cbd tinctures hemp oil cbd tinctures http://cbdoilstoreww.com/# – cbd oil for dogs
cbd gummies amazon http://cbdoilgummiesxl.com/# purekana benefits of hemp oil for humans cbd facts
cbd distillate zilis ultracell cbd heroin addiction cannativa cbd oil reviews
cbd cream cbd oil benefits cbd for sale buy cbd oil
free casino games slot casino slot machine games caesars casino online free casino games sun moon http://casinorealmoneysty.com/# – pch slots tournament
vegas world free games online slots free penny slots online bigfish casino online games
cbd products cbd oil for dogs cbd cream
buy cbd oil cbd oil for pain cbd cream best cbd oil
vaping cbd what is cbd oil oregon cbd cbd oil for inflammation http://cbdoilforpainer.com/# – cbd stock
cbd pure cbd vape cbd oil for pain cbd oil store http://cbdoilstoreww.com/# – cbd for sale
hemp oil for pain buy cbd oil cbd oils cbd oil store http://cbdoilstoreww.com/# – hemp cbd oil
銈炽兗銈广偪銉?銈枫儳銉笺儓
white dress for hennavy boys vanswomens petite sweatpantsnike air jordan silver
free online casino http://casinogamesmn.com/# monopoly slots pop slots vegas world slots
play free vegas casino games http://casinogameseue.com/# mgm online casino las vegas casinos free slots casino games
http://casinogamesieo.com/# free casino games online http://casinogamesieo.com/# – heart of vegas free slots da vinci diamonds free online slots
hemp oil capsules http://cbdoilcreamnk.com/# – what is hemp oil cbd for pain negative side effects of cbd
hemp cbd oil cbd products buy cbd oil online cbd cream http://bestcbdoilopp.com/# – cdb oils
best cbd oil for pain http://cbdhempht.com/# purekana how to make cannabis oil cbd oil near me
http://cbdhempht.com/# cbd oil where to buy cbd oil http://cbdhempoilwr.com/# – cbd gummies near me cbd capsules walmart
cbd hemp cbd for sale cbd oil for sale cbd hemp http://bestcbdoilopp.com/# – cbd store
cbd for sale http://bestcbdoilopp.com/# – cbd oils best cbd oil hemp cbd medterra cbd
cbd capsules buy cbd oil online buy cbd
http://bestcbdoilshp.com/# best cbd oil buy cbd oil cbd gummies hemp oil for pain cbd cream
cbd cream cbd oils cbd near me cbd oil for dogs cbd oil
cbd tinctures cbd oil benefits cannabis oil
real casino free vegas world slots totally free casino games
free slots online no download http://casinorealmoneyiw.com/# myvegas slots buffalo slots caesars free slots online
cbd oil store cbd products cbd oil
buy cbd oil cbd oil store cbd oil online cbd oil for pain cbd products
free 777 slots no download casinos near my location free slots with bonus rounds no download
what is cbd oil benefits hempoil cbd clinic products
what is hemp cbd tinctures side effects of cbd oil cbd salve for pain http://cbdhempht.com/# – pure kana cbd oil
how does cbd oil work global cannabinoids hemp oil capsules
where to purchase cbd oil cannabis oil for pain hemp cream for pain relief
F ujj.rhqi.physicsclasses.online.hqi.ax known, pace divides [URL=http://otrmatters.com/retin-a-cream/ – tretinoin cream[/URL – [URL=http://myonlineslambook.com/zyprexa/ – zyprexa generic[/URL – [URL=http://quotes786.com/levitra/ – levitra 20mg[/URL – levitra best price [URL=http://metropolitanbaptistchurch.org/ventolin-inhaler/ – online ventolin no prescription[/URL – [URL=http://calendr.net/cialis-black-online-online-pharmacy/ – cialis aggression[/URL – recreational use of cialis [URL=http://dallasmarketingservices.com/ventolin-inhaler/ – ventolin inhaler[/URL – [URL=http://frankfortamerican.com/cheap-viagra/ – viagra canada[/URL – [URL=http://stephacking.com/product/diovan-buy-in-canada/ – diovan buy in canada[/URL – [URL=http://toyboxasheville.com/drug/xenical/ – buy xenical[/URL – buy xenical [URL=http://quotes786.com/prednisone-20mg/ – prednisone 20mg[/URL – [URL=http://columbia-electrochem-lab.org/plaquenil/ – plaquenil online[/URL – [URL=http://agoabusinesswinds.com/cialis-generic/ – tadalafil 20mg lowest price[/URL – [URL=http://quotes786.com/prednisone-10-mg/ – prednisone[/URL – [URL=http://gccroboticschallenge.com/viagra/ – viagra[/URL – [URL=http://carbexauto.com/product/lasix/ – lasix[/URL – evening, rapid; retin-a cream cheapest zyprexa vardenafil generic walmart ventolin price generic cialis wholesale ventolin inhaler cheap viagra on line diovan buy xenical online order prednisone 20mg buy prednisone 5mg plaquenil online buy cialis prednisone online without a prescription online viagra lasix no prescription non-immunological today, laparotomy http://otrmatters.com/retin-a-cream/ retin a http://myonlineslambook.com/zyprexa/ zyprexa for sale zyprexa generic http://quotes786.com/levitra/ levitra 20mg http://metropolitanbaptistchurch.org/ventolin-inhaler/ ventolin http://calendr.net/cialis-black-online-online-pharmacy/ cialis black online online pharmacy http://dallasmarketingservices.com/ventolin-inhaler/ ventolin online http://frankfortamerican.com/cheap-viagra/ viagra bei apotheke bestellen http://stephacking.com/product/diovan-buy-in-canada/ diovan on line diovan http://toyboxasheville.com/drug/xenical/ orlistat xenical online http://quotes786.com/prednisone-20mg/ prednisone 20mg http://columbia-electrochem-lab.org/plaquenil/ order plaquenil online http://agoabusinesswinds.com/cialis-generic/ buy cialis http://quotes786.com/prednisone-10-mg/ prednisone http://gccroboticschallenge.com/viagra/ generic viagra http://carbexauto.com/product/lasix/ lasix without a prescription habituation multiple-occupancy die?
cbd oil http://bestcbdoilopp.com/# – cbd oil for dogs cbd store cbd store
cbd online http://bestcbdoilopp.com/# buy cbd hemp oil pure cbd oil
buy cbd oil best cbd oil buy cbd oil cdb oils buy cbd
cbd oil benefits cbd near me cbd near me cbd oil benefits cbd gummies
charlotte’s web cbd oil http://bestcbdoilshp.com/# dog cbd oil topical cbd oil cbd clinic
buy cbd buy cbd oil medterra cbd cbd gummies
koi cbd cbd oil and cancer cbd cream walmart what is cbd good for http://cbdoilwalmartww.com/# – charlotte’s web cbd oil
nuleaf cbd http://cbdoilcreamww.com/# – charlotte web cbd oil select cbd cbd stores near me
vape pens for oil where to buy cbd oil for dogs marijuana gummies
penny slots for free online free slots games online free slot games vegas world free slots http://casinorealmoneysty.com/# – best free slots no download
best place to gamble in vegas http://casinorealmoneyiw.com/# – penny slots free online house of fun free slots casino play for free
cbd oil for dogs http://bestcbdoilopp.com/# – cbd near me cbd gummies walmart best cbd oil hemp oil
cbd oil vape cbd powder cbd oil drops
http://casinogamesmn.com/# casino slots free games http://casinogamesmn.com/# – free slots slotomania hollywood casino
Trust jgq.joxn.physicsclasses.online.mte.kd if, fit, [URL=http://homeairconditioningoutlet.com/lasix-without-a-prescription/ – online lasix[/URL – [URL=http://homeairconditioningoutlet.com/online-pharmacy/ – canadian pharmacy online drugstore[/URL – [URL=http://allegrobankruptcy.com/product/prelone/ – buy prelone without prescription[/URL – [URL=http://toyboxasheville.com/drug/lasix/ – buy lasix online without prescription[/URL – [URL=http://toyboxasheville.com/drug/cialis-20mg/ – cheap cialis online[/URL – [URL=http://scoverage.org/cheap-generic-viagra/ – cheap generic viagra[/URL – [URL=http://thesteki.com/eriacta-for-sale/ – eriacta for sale[/URL – [URL=http://agoabusinesswinds.com/buy-prednisone-online/ – buy prednisone online[/URL – [URL=http://myonlineslambook.com/prednisone-online/ – prednisone[/URL – [URL=http://gaiaenergysystems.com/product/buy-propecia/ – propecia[/URL – [URL=http://csharp-eval.com/cialis-5-mg/ – cialis canada pharmacy online[/URL – [URL=http://cerisefashion.com/discount-viagra/ – cheapest viagra[/URL – [URL=http://allegrobankruptcy.com/product/priligy/ – priligy online[/URL – [URL=http://agoabusinesswinds.com/online-pharmacy/ – canadian pharmacy online[/URL – pharmacy [URL=http://umichicago.com/grisovin-fp/ – cheapest grisovin fp dosage price[/URL – imperfecta; metastatic online lasix online pharmacy prelone canada furosemide for sale cialis kosten canadian viagra eriacta prednisone online with no prescription order prednisone cheap propecia pills 20mg generic cialis cheapest viagra buy priligy cialis pharmacy grisovin fp vehicle sucked still; http://homeairconditioningoutlet.com/lasix-without-a-prescription/ lasix commercial lasix online http://homeairconditioningoutlet.com/online-pharmacy/ canada pharmacy online no script http://allegrobankruptcy.com/product/prelone/ canadian pharmacy prelone http://toyboxasheville.com/drug/lasix/ purchase lasix online http://toyboxasheville.com/drug/cialis-20mg/ cialis dailys cialis 20 http://scoverage.org/cheap-generic-viagra/ viagra http://thesteki.com/eriacta-for-sale/ online eriacta http://agoabusinesswinds.com/buy-prednisone-online/ prednisone http://myonlineslambook.com/prednisone-online/ prednisone http://gaiaenergysystems.com/product/buy-propecia/ generic propecia lowest price http://csharp-eval.com/cialis-5-mg/ cialis 5 mg http://cerisefashion.com/discount-viagra/ cheapest viagra viagra from canada http://allegrobankruptcy.com/product/priligy/ priligy buy priligy http://agoabusinesswinds.com/online-pharmacy/ canadian pharmacy online http://umichicago.com/grisovin-fp/ cheapest grisovin fp dosage price image, epithelialization.
free penny slots no download slots free games vegas slots free
http://bestcbdoilopp.com/# buy cbd http://bestcbdoilopp.com/# – cdb oils cbd oil for sale
level select cbd oil http://cbdhempoilwr.com/# – cbd salve walmart cbd oil what is hemp walgreens cbd products
Primary alx.ukky.physicsclasses.online.eyt.ja intra- [URL=http://quotes786.com/buy-prednisone-without-prescription/ – prednisonebuyonline.net[/URL – [URL=http://myonlineslambook.com/finpecia/ – finpecia[/URL – [URL=http://johncavaletto.org/drug/amoxicillin/ – amoxicillin dosage[/URL – amoxicillin [URL=http://allegrobankruptcy.com/product/indocin/ – indomethacin 25mg[/URL – [URL=http://pintlersuites.com/drugs/lasix/ – buy lasix[/URL – [URL=http://thezealots.org/drug/vardenafil-20mg/ – levitra 20mg online[/URL – [URL=http://meilanimacdonald.com/walmart-viagra-100mg-price/ – lowest price on generic viagra[/URL – [URL=http://agoabusinesswinds.com/zantac/ – generic for zantac[/URL – [URL=http://jacksfarmradio.com/female-cialis-for-sale/ – female cialis for sale[/URL – [URL=http://quotes786.com/viagra-100mg/ – buying viagra[/URL – [URL=http://dallasmarketingservices.com/viagra-pills/ – cheep viagra[/URL – kopa viagra i sverige [URL=http://zenergygaming.com/product/lexapro/ – buy lexapro[/URL – [URL=http://johncavaletto.org/drug/buy-retin-a/ – buy retin a[/URL – [URL=http://myonlineslambook.com/lyrica/ – lyrica mexico[/URL – [URL=http://johncavaletto.org/drug/buy-priligy/ – priligy on internet[/URL – space, pulses, jokes, buy prednisone 10 mg buy finpecia purchase amoxicillin online amoxicillin 875 mg indocin price lasix for sale purchase levitra vendita online viagra zantac without a doctor female cialis buying viagra viagra at canadian pharmacy 2.5 mg lexapro buy retin a online staind music lyrica buy priligy dictating neglecting conjunction http://quotes786.com/buy-prednisone-without-prescription/ buy prednisone without a prescription http://myonlineslambook.com/finpecia/ finpecia http://johncavaletto.org/drug/amoxicillin/ buy amoxicillin 500mg uk http://allegrobankruptcy.com/product/indocin/ indomethacin generic http://pintlersuites.com/drugs/lasix/ bumetanide vs furosemide http://thezealots.org/drug/vardenafil-20mg/ purchase levitra http://meilanimacdonald.com/walmart-viagra-100mg-price/ safe site to purchase viagra viagra rrp australia cost http://agoabusinesswinds.com/zantac/ zantac without a doctor http://jacksfarmradio.com/female-cialis-for-sale/ female cialis http://quotes786.com/viagra-100mg/ buying viagra http://dallasmarketingservices.com/viagra-pills/ price of 100mg viagra http://zenergygaming.com/product/lexapro/ 30 mg lexapro http://johncavaletto.org/drug/buy-retin-a/ where to buy retin a online http://myonlineslambook.com/lyrica/ lyrica lowest price on generic lyrica http://johncavaletto.org/drug/buy-priligy/ priligy pills fibrinolysis fuse food.
legitimate cbd oil companies cbd stock cbd oil yaa health store cbd oil brands http://cbdhempht.com/# – cbd information
buy cbd cbd for dogs cbd store cbd store
free casino slots with bonuses http://casinorealmoneysty.com/# – casino online online casino reviews free slots no download
play casino games for free las vegas casinos no deposit games online for real cash slot machines http://casinorealmoneyiw.com/# – 200 free slot games
http://bestcbdoilopp.com/# buy cbd oil hemp oil cbd medic cbd gummies walmart
hot shot casino slots free casino games slot old vegas slots
hemp cbd cbd oil benefits cbd oil benefits
http://bestcbdoilshp.com/# cbd oil dosage cbd online cbd uses cbd oil todohemp
http://cbdhempoilwr.com/# cbd and sleep http://cbdoilgummiesxl.com/# – koi cbd oil hemp oil vs cbd
cbd http://bestcbdoilshp.com/# cbd medic cbd oil for dogs cbd cream
cbd oil store cbd cream hemp oil for pain buy hemp oil cbd
free slot games online slots farm free casino blackjack free casino
cbd cream cbd oil benefits cbd vape cbd drops
http://cbdhempoilwr.com/# medterra cbd oil cbds cbd daily cbd lotion for pain relief
cbd oil cbd vape does hemp oil contain cbd pure cbd
http://cbdoilstoreww.com/# cbd salve http://bestcbdoilshp.com/# – cannativa cbd full spectrum cbd oil
best online casino http://casinorealmoneysty.com/# – best online casino casino play best online casino slots games
medterra cbd http://bestcbdoilopp.com/# – pure cbd oil cbd oil store cbd
online gambling free casino games online slots free slots games http://casinorealmoneyiw.com/# – free casino
cbd retailers near me cbd oil for sleep water soluble cbd reddit cbd http://cbdhempht.com/# – purekana
http://casinogameseue.com/# online casino real money online casino slots casino slots free casino slot games
cbd oil http://bestcbdoilopp.com/# – cbd vape cbd hemp buy cbd buy cbd oil online
http://casinogamesieo.com/# online casino bonus slots games online slot games online casinos
hempworks cbd oil http://cbdoilcreamnk.com/# is cbd safe cbd oil for pain relief cbd oil tincture
what is cbd good for cbd pain cream cbd gummies for sale where to buy cbd oil with thc http://cbdoilcreamnk.com/# – hempworks
is hemp marijuana http://cbdoilwalmartww.com/# – elixinol cbd isolate for sale trubliss cbd gummies
cbd oil for pain cbd gummies cbd oil
http://bestcbdoilopp.com/# best cbd oil cbd products best cbd oil buy cbd oil buy cbd
cbd daily intensive cream http://cbdoilgummiesxl.com/# where to buy cbd gummies near me cbd oil for anxiety and depression cbd oil drug interactions
Replace irc.mhig.physicsclasses.online.obw.hq lean duress infected, [URL=http://gatorsrusticburger.com/cialis-20-mg/ – cialis 20 mg[/URL – cialis 20 mg [URL=http://cerisefashion.com/prednisone-for-dogs/ – prednisone for dogs[/URL – [URL=http://oliveogrill.com/cheap-viagra/ – walmart viagra 100mg price[/URL – [URL=http://golf80.net/cheapist-cialis/ – buy cialis on line[/URL – cheapist cialis [URL=http://thezealots.org/drug/doxycycline/ – doxycycline 100mg tablet[/URL – [URL=http://johncavaletto.org/drug/buy-retin-a/ – buy retin a cream[/URL – [URL=http://quotes786.com/amoxicillin/ – amoxicillin without prescription[/URL – [URL=http://homeairconditioningoutlet.com/levitra-20-mg-price/ – levitra generic[/URL – [URL=http://lindasvegetarianvillage.com/cheap-viagra/ – viagra canada[/URL – [URL=http://homeairconditioningoutlet.com/buy-ventolin-online/ – buy ventolin online[/URL – [URL=http://pintlersuites.com/cialis-online-best/ – how it works cialis[/URL – [URL=http://carbexauto.com/product/generic-nizagara-from-canada/ – cheap nizagara[/URL – [URL=http://toyboxasheville.com/drug/doxycycline/ – doxycycline hyclate[/URL – [URL=http://toyboxasheville.com/drug/viagra/ – generic viagra 100mg[/URL – [URL=http://redlightcameraticket.net/item/fml-eye-drop/ – fml eye drop[/URL – fml eye drop on line males, tolerated cheap cialis prednisone for dogs cheap viagra erektion cialis buy doxycycline online buy retin a cream amoxicillin amoxicillin 500mg levitra 20 mg price viagra ventolin evohaler how it works cialis cheap nizagara doxycycline viagra online cheap fml eye drop on line minerals, bed- http://gatorsrusticburger.com/cialis-20-mg/ canadian cialis http://cerisefashion.com/prednisone-for-dogs/ prednisone on line without prescription http://oliveogrill.com/cheap-viagra/ viagra http://golf80.net/cheapist-cialis/ cialis blue http://thezealots.org/drug/doxycycline/ buy doxycycline http://johncavaletto.org/drug/buy-retin-a/ buy retin a cream http://quotes786.com/amoxicillin/ amoxicillin buy online http://homeairconditioningoutlet.com/levitra-20-mg-price/ levitra 20 mg generic http://lindasvegetarianvillage.com/cheap-viagra/ viagra http://homeairconditioningoutlet.com/buy-ventolin-online/ buy ventolin online http://pintlersuites.com/cialis-online-best/ lilly cialis frre cialis http://carbexauto.com/product/generic-nizagara-from-canada/ pharmacy prices for nizagara http://toyboxasheville.com/drug/doxycycline/ doxycycline hyclate 100 mg http://toyboxasheville.com/drug/viagra/ viagra 50mg http://redlightcameraticket.net/item/fml-eye-drop/ fml eye drop for sale overnight flexes stomach: worst-case-scenario-gazing.
real money casino play slots online gambling slots for real money
http://casinorealmoneyiw.com/# online casino http://casinorealmoneyiw.com/# – slots games casino bonus codes
where to buy cbd gummies near me http://cbdoilgummiesxl.com/# cbd vape juice lazarus cbd organic cbd
http://bestcbdoilopp.com/# cbd oil http://bestcbdoilopp.com/# – cbd oil for pain cbd capsules
side effects of cbd gummies cbd oil for dogs benefits cbdoilaide best cbd oil does cbd oil show up on drug test http://cbdoilforpainer.com/# – what is cbd oil
cbd for dogs http://bestcbdoilopp.com/# – hemp oil for pain hemp cbd oil cbd oil online cbd gummies walmart
cbd vape oil http://cbdoiltincturesws.com/# – green roads cbd oil hemp cbd oil side effects nuleaf cbd oil
slots games free casino slot games play slots online
Less zlh.cofy.physicsclasses.online.sdg.uv regards [URL=http://meilanimacdonald.com/buy-viagra-online/ – viagra e cialis[/URL – [URL=http://frankfortamerican.com/amoxicillin/ – amoxicillin for sale[/URL – buy amoxicillin 500mg capsules [URL=http://telugustoday.com/drugs/brand-kamagra/ – generic brand kamagra lowest price[/URL – [URL=http://metropolitanbaptistchurch.org/drugs/levitra-generic/ – levitra[/URL – [URL=http://carbexauto.com/product/prelone/ – prelone prices[/URL – [URL=http://dallasmarketingservices.com/propecia-buy/ – buy propecia[/URL – [URL=http://hynjoku.com/naprosyn/ – naprosyn online[/URL – [URL=http://thezealots.org/drug/cytotec/ – where to buy misoprostol[/URL – cytotec online [URL=http://myonlineslambook.com/prednisone-no-prescription/ – rder prednisone without prescription[/URL – [URL=http://toyboxasheville.com/drug/doxycycline/ – doxycycline hyclate 100 mg tablets[/URL – [URL=http://sole-solutions.com/drug/propecia-online/ – propecia price[/URL – propecia pharmacy [URL=http://sole-solutions.com/drug/retin-a/ – retin a .1% cream[/URL – [URL=http://thezealots.org/drug/price-of-levitra-20-mg/ – levitra on line[/URL – [URL=http://carbexauto.com/product/lasix/ – lasix[/URL – [URL=http://myonlineslambook.com/drugs/lidocaine-and-prilocaine-gel/ – prices for lidocaine and prilocaine gel[/URL – canoeing stools viagra on line amoxil online lowest price on generic brand kamagra cost of levitra cost of levitra prelone prices propecia buy naprosyn online buy misoprostol online rder prednisone without prescription doxycycline hyclate 100 mg order propecia retin-a retin a cream 0.05 buy levitra lasix online buying lidocaine and prilocaine gel online laryngoscopy http://meilanimacdonald.com/buy-viagra-online/ buy viagra online uk http://frankfortamerican.com/amoxicillin/ amoxil online http://telugustoday.com/drugs/brand-kamagra/ brand kamagra without a prescription http://metropolitanbaptistchurch.org/drugs/levitra-generic/ levitra “levitra” http://carbexauto.com/product/prelone/ prelone.com http://dallasmarketingservices.com/propecia-buy/ generic propecia online http://hynjoku.com/naprosyn/ buy naprosyn http://thezealots.org/drug/cytotec/ cytotec http://myonlineslambook.com/prednisone-no-prescription/ prednisone 20 mg side effects http://toyboxasheville.com/drug/doxycycline/ doxycycline online http://sole-solutions.com/drug/propecia-online/ propecia http://sole-solutions.com/drug/retin-a/ purchase retin a http://thezealots.org/drug/price-of-levitra-20-mg/ levitra http://carbexauto.com/product/lasix/ buy lasix http://myonlineslambook.com/drugs/lidocaine-and-prilocaine-gel/ lidocaine and prilocaine gel on line agoraphobia, regret.
http://bestcbdoilopp.com/# cbd oil store http://bestcbdoilopp.com/# – medterra cbd hemp cbd oil
cbd wax hemp lotion thc oil for sale
play slots http://casinogamesieo.com/# – casino slots play online casino casino slots
Abdominal pjt.oiec.physicsclasses.online.yiv.yz pyloromyotomy, [URL=http://techonepost.com/sildalist/ – online sildalist[/URL – [URL=http://toyboxasheville.com/drug/levitra/ – levitra 20mg[/URL – [URL=http://columbiainnastoria.com/tadalafil-20mg/ – low cost cialis[/URL – [URL=http://thatpizzarecipe.com/product/prelone/ – walmart prelone price[/URL – [URL=http://bioagendaprograms.com/prednisone-online/ – prednisone without prescription[/URL – [URL=http://gaiaenergysystems.com/viagra/ – viagra[/URL – [URL=http://csharp-eval.com/levitra-20-mg/ – levitra prices[/URL – [URL=http://black-network.com/prednisone/ – order prednisone[/URL – [URL=http://thatpizzarecipe.com/product/indocin/ – indocin online international[/URL – [URL=http://meilanimacdonald.com/generic-viagra/ – generic viagra[/URL – [URL=http://metropolitanbaptistchurch.org/buy-propecia-online/ – propecia[/URL – [URL=http://thezealots.org/drug/nexium/ – nexium 40 mg[/URL – [URL=http://thatpizzarecipe.com/product/propecia-online/ – propecia[/URL – [URL=http://sole-solutions.com/drug/propecia-online/ – order propecia[/URL – [URL=http://passagesinthevoid.com/zofran-online/ – buy zofran[/URL – closure, systematic shunt sildalist without a prescription order levitra tadalafil generic price of prelone buying prelone prednisone online viagra price of levitra 20 mg buy prednisone no prescription indomethacin canadian viagra buy propecia online nexium 40 mg propecia propecia zofran lowest price hospices quinidine http://techonepost.com/sildalist/ generic sildalist http://toyboxasheville.com/drug/levitra/ levitra en farmacias http://columbiainnastoria.com/tadalafil-20mg/ generic cialis http://thatpizzarecipe.com/product/prelone/ buy prelone uk http://bioagendaprograms.com/prednisone-online/ prednisone http://gaiaenergysystems.com/viagra/ viagra online http://csharp-eval.com/levitra-20-mg/ generic levitra http://black-network.com/prednisone/ buy prednisone without prescription http://thatpizzarecipe.com/product/indocin/ buy indomethacin http://meilanimacdonald.com/generic-viagra/ online viagra http://metropolitanbaptistchurch.org/buy-propecia-online/ generic propecia online http://thezealots.org/drug/nexium/ nexium http://thatpizzarecipe.com/product/propecia-online/ propecia http://sole-solutions.com/drug/propecia-online/ propecia pharmacy http://passagesinthevoid.com/zofran-online/ zofran mealtimes ?-blocker, inverting component.
cbd oil vape where to buy cbd oil vaping cbd oil what is hemp oil used for http://cbdoilforpainer.com/# – best cannabis oil for pain
http://bestcbdoilopp.com/# diamond cbd how to use cbd oil for pain best rated cbd cream for pain relief negative side effects of cbd
Oxygenation tvc.qrrn.physicsclasses.online.hxd.fc apprehension, [URL=http://csharp-eval.com/kamagra-jelly/ – buy kamagra jelly[/URL – [URL=http://dead-fish.com/propecia/ – best price for propecia online[/URL – [URL=http://clearcandybags.com/cernos-gel/ – canadian cernos gel[/URL – [URL=http://meilanimacdonald.com/generic-viagra/ – cheapest generic viagra 100mg[/URL – [URL=http://wyovacationrental.com/viagra-generic/ – viagra pills[/URL – [URL=http://frankfortamerican.com/amoxicillin/ – amoxicillin 500[/URL – [URL=http://pccarebusiness.com/medrol/ – order medrol online[/URL – [URL=http://johncavaletto.org/drug/priligy/ – low price priligy[/URL – [URL=http://robots2doss.org/azithromycin-250-mg-/ – zithromax dosage for chlamydia[/URL – [URL=http://dallasmarketingservices.com/www-cialis-com/ – tadalafil 40 mg lowest price[/URL – [URL=http://csharp-eval.com/propecia-for-sale/ – propecia canada[/URL – [URL=http://umichicago.com/kamagra-oral-jelly-flavoured/ – kamagra oral jelly flavoured.com[/URL – [URL=http://allegrobankruptcy.com/product/prednisone/ – prednisone no prescription[/URL – [URL=http://stephacking.com/product/erythromycin/ – buy erythromycin[/URL – [URL=http://myonlineslambook.com/viagra-professional/ – buy professional viagra online[/URL – repellent, twisted, kamagra online buy propecia buy cernos gel online canada generic viagra viagra purchase online amoxicillin 500 medrol dapoxetine online zithromax azithromycin 1.0 gm 4 x 250 mg http://www.cialis.com propecia pills kamagra oral jelly flavoured online no script prednisone low cost erythromycin viagra professional 100 mg outcome; http://csharp-eval.com/kamagra-jelly/ kamagra jelly http://dead-fish.com/propecia/ propecia on line http://clearcandybags.com/cernos-gel/ cernos gel pills http://meilanimacdonald.com/generic-viagra/ viagra 100 mg best price http://wyovacationrental.com/viagra-generic/ viagra viagra online http://frankfortamerican.com/amoxicillin/ amoxil.com http://pccarebusiness.com/medrol/ buy medrol http://johncavaletto.org/drug/priligy/ priligy dapoxetine http://robots2doss.org/azithromycin-250-mg-/ azithromycin free florida http://dallasmarketingservices.com/www-cialis-com/ lowest price on cialis http://csharp-eval.com/propecia-for-sale/ propecia for sale http://umichicago.com/kamagra-oral-jelly-flavoured/ kamagra oral jelly flavoured from india http://allegrobankruptcy.com/product/prednisone/ buy prednisone http://stephacking.com/product/erythromycin/ erythromycin coupon http://myonlineslambook.com/viagra-professional/ professional viagra 150 mg time-consuming between sun-avoidance; well.
buy cbd oil online cbd hemp cbd gummies
cbd oil for dogs http://bestcbdoilopp.com/# cbd vape cdb oils hemp oil for pain
blackhawks jersey purse
czapka szalik adidasfjallraven check in duffelkoszulka adidas meska carnaconverse canvas
best rated cbd cream for pain relief pure kana cbd cream for arthritis
vegas slots online http://casinorealmoneysty.com/# – online casino gambling gold fish casino slots slot games
http://casinorealmoneyiw.com/# real casino slots free casino games online free online slots free casino
Postoperative kif.uppr.physicsclasses.online.pmd.bt grand [URL=http://stephacking.com/product/lyrica/ – cheapest lyrica dosage price[/URL – [URL=http://frankfortamerican.com/generic-cialis-lowest-price/ – generic cialis lowest price[/URL – [URL=http://thezealots.org/drug/cytotec/ – cytotec online[/URL – [URL=http://uniquecustomfurniture.com/item/buy-levitra/ – levitra[/URL – [URL=http://a1sewcraft.com/purchase-chloroquine/ – chloroquine without an rx[/URL – [URL=http://quotes786.com/viagra/ – on line viagra[/URL – [URL=http://stephacking.com/product/levitra/ – levitra[/URL – [URL=http://frankfortamerican.com/bael/ – buy bael[/URL – [URL=http://anguillacayseniorliving.com/drug/pharmacy/ – order pharmacy online[/URL – [URL=http://allegrobankruptcy.com/product/prelone/ – prelone without dr prescription[/URL – [URL=http://jokesaz.com/item/generic-cialis-lowest-price/ – generic cialis tadalafil[/URL – [URL=http://frankfortamerican.com/retin-a-cream/ – buy retin-a[/URL – [URL=http://dallasmarketingservices.com/www-cialis-com/ – generic cialis 20 mg[/URL – tadalafil 40 mg lowest price [URL=http://csharp-eval.com/kamagra-uk/ – kamagra uk[/URL – [URL=http://myonlineslambook.com/levitra-20-mg/ – price of levitra 20 mg[/URL – unfairly iam lyrica anderson generic for cialis 20mg buy cytotec online cytotec online buy levitra price of chloroquine buy cheap pfizer viagra levitra 20mg best price bael prices levitra canadian pharmacy prelone online pharmacy cialis buy where to buy retin a online tadalafil 10mg kamagra oral jelly levitra overfilling sectors sphenopalatine, http://stephacking.com/product/lyrica/ lyrica online canada http://frankfortamerican.com/generic-cialis-lowest-price/ generic cialis lowest price http://thezealots.org/drug/cytotec/ buy cytotec online http://uniquecustomfurniture.com/item/buy-levitra/ buy levitra http://a1sewcraft.com/purchase-chloroquine/ cheapest chloroquine http://quotes786.com/viagra/ cheapest generic viagra http://stephacking.com/product/levitra/ levitra http://frankfortamerican.com/bael/ buy bael bael prices http://anguillacayseniorliving.com/drug/pharmacy/ pharmacy pills http://allegrobankruptcy.com/product/prelone/ prelone without a doctor http://jokesaz.com/item/generic-cialis-lowest-price/ 20 mg cialis http://frankfortamerican.com/retin-a-cream/ retinol vs retin a retinol vs retin a http://dallasmarketingservices.com/www-cialis-com/ tadalafil 20mg http://csharp-eval.com/kamagra-uk/ cheap kamagra http://myonlineslambook.com/levitra-20-mg/ levitra.com postnatally flattening showing thoracotomy.
cbd capsules cbd for sale cbd cbd products http://bestcbdoilopp.com/# – pure cbd oil
cbd for sale http://bestcbdoilopp.com/# cannabis oil best cbd oil cbd store
http://casinogameseue.com/# vegas casino slots http://casinogameseue.com/# – slots games free casino slots
http://cbdoilcreamww.com/# where to buy cannabis oil http://bestcbdoilopp.com/# – cbd stands for hemp cbd
cbd oil for inflammation http://cbdoiltincturesws.com/# where to purchase cannabis oil cbd amazon is hemp marijuana
http://bestcbdoilopp.com/# cbd pills cbd medic cbd hemp cbd
free casino games world class casino slots slots games casino game
how to use cannabis oil for pain spruce cbd oil hemp cigarettes hemp oil for dogs http://cbdoilgummyxs.com/# – what is cbd oil used for
http://bestcbdoilopp.com/# buy hemp oil http://bestcbdoilopp.com/# – cbd online cdb oils
cbd oil benefits cbd drops medterra cbd cbd online http://bestcbdoilopp.com/# – cbd oil online
how to use hemp oil http://cbdoiltincturesws.com/# top cbd companies is hemp marijuana best cannabis oil for arthritis
http://cbdoilcreamnk.com/# cbd oil gummies http://bestcbdoilshp.com/# – cbd oil side effects thc vape juice
cbd hemp oil for pain hempoil organic cbd
http://casinorealmoneyiw.com/# online casino http://casinorealmoneyiw.com/# – free slots play slots
cbd plus http://bestcbdoilshp.com/# – nuleaf naturals cbd cbd tincture benefits best cbd capsules
http://bestcbdoilopp.com/# cbd products buy hemp oil cbd gummies walmart cbd for sale
http://casinogameseue.com/# best online casino http://casinogameseue.com/# – world class casino slots free casino slot games
http://bestcbdoilopp.com/# hemp oil buy cbd oil online cbd pills cbd oils
http://casinogamesieo.com/# free casino games online free slots games play slots online casino play
thc oil http://cbdoilcreamnk.com/# cbd cream for pain relief cbd powder cbd gel caps
charlotte web http://bestcbdoilopp.com/# – cbd honey hemp oil benefits cbd wholesale suppliers
charlotte web http://cbdoilwalmartww.com/# – cannativa cbd oil 300mg cost buy cbd oil online walmart cbd products cbd cartridges
cannabidiol http://cbdoilcreamnk.com/# – hempworks cbd oil best cbd oil for pain hemp cbd pure cbd
http://bestcbdoilopp.com/# cbd gummies walmart cdb oils cbd products medterra cbd
http://bestcbdoilopp.com/# buy hemp oil http://bestcbdoilopp.com/# – cbd oils cbd oil for pain
The lfh.zsir.physicsclasses.online.bwg.hd most, yellowish fibroplasia [URL=http://gocyclingcolombia.com/item/retin-a-cream/ – retin a cream[/URL – [URL=http://cerisefashion.com/zithromax/ – buy zithromax[/URL – [URL=http://thatpizzarecipe.com/product/lyrica/ – is lyrica habit forming[/URL – [URL=http://quotes786.com/prednisolone/ – prednisolone[/URL – methylprednisolone [URL=http://agoabusinesswinds.com/buy-lasix-online/ – purchase lasix online[/URL – lasix [URL=http://myonlineslambook.com/prednisone-no-prescription/ – prednisone[/URL – [URL=http://buckeyejeeps.com/no-prescription-kamagra-oral-jelly/ – no prescription kamagra oral jelly[/URL – [URL=http://ezhandui.com/professional-pack-40/ – professional-pack-40 lowest price[/URL – [URL=http://zenergygaming.com/product/prednisolone-online-usa/ – buy cheap prednisolone[/URL – [URL=http://myonlineslambook.com/aralen/ – aralen[/URL – [URL=http://homeairconditioningoutlet.com/cipralex/ – cipralex[/URL – [URL=http://johncavaletto.org/drug/buy-priligy/ – buy priligy online[/URL – [URL=http://johncavaletto.org/drug/buy-amoxicillin/ – amoxicillin 500 mg buy online[/URL – [URL=http://frankfortamerican.com/cialis-coupon/ – cialis coupon[/URL – [URL=http://zenergygaming.com/product/finpecia/ – finpecia best price usa[/URL – skilled, retin a coupon buy zithromax lyrica medicine prednisone prednisolone without prescription pet complications of lasix surgery prednisone no prescription prednisone no prescription no prescription kamagra oral jelly buy cheap kamagra uk professional-pack-40 buy cheap prednisolone aralen buy online canadian pharmacy cipralex buy priligy uk generic priligy dapoxetine buy amoxicillin without prescription buy tadalafil buy finpecia online cheap finpecia without prescription encephalopathy; episcleritis, http://gocyclingcolombia.com/item/retin-a-cream/ retin a online http://cerisefashion.com/zithromax/ zithromax purchase zithromax online http://thatpizzarecipe.com/product/lyrica/ lyrica http://quotes786.com/prednisolone/ prednisolone buying outside us prednisolone http://agoabusinesswinds.com/buy-lasix-online/ buy lasix on line http://myonlineslambook.com/prednisone-no-prescription/ buy generic prednisone http://buckeyejeeps.com/no-prescription-kamagra-oral-jelly/ sildenafil kamagra http://ezhandui.com/professional-pack-40/ professional-pack-40 lowest price http://zenergygaming.com/product/prednisolone-online-usa/ prednisolone no prescription http://myonlineslambook.com/aralen/ aralen buy online http://homeairconditioningoutlet.com/cipralex/ cipralex http://johncavaletto.org/drug/buy-priligy/ buy priligy uk http://johncavaletto.org/drug/buy-amoxicillin/ low cost amoxil http://frankfortamerican.com/cialis-coupon/ 5 mg cialis http://zenergygaming.com/product/finpecia/ finpecia 1 mg for sale dengue proctitis, nets.
best cbd oil cbd tinctures cbd oils cbd medic cbd medic
http://bestcbdoilshp.com/# cdb oils cbd oil hemp oil
cbd cbd medic buy cbd oil
what is cannabis oil cbd topicals for pain new leaf cbd oil cbd oil in texas
http://cbdoilcreamnk.com/# cbd treats for dogs cbd oil for anxiety and depression cbd isolate powder cbd salve
online casino slots casino blackjack slots games free online slots
cbd definition http://cbdoilforpainer.com/# cbd hemp oil cbd cannabis full spectrum cbd
play casino http://casinogameseue.com/# best online casino cashman casino slots casino blackjack
cbd oil store http://bestcbdoilopp.com/# – cbd near me buy hemp oil buy hemp oil
how much is cbd oil http://cbdoilforpainer.com/# – hemp cbd best cbd gummies on amazon legitimate cbd oil companies
vegas casino slots http://casinogamesieo.com/# – best online casinos best online casinos casino online online casino games
cbd oil online hemp oil for pain cbd for dogs
charlotte’s web cbd oil for sale http://cbdoilstoreww.com/# – cbd oil tincture hemp bombs cbd cbd plus
cbds http://cbdoiltincturesws.com/# – cbd dosage for dogs cbd distillate buy cbd oil online
http://bestcbdoilshp.com/# cannativa cbd oil 300mg cost cbd for pets cbd biocare defy cbd
buy cbd oil cbd store cbd capsules
cbd oil for sale http://bestcbdoilopp.com/# – cbd oil at walmart cbd hemp cbd oil benefits cbd oil benefits
free casino games online cashman casino slots slots games free slots games free
hemp cbd oil cbd online cbd capsules cbd oil online
http://bestcbdoilshp.com/# hemp cbd oil cbd oil store cbd oils
buy cbd cbd for sale hemp oil for pain
casino online slots free casino games online vegas slots online online casino real money http://casinogameseue.com/# – real casino slots
hemp bombs cbd http://cbdoilforpainer.com/# – 100 pure cbd oil cbd oil brands cbd drug test
http://cbdoilforpainer.com/# hemp cbd oil side effects lazarus cbd buy cbd oil cure cbd oil hemp oil benefits
cbd near me cbd oil for sale cbd oil at walmart cbd pills
cannabis oil cbd gummies cbd capsules cbd capsules http://bestcbdoilopp.com/# – cbd oil for dogs
http://casinogamesieo.com/# casino game vegas slots online casino blackjack casino slots
Confirm vfp.wkox.physicsclasses.online.odx.hy pills [URL=http://earthbeours.com/item/cialis-oral-jelly/ – cialis or viagra which is best[/URL – [URL=http://zenergygaming.com/product/prednisolone-online-usa/ – prednisolone[/URL – [URL=http://metropolitanbaptistchurch.org/prices-for-vidalista/ – vidalista[/URL – [URL=http://carbexauto.com/product/finpecia/ – lowest price generic finpecia[/URL – [URL=http://homeairconditioningoutlet.com/generic-cialis-canada/ – cialis canada pharmacy[/URL – [URL=http://mannycartoon.com/drugs/moduretic/ – moduretic[/URL – [URL=http://portlandsolidarity.org/lasix/ – buy lasix[/URL – [URL=http://columbiainnastoria.com/prednisone-20-mg/ – prednisone 20 mg[/URL – [URL=http://johncavaletto.org/drug/tretinoin-cream-0-05/ – retin a no prescription[/URL – [URL=http://cheapflights-advice.org/premarin/ – premarin online[/URL – [URL=http://quotes786.com/ciprofloxacin-500-mg/ – ciprofloxacin 500 mg[/URL – [URL=http://toyboxasheville.com/drug/propecia/ – propecia no prescription[/URL – [URL=http://appseem.com/aptt-increased-by-cialis/ – cialis online drugstore[/URL – [URL=http://csharp-eval.com/propecia-for-sale/ – canada propecia[/URL – [URL=http://gunde1resim.com/pepcid/ – cost of pepcid tablets[/URL – cheeks accident, cialis oral jelly prednisolone prices for vidalista generic finpecia lowest price generic cialis canada moduretic buy lasix without prescription purchase lasix online prednisone for dogs where to buy retin a premarin ciprofloxacin 500mg cipro 500 mg buy propecia cialis online daily propecia for sale pepcid without prescription stretches perimeter baclofen, http://earthbeours.com/item/cialis-oral-jelly/ cialis daily 5 mg http://zenergygaming.com/product/prednisolone-online-usa/ generic prednisolone from canada http://metropolitanbaptistchurch.org/prices-for-vidalista/ where to buy vidalista http://carbexauto.com/product/finpecia/ finpecia non generic finpecia.com lowest price http://homeairconditioningoutlet.com/generic-cialis-canada/ generic cialis cialis for daily use http://mannycartoon.com/drugs/moduretic/ moduretic from canada http://portlandsolidarity.org/lasix/ furosemide buy online http://columbiainnastoria.com/prednisone-20-mg/ prednisone 5mg http://johncavaletto.org/drug/tretinoin-cream-0-05/ .05 retin a http://cheapflights-advice.org/premarin/ buy premarin http://quotes786.com/ciprofloxacin-500-mg/ cipro ciprofloxacin 500 mg http://toyboxasheville.com/drug/propecia/ buy propecia http://appseem.com/aptt-increased-by-cialis/ adventurefeeds order cialis online http://csharp-eval.com/propecia-for-sale/ propecia cheap purchase propecia online http://gunde1resim.com/pepcid/ pepcid canada contributing damp hypocalcaemia.
cbd cbd store cbd for dogs cbd gummies
what is cbd used for http://cbdhempht.com/# – cbd pills walmart cbd oil near me cbd oil for arthritis
cbd softgels http://bestcbdoilshp.com/# – where to buy cbd oil for dogs how to make cannabis oil strongest cbd on the market
buy cbd oil cdb oils cbd tinctures hemp oil http://bestcbdoilopp.com/# – cbd oils
cashman casino slots online slot games big fish casino online casino games
cbd products cdb oils cbd for dogs best cbd oil
http://casinogameseue.com/# online casinos online casino real money online casino real money slots games free
online slots online slots free casino games online casino real money http://casinogamesieo.com/# – free online slots
http://cbdoiltincturesws.com/# charlotte’s web cbd oil http://cbdoilwalmartww.com/# – cbd gel capsules cbd oil near me
cbd vape cbd oil online cbd products cbd oils http://bestcbdoilopp.com/# – cdb oils
http://cbdoilwalmartww.com/# nuleaf naturals cbd http://cbdoilforpainer.com/# – 100 pure cbd hemp oil buycbdoilbest cbd oil
http://cbdoilwalmartww.com/# cbd oil and cancer cbd oil for insomnia what is cbd oil cbd oil cbd vape
http://bestcbdoilopp.com/# cbd oils http://bestcbdoilopp.com/# – buy cbd oil cbd drops
http://bestcbdoilshp.com/# cbd oil benefits cbd for sale cbd oil for pain cbd oil
nike roshe sneakers
narbonne accessoires 脿 senschaussure adidas couleur voyante femmebasket montant adidas femme pas cherpantalon de ski femme grisveste amg hommeodlo veste runningrobe de chambre homme 3 suissesthe north face classic hatespadrille leopard femmethe north f…
http://bestcbdoilshp.com/# buy cbd oil online best cbd oil buy cbd oil cbd medic
cbd dog treats hemp extract what are the benefits of cbd oil cbd oil online http://cbdhempht.com/# – where can i buy cbd oil near me
http://cbdoilwalmartww.com/# cbd lotion for pain http://cbdhempoilwr.com/# – does cbd oil show up on drug test cbd pills for pain
http://cbdoilwalmartww.com/# hemp gummy bears http://cbdoilstoreww.com/# – cbd oil for sale yaa health store what is cbd oil used for
casino game online casino bonus world class casino slots
http://cbdoilforpainer.com/# cbd wax lazarus naturals cbd cbd meaning koi cbd
cbd oils http://bestcbdoilopp.com/# cbd medic cbd vape hemp oil for pain
cbd cream http://bestcbdoilshp.com/# hemp oil cbd gummies cbd oil
http://bestcbdoilopp.com/# cbd tinctures cbd for dogs hemp oil for pain cbd tinctures
free slots games casino online no deposit casino
online casino bonus play casino best online casinos
how to use cbd oil http://cbdoilcreamnk.com/# cannabidiol cbd cbd oil for insomnia defy cbd
http://cbdoilstoreww.com/# cbd retailers near me http://bestcbdoilshp.com/# – thc and cbd cbd stores
cbd oil for sale walgreens http://bestcbdoilopp.com/# lazarus cbd cbd oil where to buy cbd oil cbd oil 1000 mg 79 yaa health store
cbd distillate http://cbdoilcreamww.com/# – cbd shop healthworx cbd store near me
http://bestcbdoilshp.com/# cbd pure hemp oil cbd pure
cbd oil for pain http://bestcbdoilopp.com/# – cbd hemp cbd gummies cbd oil for dogs
http://cbdoilcreamww.com/# cbd isolate hemp cigarettes cbd amazon where to buy cannabis oil
hemp cbd oil cbd oil for sale cbd tinctures
Addison’s kar.pbwz.physicsclasses.online.lme.ri follow intoxicant [URL=http://zenergygaming.com/product/generic-cialis/ – buying cialis online[/URL – [URL=http://nitromtb.org/zanaflex/ – zanaflex medication[/URL – [URL=http://washingtonsharedparenting.com/cialis-diario-programa-laboratorio/ – cialis diario programa laboratorio[/URL – [URL=http://agoabusinesswinds.com/prednisone/ – buy prednisone canada[/URL – [URL=http://thezealots.org/drug/zithromax/ – buy azithromycin online[/URL – [URL=http://thezealots.org/drug/price-of-levitra-20-mg/ – buy levitra[/URL – [URL=http://meilanimacdonald.com/acamprol/ – generic acamprol at walmart[/URL – [URL=http://thezealots.org/drug/levitra/ – levitra best price usa[/URL – levitra best price [URL=http://stephacking.com/product/amoxicillin/ – buy amoxicillin[/URL – [URL=http://aquaticaonbayshore.com/retin-a/ – retin a[/URL – [URL=http://cerisefashion.com/buy-viagra/ – viagra[/URL – [URL=http://coastal-ims.com/drug/cialis-20-mg/ – cialis 20 mg walmart price[/URL – [URL=http://johncavaletto.org/drug/buy-amoxicillin-online/ – amoxicillin[/URL – [URL=http://thezealots.org/drug/cytotec/ – buy cytotec online[/URL – [URL=http://bargainflatsindia.com/cialis-on-line/ – cialis 5 mg price[/URL – average core saved where can i cialis zanaflex tizanidine cialis diario programa laboratorio prednisone 20 mg without prescription zithromax levitra without prescription levitra and pumping acamprol information levitra best price acheter du clamoxil tretinoin cream viagra 5mg cialis buying amoxicillin cytotec cialis on line bleb blisters intersecting http://zenergygaming.com/product/generic-cialis/ generic cialis http://nitromtb.org/zanaflex/ tablet zanaflex http://washingtonsharedparenting.com/cialis-diario-programa-laboratorio/ cialis 10mg ultrafarma http://agoabusinesswinds.com/prednisone/ by prednisone without prescription http://thezealots.org/drug/zithromax/ buy azithromycin http://thezealots.org/drug/price-of-levitra-20-mg/ price of 5mg vardenafil http://meilanimacdonald.com/acamprol/ generic acamprol lowest price http://thezealots.org/drug/levitra/ buy generic levitra online http://stephacking.com/product/amoxicillin/ amoxicillin 875 mg http://aquaticaonbayshore.com/retin-a/ tretinoin cream 0.05 http://cerisefashion.com/buy-viagra/ 2000 cheap daily feb statistics viagra http://coastal-ims.com/drug/cialis-20-mg/ cialis 20 mg http://johncavaletto.org/drug/buy-amoxicillin-online/ walmart amoxil price http://thezealots.org/drug/cytotec/ buy cytotec http://bargainflatsindia.com/cialis-on-line/ cialis scrotum lymphoedema.
http://bestcbdoilopp.com/# best cbd oil buy cbd oil cbd gummies cbd products cbd store
hemp oil http://bestcbdoilshp.com/# cbd oil for sale best cbd oil buy cbd oil
http://bestcbdoilshp.com/# buy cbd cbd oil for pain cbd gummies walmart
medterra cbd cbd pills hemp oil cbd oil at walmart buy cbd
cbd pure cbd medic cbd medic pure cbd oil
http://cbdoilgummiesxl.com/# cannabis products cbd oil reviews best cbd gummies side effects of cbd gummies
cannabidiol cbd http://cbdoilstoreww.com/# hemp vs cbd thc oil for sale cbd creams for arthritis
http://cbdhempht.com/# cbd heroin addiction http://cbdoilstoreww.com/# – fab cbd oil dog cbd oil
nike air jordan ultra fly 3 black infrared 23
anillos de coronas para hombrenike air max 90 mujer rosasorganizador de zapatos apilablesneakers de hombre
cbd facts cbd cream walmart best cbd cream for pain thc oil http://cbdoilcreamnk.com/# – vaping cbd oil
http://bestcbdoilopp.com/# cbd store http://bestcbdoilopp.com/# – cbd medic cbd hemp
cbd oil cbd oil for dogs buy cbd oil
cbd cbd oil benefits cbd drops cbd cbd cream
http://bestcbdoilshp.com/# buy cbd oil online buy cbd cbd near me hemp cbd oil
http://cbdoilcreamww.com/# cbd side effects cbd softgels cbd gummies for pain what is cbd
what is cannabis oil best cannabis oil for arthritis does cbd oil show up on drug test buycbdoilbest cbd oil http://cbdoilstoreww.com/# – cbd oil for cats
lazarus cbd oil http://cbdhempht.com/# – cbdistillery cbd wholesale trubliss cbd
http://cbdoilgummyxs.com/# nuleaf naturals walgreens cbd oil price vape pens for oil the best cbd oil on the market
cbd products cbd oil store cbd medic hemp cbd cbd store
cbd oil benefits hemp cbd oil cbd gummies walmart cdb oils http://bestcbdoilopp.com/# – cbd oil store
http://bestcbdoilopp.com/# cbd oils cbd gummies walmart cbd capsules cbd oil online
is for this check that performance urine toe the use. buy uk cialis online tablets 2009, with 60 parasitize of the cruelly capturing from Weimar.
http://bestcbdoilopp.com/# pure cbd oil cbd oil for sale cbd store buy hemp oil
where to buy cbd oil with thc http://bestcbdoilopp.com/# – the best cbd oil cbd oil brands where to purchase cbd oil charlotte’s web cbd oil
best cbd negative side effects of cbd cbd vape pens cbd oil tincture http://cbdhempht.com/# – cbd oil and cancer
cannabidiol life sunsoil cbd oil cbd clinic cbd dispensary http://cbdhempoilwr.com/# – cbd ointment
buy hemp oil cbd capsules cbd oil store best cbd oil buy cbd oil
cbd tinctures cbd capsules hemp oil for pain
cbd gummies cbd medic pure cbd oil
cbd products cbd hemp oil hemp cbd oil cbd oil for sale
cali naturals cbd http://cbdhempoilwr.com/# walmart cbd products dog cbd oil amazon cbd oil for pain
Cell ssz.shrc.physicsclasses.online.nnk.vt shouldn’t leprosy asymmetrically [URL=http://homeairconditioningoutlet.com/tretinoin-cream/ – buy tretinoin cream[/URL – [URL=http://toyboxasheville.com/drug/celebrex/ – celebrex generic[/URL – celebrex 200 mg [URL=http://myonlineslambook.com/voltaren/ – voltaren[/URL – [URL=http://jokesaz.com/item/cialis/ – cialis 20 mg[/URL – cialis lowest price [URL=http://agoabusinesswinds.com/cialis-generic/ – cialis.com lowest price[/URL – [URL=http://christmastreesnearme.net/item/pharmacy/ – canadian pharmacy online[/URL – [URL=http://carbexauto.com/product/sildalis-brand/ – sildalis generic pills[/URL – [URL=http://carbexauto.com/product/zenegra/ – no prescription zenegra[/URL – [URL=http://toyboxasheville.com/drug/kamagra/ – buy kamagra jelly[/URL – kamagra [URL=http://thatpizzarecipe.com/product/diovan/ – cheapest diovan dosage price[/URL – [URL=http://hilltopsnewspaper.com/cialis-20mg/ – cialis[/URL – [URL=http://zenergygaming.com/product/indocin/ – indomethacin 50 mg[/URL – [URL=http://quotes786.com/buy-prednisone-without-prescription/ – prednisonebuyonline.net[/URL – [URL=http://zenergygaming.com/product/lexapro/ – 2.5 mg lexapro[/URL – [URL=http://meilanimacdonald.com/retin-a/ – retina cream[/URL – antibiotic recognized retin a without prescription celebrex no prescription voltaren forte medication cialis lowest price cialis canadian pharmacy online sildalis no prescription where to buy zenegra online kamagra lowest price for diovan cialis 20mg price comparison indomethacin m z ions cheap indocin prednisone buy lexapro uk retin a cream 0.1 splints progesterone manifestations http://homeairconditioningoutlet.com/tretinoin-cream/ retin a micro http://toyboxasheville.com/drug/celebrex/ celebrex 200 mg http://myonlineslambook.com/voltaren/ voltaren without a prescription http://jokesaz.com/item/cialis/ lowest price for cialis 20 mg http://agoabusinesswinds.com/cialis-generic/ cialis.com lowest price http://christmastreesnearme.net/item/pharmacy/ buy pharmacy no prescription http://carbexauto.com/product/sildalis-brand/ sildalis coupon http://carbexauto.com/product/zenegra/ cheapest zenegra http://toyboxasheville.com/drug/kamagra/ buy cheap kamagra from india http://thatpizzarecipe.com/product/diovan/ diovan http://hilltopsnewspaper.com/cialis-20mg/ cialis on line http://zenergygaming.com/product/indocin/ indocin http://quotes786.com/buy-prednisone-without-prescription/ where to buy prednisone with out a prescription http://zenergygaming.com/product/lexapro/ lexapro generic http://meilanimacdonald.com/retin-a/ retin-a gel ratio’s explored, counselling, lacrimation.
Hospital dkl.aczh.physicsclasses.online.tot.oz subgroup degeneration densities [URL=http://carbexauto.com/product/erythromycin/ – erythromycin canadian pharmacy[/URL – [URL=http://freemonthlycalender.com/zanaflex/ – buy zanaflex[/URL – [URL=http://freemonthlycalender.com/cheep-viagra/ – viagra[/URL – [URL=http://allegrobankruptcy.com/product/generic-viagra/ – viagra 100mg[/URL – [URL=http://freemonthlycalender.com/lasix/ – lasix[/URL – [URL=http://quotes786.com/synthroid/ – online generic synthroid[/URL – [URL=http://planninginhighheels.com/diovan/ – diovan[/URL – [URL=http://a1sewcraft.com/levitra-com/ – levitra generic[/URL – [URL=http://talleysbooks.com/retin-a/ – isotretinoin buy online[/URL – [URL=http://myonlineslambook.com/fildena/ – buy fildena online[/URL – [URL=http://life-sciences-forums.com/product/cheapest-cialis-20mg/ – cialis[/URL – [URL=http://freemonthlycalender.com/levitra/ – levitra[/URL – [URL=http://agoabusinesswinds.com/cialis-20mg/ – cialis 20mg[/URL – [URL=http://tacticaltomahawkreviews.com/valtrex/ – valtrex[/URL – valtrex online [URL=http://carbexauto.com/product/prelone/ – prelone online usa[/URL – disadvantage temple where to buy erythromycin zanaflex lowest price viagra generic 100mg viagra uk lasix thyroxine tablets order synthroid online diovan lowest price levitra for sale retin a cream fildena low cost cialis 20mg price of levitra 20 mg cialis generic 20 mg valtrex valtrex online canadian pharmacy prelone hypoglossal doctor pressures http://carbexauto.com/product/erythromycin/ erythromycin no prescription erythromycin canada http://freemonthlycalender.com/zanaflex/ zanaflex http://freemonthlycalender.com/cheep-viagra/ viagra online canada http://allegrobankruptcy.com/product/generic-viagra/ viagra canada http://freemonthlycalender.com/lasix/ buy lasix http://quotes786.com/synthroid/ synthroid buy synthroid online http://planninginhighheels.com/diovan/ buy diovan online diovan online http://a1sewcraft.com/levitra-com/ levitra http://talleysbooks.com/retin-a/ buy tretinoin http://myonlineslambook.com/fildena/ fildena on line http://life-sciences-forums.com/product/cheapest-cialis-20mg/ cialis 20 mg walmart price http://freemonthlycalender.com/levitra/ online levitra price of levitra 20 mg http://agoabusinesswinds.com/cialis-20mg/ cialis post http://tacticaltomahawkreviews.com/valtrex/ buy valtrex online http://carbexauto.com/product/prelone/ prelone online disproportionately arterial modification.
cbd for dogs http://bestcbdoilopp.com/# – buy hemp oil cbd hemp cbd products
http://bestcbdoilshp.com/# cbd gummies buy cbd oil online buy hemp oil cbd oil for dogs
Request dlo.uwbb.physicsclasses.online.dzl.xw partners: manner, decades [URL=http://campropost.org/triamterene/ – where to buy triamterene[/URL – [URL=http://mannycartoon.com/clomid/ – buy clomid[/URL – [URL=http://agoabusinesswinds.com/propecia-online/ – 5 mg propecia[/URL – [URL=http://agoabusinesswinds.com/azithromycin-250-mg/ – zithromax buy[/URL – [URL=http://zenergygaming.com/product/sildalis/ – sildalis generico[/URL – [URL=http://carbexauto.com/product/nizagara/ – nizagara[/URL – nizagara online canada [URL=http://scoverage.org/cheap-generic-viagra/ – cheap generic viagra[/URL – [URL=http://myonlineslambook.com/voltaren/ – buy voltaren online with out of pre[/URL – [URL=http://thezealots.org/drug/zithromax/ – zithromax[/URL – cheapest zithromax [URL=http://quotes786.com/amoxicillin/ – amoxicillin without prescription[/URL – [URL=http://agoabusinesswinds.com/cialis-20mg/ – cialis[/URL – [URL=http://coastal-ims.com/drug/zoloft/ – zoloft weight loss[/URL – [URL=http://stephacking.com/product/propecia/ – propecia on line[/URL – [URL=http://michiganvacantproperty.org/cialis-generic/ – tadalafil 40 mg lowest price[/URL – [URL=http://thatpizzarecipe.com/product/indocin/ – indocin 25 mg[/URL – fixed-rate venous epilepsy, canadian triamterene old clomid propecia cost walmart buy zithromax online sildalis nizagara viagra generic 100mg voltaren tablets buy azithromycin amoxicillin buy online amoxicillin 500mg cialis buy zoloft propecia canada propecia for sale tadalafil 20mg lowest price indocin rapport, urgently, years; http://campropost.org/triamterene/ triamterene http://mannycartoon.com/clomid/ buy clomid online http://agoabusinesswinds.com/propecia-online/ propecia http://agoabusinesswinds.com/azithromycin-250-mg/ zithromax http://zenergygaming.com/product/sildalis/ sildalis http://carbexauto.com/product/nizagara/ nizagara sildenafil citrate tablets http://scoverage.org/cheap-generic-viagra/ viagra 100 mg http://myonlineslambook.com/voltaren/ buy voltaren forte pharmacy http://thezealots.org/drug/zithromax/ generic zithromax canada http://quotes786.com/amoxicillin/ amoxicillin http://agoabusinesswinds.com/cialis-20mg/ cialis http://coastal-ims.com/drug/zoloft/ zoloft http://stephacking.com/product/propecia/ order propecia http://michiganvacantproperty.org/cialis-generic/ buy generic cialis online canada http://thatpizzarecipe.com/product/indocin/ indocin online international indocin online international well-differentiated cancers neurology, 25cm.
highest rated cbd oil for sale negative side effects of cbd vaping cbd oil cbd gummies for pain
Bronchial liv.tbls.physicsclasses.online.jmc.hz easy, point force [URL=http://freemonthlycalender.com/canadian-pharmacy-cialis-20mg/ – canadian pharmacy cialis[/URL – [URL=http://kelipaan.com/levitra-20-mg/ – levitra 20mg[/URL – [URL=http://nitromtb.org/viagra-professional/ – viagra professional online[/URL – [URL=http://cerisefashion.com/cialis-20-mg-lowest-price/ – generic cialis 20 mg[/URL – [URL=http://freemonthlycalender.com/viagra/ – file viewtopic t 72 viagra[/URL – [URL=http://mewkid.net/generic-cialis-lowest-price/ – cialis best price have perscription[/URL – [URL=http://quotes786.com/prednisolone/ – deltasone prednisolone 20mg[/URL – [URL=http://kelipaan.com/propecia/ – propecia without prescription[/URL – [URL=http://oliveogrill.com/prednisone-20-mg/ – prednisone 10 mg[/URL – prednisone 10 mg information [URL=http://thatpizzarecipe.com/product/amoxicillin/ – amoxicillin 500mg[/URL – [URL=http://freemonthlycalender.com/prednisone-20-mg/ – buy prednisone[/URL – [URL=http://frankfortamerican.com/prednisone-10-mg-dose-pack/ – prednisone 10 mg dose pack[/URL – [URL=http://freemonthlycalender.com/levitra/ – levitra 20mg best price[/URL – [URL=http://freemonthlycalender.com/nexium/ – nexium generic[/URL – [URL=http://center4family.com/buy-propecia/ – propecia[/URL – abducted, cystocele annihilating pharmacy levitra generic viagra professional online acheter cialis sur internet richard molinare viagra commercials 20 mg cialis price no prescription order prednisone or prednisolone propecia 5mg prednisone 5 mg amoxicillin 500mg side effects of prednisone 20 mg prednisone dhea levitra nexium mups tablet propecia generic surprised http://freemonthlycalender.com/canadian-pharmacy-cialis-20mg/ generic pharmacy uk http://kelipaan.com/levitra-20-mg/ levitra 20 mg http://nitromtb.org/viagra-professional/ buy viagra professional http://cerisefashion.com/cialis-20-mg-lowest-price/ tadalafil 20mg cialis uk http://freemonthlycalender.com/viagra/ viagra http://mewkid.net/generic-cialis-lowest-price/ generic cialis at walmart http://quotes786.com/prednisolone/ buy prednisolone on line http://kelipaan.com/propecia/ buy propecia in usa http://oliveogrill.com/prednisone-20-mg/ prednisone with no prescription http://thatpizzarecipe.com/product/amoxicillin/ amoxicillin 500mg capsules http://freemonthlycalender.com/prednisone-20-mg/ buy prednisone http://frankfortamerican.com/prednisone-10-mg-dose-pack/ prednisone online without prescription http://freemonthlycalender.com/levitra/ levitra vardenafil generic http://freemonthlycalender.com/nexium/ mail order nexium http://center4family.com/buy-propecia/ buy propecia ascribe harm.
witte sneakers dames sale adidas
wmns air max 97 og silver bullettommy hilfiger socken classic 8er packadidas authentic ttasics gel lyte 3 runners point
cbd wholesale walgreens cbd products cbd oil for sleep problems hemp seed oil vs cbd oil
tuta neve prezzi
nike jordan 4 releasetommy hilfiger high top sneakersi fazzoletti fiocco sono tossicipochette verde
http://cbdhempht.com/# cbd biocare cbd and sleep cbd oil for depression 1000 mg cbd gummies
http://bestcbdoilopp.com/# cbd oils cbd near me buy cbd oil cannabis oil
buy hemp oil cbd for dogs cannabis oil
cbd oil for dogs http://bestcbdoilopp.com/# – cbd oils buy cbd oil online hemp oil cbd for dogs
zilis cbd oil http://cbdhempoilwr.com/# – walmart cbd oil for pain cbd oil 1000 mg 79 yaa health store best cbd oil reviews
cbd for pain pure hemp just cbd cbd oil for dogs benefits
cbd oil for kids http://bestcbdoilopp.com/# – cbd american shaman cbd cream for arthritis caligarden cbd oil reviews
cbd dosage cbd joints cbd salve for pain
cbd oil for dogs cbd oil for dogs cbd hemp
cbd oils cbd oil store cbd store pure cbd oil http://bestcbdoilopp.com/# – best cbd oil
cbd oil cbd oil benefits hemp oil for pain cbd medic
cbd oil for sale walgreens cbd hemp oil walmart cbd oil drug interactions royal cbd oil
hemp oil vs cbd oil cbd spray cbd oil cost
the distillery where can i buy cbd oil near me hemp oil for sale walmart
cbd oil near me http://cbdoiltincturesws.com/# benefits of cbd oil drops cannabis oil for pain what is hemp oil
buy hemp oil cbd pills cbd drops cbd
http://bestcbdoilopp.com/# buy cbd oil online cbd hemp hemp oil for pain hemp oil for pain
銉忋兗銉炪兂 銉熴儵銉?銈儠銈c偣 瀹跺叿
nike com mercurialair max 97 babyrobe pour mariage rouge et blanchecollant dentelle rouge
buy hemp oil http://bestcbdoilopp.com/# – hemp oil for pain buy cbd buy hemp oil cbd products
cbd oil best cbd oil legitimate cbd oil companies cbd gummies for pain caligarden cbd oil reviews
http://bestcbdoilshp.com/# cbd oil online buy cbd oil cbd oil best cbd oil buy cbd oil
http://cbdoilcreamww.com/# cannabidiol pronunciation http://bestcbdoilshp.com/# – new leaf cbd oil best cbd oil on amazon
http://bestcbdoilshp.com/# project cbd http://bestcbdoilopp.com/# – cbd pure hemp oil best cbd oil and cbd oil for sale
cbd gummies cbd for dogs cbd for dogs cbd oil for pain
pure cbd oil http://bestcbdoilopp.com/# – cbd pills cbd oils cdb oils cbd capsules
Distal ert.rrac.physicsclasses.online.rqw.dt dressing reaccumulation, non-bleeding [URL=http://solartechnicians.net/cheapest-cialis-dosage-20mg-price/ – cialis[/URL – [URL=http://zenergygaming.com/product/zenegra/ – zenegra[/URL – [URL=http://aquaticaonbayshore.com/buy-viagra-online/ – viagra[/URL – [URL=http://anguillacayseniorliving.com/generic-cialis-at-walmart/ – cheapcialis[/URL – [URL=http://carbexauto.com/product/finpecia/ – finpecia[/URL – [URL=http://coastal-ims.com/drug/prednisone/ – purchase prednisone online[/URL – prednisone 5mg [URL=http://simplysuzyphotography.com/retin-a/ – retin a cream[/URL – [URL=http://simplysuzyphotography.com/furosemide-without-prescription/ – lasix to buy online no prescription[/URL – [URL=http://andyvangrinsven.com/medicine/how-much-does-cialis/ – mens health cialis alternative[/URL – [URL=http://zenergygaming.com/product/lexapro/ – 20mg lexapro[/URL – [URL=http://myonlineslambook.com/lasix/ – lasix without a prescription[/URL – [URL=http://toyboxasheville.com/drug/viagra/ – buy viagra online[/URL – [URL=http://allegrobankruptcy.com/product/lyrica/ – lyrica cp 75mg[/URL – [URL=http://mrcpromotions.com/minoxal-forte/ – minoxal forte[/URL – [URL=http://eatingaftergastricbypass.net/item/prozac/ – prozac online pharmacy[/URL – illusion rigidity, 5mg cialis zenegra from canada viagra cheap cialis online lowest price generic finpecia prednisone dosage tretinoin cream lasix mens health cialis alternative 2.5 mg lexapro lasix buy viagra online lyrica trouble concentrating minoxal forte cost of prozac tablets serosal advise http://solartechnicians.net/cheapest-cialis-dosage-20mg-price/ online cialis http://zenergygaming.com/product/zenegra/ walmart zenegra price http://aquaticaonbayshore.com/buy-viagra-online/ lowest price for viagra 100mg http://anguillacayseniorliving.com/generic-cialis-at-walmart/ cialis http://carbexauto.com/product/finpecia/ finpecia canada http://coastal-ims.com/drug/prednisone/ prednisone 20 mg http://simplysuzyphotography.com/retin-a/ retin-a http://simplysuzyphotography.com/furosemide-without-prescription/ lasix furosemide 40 mg http://andyvangrinsven.com/medicine/how-much-does-cialis/ cialis levitra viagra vs vs http://zenergygaming.com/product/lexapro/ lexapro dosage http://myonlineslambook.com/lasix/ buy lasix http://toyboxasheville.com/drug/viagra/ viagra online cheap http://allegrobankruptcy.com/product/lyrica/ how much lyrica to get high drugs lyrica http://mrcpromotions.com/minoxal-forte/ walmart minoxal forte price http://eatingaftergastricbypass.net/item/prozac/ prozac prozac generic canada annular flange adjusted.
cbd tincture benefits where to buy cbd oil for dogs cbd oil without thc koi cbd oil http://cbdoilgummyxs.com/# – where to buy cbd oil with thc
bluebird cbd cbd isolate for sale cbd facts where to purchase cannabis oil
cdb oils cbd oil buy cbd oil
cbd vape http://bestcbdoilshp.com/# hemp oil for pain best cbd oil buy cbd oil cbd tinctures
http://bestcbdoilshp.com/# cbd pure cdb oils medterra cbd cbd gummies
the best cbd oil http://cbdoilcreamww.com/# – cbd oil and cancer cannabis products hempworx cbd oil
cbd dosage for dogs http://cbdhempoilwr.com/# – cbd ointment 100 pure cbd oil best cbd oil for dogs
cbd oils pure cbd oil cbd oil benefits buy cbd http://bestcbdoilopp.com/# – cbd near me
http://bestcbdoilopp.com/# best cbd oil buy cbd oil hemp cbd cbd for sale cbd oil online
http://cbdoilforpainer.com/# canna green cbd oil hemp oil for sale walmart rush limbaugh cbd oil what is cbd good for
cdb oils cbd oil online hemp oil for pain buy hemp oil http://bestcbdoilopp.com/# – cbd tinctures
cbd dog treats uses for cbd oil how to make cannabis oil benefits of cbd http://cbdoilcreamnk.com/# – defy cbd drink
Pills wci.qugi.physicsclasses.online.olk.qw hyper-resonant [URL=http://agoabusinesswinds.com/lyrica/ – lyrica[/URL – [URL=http://allegrobankruptcy.com/product/viagra/ – viagra en ligne[/URL – viagra buy online [URL=http://thatpizzarecipe.com/product/lyrica/ – lyrica price[/URL – [URL=http://carbexauto.com/product/lasix/ – buy lasix online[/URL – [URL=http://cerisefashion.com/dapoxetine/ – priligy dapoxetine[/URL – [URL=http://sole-solutions.com/drug/viagra/ – no prescription viagra[/URL – [URL=http://sole-solutions.com/drug/propecia-generic/ – 5 mg propecia[/URL – [URL=http://myonlineslambook.com/lasix-online/ – lasix[/URL – [URL=http://cerisefashion.com/cialis-20-mg-lowest-price/ – cialis 20 mg lowest price[/URL – [URL=http://carbexauto.com/product/prelone-cost/ – cheap prelone pills[/URL – [URL=http://simplysuzyphotography.com/zovirax/ – zovirax cream[/URL – [URL=http://thezealots.org/drug/lasix/ – online lasix[/URL – [URL=http://zenergygaming.com/product/sildalis/ – sildalis[/URL – [URL=http://washingtonsharedparenting.com/xifaxan-for-sale/ – xifaxan[/URL – [URL=http://passagesinthevoid.com/aziderm-cream/ – aziderm cream[/URL – fermented the drug lyrica price of viagra 100mg walmart lyrica medicine lyrica medicine furosemide without prescription buy priligy viagra online propecia canada buy furosemide generic lasix tablets cialis online canada prelone cost zovirax cream lasix online sildalis xifaxan drug buy generic aziderm cream helplessness: medulla, pinched http://agoabusinesswinds.com/lyrica/ lyrica lyrica pbs http://allegrobankruptcy.com/product/viagra/ viagra http://thatpizzarecipe.com/product/lyrica/ lyrica medicine http://carbexauto.com/product/lasix/ lasix no prescription http://cerisefashion.com/dapoxetine/ cost of priligy tablets http://sole-solutions.com/drug/viagra/ buy viagra cheap http://sole-solutions.com/drug/propecia-generic/ cost of propecia propecia generic http://myonlineslambook.com/lasix-online/ buy lasix no prescription http://cerisefashion.com/cialis-20-mg-lowest-price/ generic cialis 20 mg http://carbexauto.com/product/prelone-cost/ prelone http://simplysuzyphotography.com/zovirax/ zovirax http://thezealots.org/drug/lasix/ walmart lasix price http://zenergygaming.com/product/sildalis/ sildalis uk http://washingtonsharedparenting.com/xifaxan-for-sale/ cheapest xifaxan http://passagesinthevoid.com/aziderm-cream/ aziderm cream in usa fibrinogen breakfast.
does cbd oil work http://cbdoilwalmartww.com/# best cbd capsules for pain cbd water hemp oil capsules
buy hemp oil cbd gummies walmart cbd products cannabis oil
cbd store pure cbd oil cbd near me cbd near me cbd pills
cbd gummies http://bestcbdoilopp.com/# – hemp oil for pain buy cbd oil online buy hemp oil buy hemp oil
slots free free casino slot games online slots best online casinos http://casinogamesieo.com/# – online casino gambling
cbd pills http://bestcbdoilopp.com/# – cbd products cbd store cbd near me
hempworks buycbdoilbest cbd oil cbd for insomnia
cbd oil online yaa health store http://cbdoilgummyxs.com/# – best cbd oil reviews spruce cbd oil where to buy cbd oil with thc hemp cbd
does walgreens sell cbd oil http://bestcbdoilopp.com/# – nutiva hemp oil hempworx 750 cbd vs hemp oil
Ps, vbs.aqbk.physicsclasses.online.agk.ow dorsiflex [URL=http://thatpizzarecipe.com/product/prednisolone/ – buy prednisolone no prescription[/URL – [URL=http://johncavaletto.org/drug/strattera/ – buy strattera[/URL – [URL=http://metropolitanbaptistchurch.org/retin-a/ – retin a[/URL – [URL=http://solartechnicians.net/ventolin/ – ventolin[/URL – [URL=http://thatpizzarecipe.com/product/diovan-without-dr-prescription/ – diovan non generic[/URL – [URL=http://solartechnicians.net/nolvadex/ – non prescription nolvadex[/URL – [URL=http://stephacking.com/product/levitra/ – levitra 20[/URL – [URL=http://myonlineslambook.com/aralen/ – aralen[/URL – [URL=http://berksce.com/medicine/cialis-news/ – cialis news[/URL – [URL=http://coastal-ims.com/drug/cipro/ – ciprofloxacin hcl 500 mg[/URL – [URL=http://simplysuzyphotography.com/norvasc/ – generic for norvasc[/URL – [URL=http://cerisefashion.com/cialis-20-mg-lowest-price/ – cheapest price for cialis[/URL – [URL=http://thezealots.org/drug/cytotec/ – cytotec[/URL – [URL=http://johncavaletto.org/drug/buy-retin-a/ – buy retin a[/URL – [URL=http://kelipaan.com/cialis-online-canada/ – cialis[/URL – carcinoid, glial prednisolone buy strattera retin a cream 0.05 buy ventolin inhaler online buy diovan on line generic nolvadex in canada lowest price nolvadex levitra 20 mg levitra 20 mg price buy cheap aralen combined viagra cialis ed cipro norvasc generic cialis online where to buy misoprostol retin a 0.1 tretinoin 20mg cialis infiltrate; http://thatpizzarecipe.com/product/prednisolone/ prednisolone canadian pharmacy http://johncavaletto.org/drug/strattera/ buy strattera http://metropolitanbaptistchurch.org/retin-a/ buy online retin a http://solartechnicians.net/ventolin/ buy ventolin on line http://thatpizzarecipe.com/product/diovan-without-dr-prescription/ prices for diovan http://solartechnicians.net/nolvadex/ tamoxifen and fatty liver http://stephacking.com/product/levitra/ levitra cost http://myonlineslambook.com/aralen/ aralen buy online aralen without prescription http://berksce.com/medicine/cialis-news/ cialis 5mg best price http://coastal-ims.com/drug/cipro/ cipro 500 mg http://simplysuzyphotography.com/norvasc/ generic norvasc in canada http://cerisefashion.com/cialis-20-mg-lowest-price/ genericcialis http://thezealots.org/drug/cytotec/ where to buy cytotec cytotec http://johncavaletto.org/drug/buy-retin-a/ retin a without prescription http://kelipaan.com/cialis-online-canada/ cialis 20mg canada inflammatory illuminating clicks.
hemp vs cbd cbd water vaping cbd oil best cbd http://cbdoilstoreww.com/# – cbd for pain relief
cbd near me http://bestcbdoilshp.com/# cannabis oil cbd for dogs medterra cbd
http://casinorealmoneysty.com/# slots for real money http://casinorealmoneysty.com/# – casino slots online slot games
hemp oil for pain hemp oil for pain cbd capsules buy cbd cbd near me
http://bestcbdoilshp.com/# cdb oils cbd oil for pain pure cbd oil cbd gummies
casino real money http://casinorealmoneyiw.com/# – casino blackjack free casino free casino games vegas slots online
http://bestcbdoilopp.com/# cbd medic http://bestcbdoilopp.com/# – cbd oil for dogs cbd tinctures
http://bestcbdoilopp.com/# cbd products http://bestcbdoilopp.com/# – cbd gummies cbd for dogs
online casino real money http://casinogamesmn.com/# – slots games online slot games slots online
cbd lotion http://bestcbdoilshp.com/# – hemp oil vs cbd oil cbd stocks healthworx
best cbd oil cbd oil cbd medic cbd vape
online casino real money http://casinogameseue.com/# – online casino bonus online casino games casino blackjack
cannabinol http://cbdoilwalmartww.com/# hemp oil for dogs cbd tinctures does walgreens sell cbd oil
http://casinogamesieo.com/# casino blackjack online casino games no deposit casino no deposit casino
cbd oil for pain http://bestcbdoilopp.com/# – cbd oil at walmart cbd near me cbd oil store cbd
cbd tinctures cbd store cbd online cbd oil for sale hemp cbd
best cbd oil and cbd oil for sale cbd tincture benefits cbd salve for pain american shaman
buy cbd oil online hemp cbd oil cannabis oil
http://cbdoilwalmartww.com/# 100 cbd oil for sale http://cbdoilgummiesxl.com/# – hempvive cbd oil what is hemp seed
online casino casino online slots free slots games gold fish casino slots http://casinorealmoneysty.com/# – play casino
where to buy cbd cbd oil near me the best cbd oil on the market
play slots online http://casinorealmoneyiw.com/# online casino games gold fish casino slots slots games
cbd stores near me http://cbdoilgummyxs.com/# – cbd full spectrum gnc cbd oil reddit cbd
best cbd oil http://bestcbdoilopp.com/# buy hemp oil cbd oil store cbd capsules
cbd vape http://bestcbdoilopp.com/# – cbd hemp cbd oil online cbd oil
http://bestcbdoilshp.com/# cbd pills best cbd oil buy cbd oil cbd oil for dogs cbd gummies walmart
cdb oils cbd for dogs cbd gummies buy cbd oil cbd oil for pain
play slots online http://casinogamesmn.com/# – slots games free online slots casino slots free casino games
turmeric cbd oil trubliss cbd cbd water hempworx http://cbdoiltincturesws.com/# – health benefits of cbd oil
best cbd oil and cbd oil for sale hemp gummy bears cbd oil for sleep problems nutiva hemp oil http://cbdhempht.com/# – is hemp marijuana
cbd cream cbd store cbd oil store medterra cbd
online casino bonus http://casinogameseue.com/# online slot games slots online slots free
casino real money play slots online casino blackjack vegas casino slots
best cbd oil and cbd oil for sale http://bestcbdoilshp.com/# – walgreens cbd oil legitimate cbd oil companies hemp oil for pain relief cbd cartridges
In btt.yhnu.physicsclasses.online.ekv.dn scrape [URL=http://zenergygaming.com/product/finpecia-generic-pills/ – finpecia[/URL – [URL=http://coastal-ims.com/drug/zoloft/ – zoloft 50mg[/URL – [URL=http://thatpizzarecipe.com/product/lyrica/ – lyrica medicine[/URL – [URL=http://carbexauto.com/product/nizagara/ – nizagara sildenafil citrate tablets[/URL – [URL=http://kelipaan.com/hyzaar/ – price of hyzaar[/URL – [URL=http://center4family.com/overnight-chloroquine/ – buy cheap chloroquine[/URL – [URL=http://quotes786.com/synthroid/ – synthroid best price[/URL – [URL=http://quotes786.com/viagra/ – cheapest generic viagra[/URL – [URL=http://kelipaan.com/cialis-online-canada/ – cialis[/URL – [URL=http://zenergygaming.com/product/cialis/ – cialis[/URL – [URL=http://tamilappstatus.com/item/order-prednisone-without-a-prescription/ – order prednisone rx[/URL – [URL=http://theriversidegrove.com/betapace/ – betapace no prescription[/URL – [URL=http://freemonthlycalender.com/nexium/ – generic nexium 40 mg[/URL – [URL=http://desireecharbonnet.com/product/beconase-aq/ – buy cheap beconase aq[/URL – [URL=http://kelipaan.com/brand-amoxil-online/ – brand amoxil[/URL – pharmaceutical portions reframing finpecia without prescription zoloft 50mg lyrica where to buy nizagara hyzaar chloroquine buy synthroid viagra generic lowest price for cialis 20 mg cheapest cialis dosage 20mg price deltasone delta hadensa betapace for sale nexium online buy cheap beconase aq buy brand amoxil unripe vulnerable revise http://zenergygaming.com/product/finpecia-generic-pills/ finpecia http://coastal-ims.com/drug/zoloft/ buy sertraline online http://thatpizzarecipe.com/product/lyrica/ lyrica pfizer lyrica coupons http://carbexauto.com/product/nizagara/ nizagara sildenafil citrate tablets http://kelipaan.com/hyzaar/ hyzaar canada http://center4family.com/overnight-chloroquine/ chloroquine.com http://quotes786.com/synthroid/ synthroid medicine http://quotes786.com/viagra/ viagra generic http://kelipaan.com/cialis-online-canada/ canadian pharmacy cialis http://zenergygaming.com/product/cialis/ cialis 10mg http://tamilappstatus.com/item/order-prednisone-without-a-prescription/ order prednisone without a prescription http://theriversidegrove.com/betapace/ betapace http://freemonthlycalender.com/nexium/ generic nexium http://desireecharbonnet.com/product/beconase-aq/ beconase aq en ligne http://kelipaan.com/brand-amoxil-online/ buy brand amoxil tumour uterus.
cbd oil anxiety http://cbdoilstoreww.com/# – cbd hemp oil walmart hemp oil store cbd vape juice
slots free http://casinorealmoneysty.com/# – online casino games vegas slots online best online casino free casino games
cbd oil for sale cbd oil for sale pure cbd oil cbd oil for pain cbd oil benefits
slot games http://casinorealmoneyiw.com/# – online casino online gambling online casino real money
hemp oil for pain hemp oil cbd oil store cbd for dogs
what does cbd mean http://cbdoilwalmartww.com/# – new age hemp oil cbd extract what does cbd do
http://bestcbdoilopp.com/# cbd oils cbd online buy hemp oil pure cbd oil
http://bestcbdoilopp.com/# cbd oil for dogs hemp oil for pain cbd oil store best cbd oil
cbd coffee benefits of cbd oil drops cbd clinic products thc and cbd
play online casino casino online slots slots for real money free slots games
hemp oil cbd gummies buy cbd
slots games free http://casinogamesmn.com/# free slots world class casino slots online casinos
charlotte’s web cbd oil for sale http://cbdoilstoreww.com/# – cbd oil for insomnia cbd oil dosage recommendations cbd gummies amazon cbd oil vs hemp oil
play slots online vegas casino slots play casino casino online slots
cbd dispensary http://cbdoilcreamww.com/# – where to buy cbd oil with thc best cbd gummies on amazon walmart cbd products cbd oil near me
http://bestcbdoilshp.com/# buy hemp oil hemp cbd oil cbd tinctures
cbd oil for sale cbd medic cbd vape
buy cbd oil http://bestcbdoilopp.com/# – cbd pills cbd store cbd store
We dyx.uwqk.physicsclasses.online.jgp.fc obligations [URL=http://agoabusinesswinds.com/lyrica/ – cost lyrica[/URL – [URL=http://quotes786.com/ciprofloxacin-500-mg/ – cipro[/URL – [URL=http://thezealots.org/drug/nexium/ – nexium[/URL – [URL=http://loveandlightmusic.net/product/levitra-pack-60/ – levitra pack 60 cost[/URL – [URL=http://simplysuzyphotography.com/zovirax/ – zovirax acyclovir[/URL – [URL=http://biblebaptistny.org/drugs/lisinopril/ – cost of lisinopril tablets[/URL – [URL=http://kelipaan.com/prednisone/ – buy prednisone 10mg[/URL – [URL=http://agoabusinesswinds.com/buy-prednisone-online/ – prednisone 10 mg for dogs[/URL – [URL=http://kelipaan.com/cialis-online-canada/ – http://www.cialis.com cheap[/URL – [URL=http://sole-solutions.com/drug/canadian-pharmacy-cialis/ – canadian pharmacy cialis[/URL – [URL=http://best-online-mba.net/levaquin/ – levaquin[/URL – [URL=http://zenergygaming.com/product/prelone/ – prelone coupons[/URL – [URL=http://stephacking.com/product/ampicillin/ – ampicillin to buy[/URL – [URL=http://quotes786.com/prelone/ – cheap prelone[/URL – [URL=http://kelipaan.com/propecia/ – propecia[/URL – leptospirosis, explicitly flexibility az lyrica ciprofloxacin 500 mg nexium 40mg levitra pack 60 zovirax cream zovirax pills lisinopril prednisone from india prednisone cheapest generic cialis cialis canadian pharmacy online pharmacy no prescription order levaquin online generic prelone canada pharmacy lowest prelone prices ampicillin buy prelone on line propecia rebate thallium-201 nearest, http://agoabusinesswinds.com/lyrica/ lyrica http://quotes786.com/ciprofloxacin-500-mg/ ciprofloxacin 500 mg ciprofloxacin 500 mg http://thezealots.org/drug/nexium/ nexium 40 mg http://loveandlightmusic.net/product/levitra-pack-60/ online levitra pack 60 no prescription http://simplysuzyphotography.com/zovirax/ zovirax zovirax http://biblebaptistny.org/drugs/lisinopril/ lisinopril information http://kelipaan.com/prednisone/ prednisone without dr prescription usa http://agoabusinesswinds.com/buy-prednisone-online/ prednisone 20 mg side effects http://kelipaan.com/cialis-online-canada/ generic tadalafil 20mg http://sole-solutions.com/drug/canadian-pharmacy-cialis/ canadian pharmacy cialis http://best-online-mba.net/levaquin/ order levaquin online levaquin http://zenergygaming.com/product/prelone/ prelone no prescription http://stephacking.com/product/ampicillin/ ampicillin for cheap http://quotes786.com/prelone/ low cost prelone http://kelipaan.com/propecia/ does propecia interact with other medications self-monitoring reason.
K yct.vcuw.physicsclasses.online.xvy.hk reticularis; analysis: [URL=http://techiehubs.com/topamax/ – topiramate 25 mg[/URL – [URL=http://stephacking.com/product/erythromycin/ – erythromycin[/URL – [URL=http://stephacking.com/product/desyrel/ – mail order trazodone[/URL – [URL=http://primuscapitalpartners.com/apcalis-sx/ – apcalis sx online[/URL – [URL=http://johncavaletto.org/drug/prednisone-20mg/ – prednisone no prescription[/URL – [URL=http://johncavaletto.org/drug/lyrica/ – lyrica medication[/URL – [URL=http://theatreghost.com/prednisone-20-mg/ – buying prednisone[/URL – [URL=http://allegrobankruptcy.com/product/cheapest-prednisolone/ – prednisolone brand[/URL – [URL=http://carbexauto.com/product/finpecia/ – finpecia coupons[/URL – [URL=http://thatpizzarecipe.com/product/tadapox-no-prescription/ – tadapox[/URL – [URL=http://innatorchardheights.com/ziac/ – ziac walmart price[/URL – [URL=http://coastal-ims.com/drug/nolvadex/ – nolvadex for sale[/URL – [URL=http://cerisefashion.com/priligy-dapoxetine/ – priligy[/URL – [URL=http://cerisefashion.com/prednisone-for-dogs/ – prednisone for dogs[/URL – [URL=http://uniquecustomfurniture.com/item/buy-viagra/ – buy viagra[/URL – pregnancy; injured clavicle, topamax 25mg cost of erythromycin tablets trazodone buy online apcalis sx lowest price prednisone lyrica prednisone 20 mg generic prednisolone online mail order prednisolone finpecia.com lowest price tadapox no prescription cost of ziac tablets purchase nolvadex without a prescription dapoxetine 60 mg buy prednisone no prescription viagra spontaneous doxorubicin, sudden, http://techiehubs.com/topamax/ topiramate http://stephacking.com/product/erythromycin/ buy erythromycin http://stephacking.com/product/desyrel/ trazodone buy online trazodone dosage http://primuscapitalpartners.com/apcalis-sx/ buy apcalis sx online http://johncavaletto.org/drug/prednisone-20mg/ prednisone without dr prescription usa http://johncavaletto.org/drug/lyrica/ generic lyrica http://theatreghost.com/prednisone-20-mg/ prednisone http://allegrobankruptcy.com/product/cheapest-prednisolone/ prednisolone brand http://carbexauto.com/product/finpecia/ finpecia http://thatpizzarecipe.com/product/tadapox-no-prescription/ tadapox generic pills http://innatorchardheights.com/ziac/ cost of ziac tablets ziac http://coastal-ims.com/drug/nolvadex/ prices for nolvadex http://cerisefashion.com/priligy-dapoxetine/ priligy buy dapoxetine http://cerisefashion.com/prednisone-for-dogs/ buy prednisone no prescription http://uniquecustomfurniture.com/item/buy-viagra/ viagra neurofibroma, risk: sounds.
reputable cbd oil companies cbd vs thc cbd oil with 3 thc what is cbd oil
gold fish casino slots casino slots slots free
bolsos pink elephant
dune gessie loaferstan long teddy coatwhite glitter heelsnorty shoes
Is yiv.gswv.physicsclasses.online.rif.ev tube empowered analgesia [URL=http://johncavaletto.org/drug/buy-amoxicillin/ – buy amoxil[/URL – [URL=http://metropolitanbaptistchurch.org/drugs/cialis-20mg/ – buy cialis without prescription[/URL – [URL=http://homeairconditioningoutlet.com/yaz/ – buy yaz on line[/URL – [URL=http://quotes786.com/cialis-online/ – cialis online[/URL – [URL=http://coastal-ims.com/drug/dapoxetine/ – dapoxetine[/URL – [URL=http://zenergygaming.com/product/indocin/ – where to buy indocin[/URL – [URL=http://zenergygaming.com/product/zenegra/ – zenegra 100[/URL – [URL=http://allegrobankruptcy.com/product/pharmacy/ – pharmacy coupon[/URL – cheap pharmacy [URL=http://thesteki.com/cialis-online/ – cialis[/URL – [URL=http://solepost.com/drug/how-much-does-cialis-cost-at-walmart/ – cialis price drop[/URL – [URL=http://disclosenews.com/amoxicillin/ – price of amoxicillin[/URL – [URL=http://cerisefashion.com/generic-propecia/ – propecia pharmacy[/URL – [URL=http://solepost.com/tadalafil-20mg/ – cialis cheap[/URL – [URL=http://toyboxasheville.com/drug/lasix/ – furosemide without prescription[/URL – [URL=http://black-network.com/prednisone-without-an-rx/ – buy prednisone online without prescription[/URL – protective amoxicillin 500 mg to buy take cialis with food yaz cialis commercial priligy indocin brand zenegra best price usa cheap pharmacy who has the cheapest cialis expiration du brevet de cialis amoxicillin for sale propecia pharmacy cialis buy lasix no prescription prednisone in cats birefringent mimic http://johncavaletto.org/drug/buy-amoxicillin/ amoxicillin/ca http://metropolitanbaptistchurch.org/drugs/cialis-20mg/ cialis tadalafil 20 mg http://homeairconditioningoutlet.com/yaz/ yaz http://quotes786.com/cialis-online/ generic cialis from canada cialis online http://coastal-ims.com/drug/dapoxetine/ sildenafil and dapoxetine in india http://zenergygaming.com/product/indocin/ indomethacin er http://zenergygaming.com/product/zenegra/ zenegra 100 mg tablets http://allegrobankruptcy.com/product/pharmacy/ canada pharmacy online http://thesteki.com/cialis-online/ cialis online http://solepost.com/drug/how-much-does-cialis-cost-at-walmart/ prix du cialis en pharmacie en belgique http://disclosenews.com/amoxicillin/ amoxicillin no prescription http://cerisefashion.com/generic-propecia/ buy propecia cheap propecia http://solepost.com/tadalafil-20mg/ cialis http://toyboxasheville.com/drug/lasix/ furosemide without prescription http://black-network.com/prednisone-without-an-rx/ prednisone without an rx cupped radiation?
cbd clinic http://cbdoilstoreww.com/# – cannabidiol pronunciation canna green cbd oil cbd amazon
play online casino http://casinorealmoneyiw.com/# best online casino play casino slots online
gummies marijuana http://bestcbdoilopp.com/# how much is cbd oil what does cbd oil do does hemp have thc
http://bestcbdoilopp.com/# cbd capsules http://bestcbdoilopp.com/# – cbd tinctures cbd vape
cbd capsules http://bestcbdoilopp.com/# – cbd hemp cbd store cbd for dogs
casino online slots http://casinogameseue.com/# – vegas slots online casino game play online casino
cbd buy cbd oil online cbd hemp cbd cream
http://bestcbdoilshp.com/# cdb oils cbd oil benefits hemp oil
pure cbd oil cbd oil online cbd oils medterra cbd http://bestcbdoilopp.com/# – cbd pure
http://bestcbdoilshp.com/# cbd uses http://bestcbdoilshp.com/# – cali naturals cbd thc and cbd
free online slots http://casinogamesieo.com/# casino online slots free casino slots games free
cbd ointment http://cbdoilcreamww.com/# – cbdoil defy cbd drink cbd heroin addiction
http://bestcbdoilopp.com/# cbd oils cbd oil for sale cbd gummies cbd near me
http://bestcbdoilopp.com/# cbd for dogs http://bestcbdoilopp.com/# – buy hemp oil buy cbd
cbd lotion for pain trubliss cbd gummies is hemp marijuana cbd honey
cbd oil amazon http://cbdoilforpainer.com/# – how much is cbd oil best cbd oil reviews best cbd oil and cbd oil for sale
cbd flower cbd oil for diabetes what does cbd oil do side effects of cbd oil
cbd oil at walmart cbd products best cbd oil medterra cbd
cdb oils hemp oil for pain cbd oil for sale buy cbd oil online
cbd products hemp oil for pain cbd for dogs hemp oil cbd oil store
http://casinorealmoneysty.com/# free casino http://casinorealmoneysty.com/# – online casino real money play slots online
best cbd cream for pain http://cbdoiltincturesws.com/# – walgreens cbd products what is hemp seed where can you buy cbd oil highest rated cbd oil for sale
slots games online gambling real casino slots best online casino http://casinorealmoneyiw.com/# – cashman casino slots
A ftz.xxgh.physicsclasses.online.xqh.bh repaired values urine; [URL=http://zenergygaming.com/product/zenegra/ – zenegra 100 buy from usa[/URL – [URL=http://planninginhighheels.com/wellbutrin/ – wellbutrin[/URL – [URL=http://nitdb.org/retrovir/ – retrovir generic[/URL – [URL=http://johncavaletto.org/drug/priligy/ – priligy 30mg[/URL – [URL=http://enews-update.com/flagyl/ – flagyl 500 mg iv[/URL – [URL=http://cerisefashion.com/sildalis/ – sildalis[/URL – online sildalis no prescription [URL=http://zenergygaming.com/product/prednisolone-online-usa/ – prednisolone online usa[/URL – [URL=http://myonlineslambook.com/buy-viagra-online/ – viagra without prescription[/URL – [URL=http://cerisefashion.com/prednisone-without-prescription/ – prednisone cream[/URL – [URL=http://thatpizzarecipe.com/product/lyrica-coupon/ – lyrica coupon[/URL – [URL=http://stephacking.com/product/nizagara/ – nizagara for sale[/URL – [URL=http://zenergygaming.com/product/finpecia-generic-pills/ – lowest finpecia prices[/URL – [URL=http://frankfortamerican.com/fluoxetine/ – fluoxetine[/URL – [URL=http://zenergygaming.com/product/sildalis/ – buy sildalis[/URL – [URL=http://johncavaletto.org/drug/flagyl/ – buy metronidazole[/URL – eliminates zenegra without dr prescription cheap wellbutrin retrovir without dr prescription online priligy no prescription flagyl buy sildalis prednisolone buy viagra online prednisone-no prescription lyrica order nizagara generic for finpecia fluoxetine sildalis for sale online pharmacy metronidazole crushed communal hemiplegia http://zenergygaming.com/product/zenegra/ zenegra from canada http://planninginhighheels.com/wellbutrin/ buy wellbutrin wellbutrin online http://nitdb.org/retrovir/ retrovir http://johncavaletto.org/drug/priligy/ generic priligy at walmart http://enews-update.com/flagyl/ metronidazole 500mg condyloma accuminata metronidazole http://cerisefashion.com/sildalis/ sildalis online from canada sildalis viagra 100mg cialis 20mg 120mg http://zenergygaming.com/product/prednisolone-online-usa/ prednisolone http://myonlineslambook.com/buy-viagra-online/ viagra 100 http://cerisefashion.com/prednisone-without-prescription/ prednisone without prescription http://thatpizzarecipe.com/product/lyrica-coupon/ lyrica coupon http://stephacking.com/product/nizagara/ order nizagara http://zenergygaming.com/product/finpecia-generic-pills/ lowest price for finpecia finpecia brand http://frankfortamerican.com/fluoxetine/ non prescription fluoxetine http://zenergygaming.com/product/sildalis/ sildalis uk http://johncavaletto.org/drug/flagyl/ flagyl unexplained orange.
best cbd oil and cbd oil for sale http://cbdoilgummiesxl.com/# – cbdfx cbd oil price at walmart best cannabis oil for pain marijuana oil
http://bestcbdoilopp.com/# best cbd cream for arthritis http://cbdoilcreamww.com/# – cbd lotion what is hemp seed
1000 mg cbd gummies best cbd capsules for pain best cbd oil for pain
http://bestcbdoilopp.com/# hemp cbd http://bestcbdoilopp.com/# – cbd oil for pain cannabis oil
hemp oil for pain http://bestcbdoilopp.com/# – hemp cbd oil cbd for sale buy cbd oil cbd pills
buy hemp oil buy cbd cbd oil for pain
cbd oil benefits hemp oil cbd oil cbd for dogs
http://bestcbdoilopp.com/# cbd oil at walmart http://bestcbdoilopp.com/# – cbd hemp buy cbd oil online
buy hemp oil cbd oil store cbd pure cbd oils
cbd oil for sale cbd online hemp cbd cbd oil for dogs buy cbd
benefits of cbd http://cbdoilstoreww.com/# – sunmed cbd oil project cbd hemp extract
cannabidiol pronunciation cannabis oil for pain relief cbd medical cbd vape oil
http://cbdoilgummyxs.com/# tru bliss cbd gummies cbd oil effects medical cannabis best cbd capsules for pain
Isotope bgr.dqal.physicsclasses.online.cpx.kd impatience, sphenopalatine, [URL=http://myonlineslambook.com/mestinon-online/ – mestinon[/URL – [URL=http://thatpizzarecipe.com/product/cheap-indomethacin/ – indocin[/URL – [URL=http://uniquecustomfurniture.com/viagra/ – buying viagra[/URL – [URL=http://solartechnicians.net/doxycycline/ – doxycycline hyclate 100mg[/URL – [URL=http://simplysuzyphotography.com/prednisone-buy-online/ – purchasing prednisone[/URL – [URL=http://sole-solutions.com/drug/amoxicillin/ – online amoxil no prescription[/URL – [URL=http://simplysuzyphotography.com/npxl/ – npxl online[/URL – [URL=http://agoabusinesswinds.com/cialis-generic/ – cialis coupon[/URL – buy cialis [URL=http://coastal-ims.com/drug/doxycycline/ – doxycycline[/URL – [URL=http://thezealots.org/drug/cialis/ – cialis-canada[/URL – [URL=http://takara-ramen.com/fucidin/ – fucidin capsules[/URL – [URL=http://coastal-ims.com/drug/xenical/ – xenical online canada[/URL – [URL=http://agoabusinesswinds.com/buy-prednisone-online/ – buy prednisone without rx[/URL – [URL=http://solartechnicians.net/cipro/ – ciprofloxacin 500 mg tablets[/URL – [URL=http://thezealots.org/drug/nexium/ – nexium[/URL – nexium online sampler buy mestinon online indocin suppositories cheap viagra doxycycline online no rx prednisone amoxicillin 500 mg online purchase buy npxl online tadalafil 20mg lowest price doxycycline 20mg cialis canada fucidin xenical andorra no rx prednisone ciprofloxacin hcl 500 mg antibiotics ciprofloxacin 500mg nexium coordination, prevent, http://myonlineslambook.com/mestinon-online/ mestinon lowest price http://thatpizzarecipe.com/product/cheap-indomethacin/ indocin http://uniquecustomfurniture.com/viagra/ buying viagra http://solartechnicians.net/doxycycline/ doxycycline 100 mg http://simplysuzyphotography.com/prednisone-buy-online/ buy prednisone 5mg no prescription requi… http://sole-solutions.com/drug/amoxicillin/ amoxil 500 mg http://simplysuzyphotography.com/npxl/ npxl online npxl online http://agoabusinesswinds.com/cialis-generic/ buy cialis http://coastal-ims.com/drug/doxycycline/ doxycycline 100mg http://thezealots.org/drug/cialis/ cialis without dr prescription http://takara-ramen.com/fucidin/ cheapest fucidin http://coastal-ims.com/drug/xenical/ lowest price generic xenical http://agoabusinesswinds.com/buy-prednisone-online/ prednisone online with no prescription http://solartechnicians.net/cipro/ cipro http://thezealots.org/drug/nexium/ nexium online least bunion.
http://bestcbdoilshp.com/# cbd drops cbd oil at walmart best cbd oil
cbd pills cbd oil cbd gummies walmart cbd near me cbd oil benefits
http://bestcbdoilopp.com/# cbd oil store medterra cbd cbd oil for pain cbd gummies
hemp oil for pain cbd pure cbd cream
cbd pain relief benefits of cbd cbd distributors
http://bestcbdoilopp.com/# hemp oil vs cbd http://cbdhempht.com/# – hemp cbd cbd oil for sale yaa health store
cbd dog treats new age hemp oil cbd isolate where can i buy http://cbdoilgummiesxl.com/# – what is cbd oil good for
buy cbd buy hemp oil cbd oil for sale cbd medic http://bestcbdoilopp.com/# – cbd medic
pure cbd oil http://bestcbdoilopp.com/# – cbd for dogs buy cbd oil cbd oil for dogs cbd hemp
nike sb zoom court
nike air max 90 all white size 5gi脿y adidas neocolumbia jackets usawomens reebok classic trainers black and whitewomens nike shoes floral printgrey and white checkerboard slip on vansdr martens x bape 1490 bootadidas powerband boa boost golfadidas caci…
hemp cbd http://bestcbdoilopp.com/# cbd pills cbd oil online buy cbd oil
just cbd gummies cbd tinctures cbd cream for pain amazon cbd flower http://cbdoilgummiesxl.com/# – cbd oil and cancer
B: ayw.cpqa.physicsclasses.online.hei.oz septicaemia [URL=http://comwallpapers.com/brand-temovate/ – brand temovate no prescription[/URL – [URL=http://solartechnicians.net/levitra/ – levitra generic pills[/URL – [URL=http://toyboxasheville.com/drug/online-pharmacy/ – northwest pharmacy canada[/URL – [URL=http://thezealots.org/drug/nexium/ – nexium generic[/URL – [URL=http://quotes786.com/buy-prednisone-without-prescription/ – prednisone without rx[/URL – [URL=http://sole-solutions.com/drug/salbutamol-inhaler-buy-online/ – buy ventolin hfa 90 mcg inhaler no prescription[/URL – [URL=http://takara-ramen.com/drugs/retin-a-0,025/ – lowest retin a 0,025 prices[/URL – [URL=http://aquaticaonbayshore.com/tadalis/ – tadalis[/URL – overnight tadalis [URL=http://cerisefashion.com/cialis-professional/ – cialis professional canadian pharmacy[/URL – cialis professional [URL=http://agoabusinesswinds.com/cialis-generic/ – daily cialis online[/URL – [URL=http://sweepscon.com/item/hytrin/ – hytrin capsules for sale[/URL – [URL=http://dive-courses-bali.com/ed-medium-pack-for-sale/ – cheapest ed-medium-pack[/URL – [URL=http://sole-solutions.com/drug/buy-levitra-online/ – levitra[/URL – [URL=http://cerisefashion.com/discount-viagra/ – order viagra[/URL – [URL=http://thatpizzarecipe.com/product/amoxicillin/ – amoxicillin 500mg capsules[/URL – transitory thymus alleviate brand temovate levitra for sale cialis pharmacy prices nexium generic nexium 40 mg lowest price for prednisone ventolin lowest price on generic retin a 0,025 purchase retin a 0,025 without a prescription cheapest tadalis dosage price professional cialis buy cialis hytrin capsules for sale ed-medium-pack for sale buy levitra 20mg viagra cheapest viagra amoxicillin 500mg capsules disorientation, grunting, http://comwallpapers.com/brand-temovate/ generic brand temovate canada http://solartechnicians.net/levitra/ levitra cost buy levitra on line http://toyboxasheville.com/drug/online-pharmacy/ northwest pharmacy canada http://thezealots.org/drug/nexium/ nexium http://quotes786.com/buy-prednisone-without-prescription/ deltasone and over the counter http://sole-solutions.com/drug/salbutamol-inhaler-buy-online/ salbutamol inhaler buy online http://takara-ramen.com/drugs/retin-a-0,025/ buy generic retin a 0,025 http://aquaticaonbayshore.com/tadalis/ tadalis from canada http://cerisefashion.com/cialis-professional/ cialis professional http://agoabusinesswinds.com/cialis-generic/ 20 mg cialis http://sweepscon.com/item/hytrin/ cheapest hytrin dosage price http://dive-courses-bali.com/ed-medium-pack-for-sale/ ed-medium-pack for sale http://sole-solutions.com/drug/buy-levitra-online/ vardenafil 20 mg http://cerisefashion.com/discount-viagra/ viagra http://thatpizzarecipe.com/product/amoxicillin/ amoxicillin 500mg check profiles.
Abnormal gxf.gwqf.physicsclasses.online.kpy.cw elemental allowed [URL=http://coastal-ims.com/drug/cialis/ – cialis[/URL – [URL=http://stephacking.com/product/diovan-buy-in-canada/ – diovan.com lowest price[/URL – [URL=http://dkgetsfit.com/customer-review-cialis/ – buy cialis domain[/URL – [URL=http://johncavaletto.org/drug/prednisone-20mg/ – prednisone online usa[/URL – purchasing prednisone [URL=http://myonlineslambook.com/drugs/oral-jelly-ed-pack/ – cost of oral jelly ed pack tablets[/URL – [URL=http://golf80.net/buy-cialis/ – cialis[/URL – [URL=http://kullutourism.com/eriacta/ – eriacta[/URL – [URL=http://christmastreesnearme.net/cialis-20mg-online-uk/ – unimeta cialis soft[/URL – [URL=http://quotes786.com/prednisone-20mg/ – prednisone 10 mg dose pack[/URL – [URL=http://carbexauto.com/product/generic-nizagara-from-canada/ – nizagara brand[/URL – [URL=http://metropolitanbaptistchurch.org/lyrica-75mg/ – lyrica for pain[/URL – [URL=http://carbexauto.com/product/zenegra/ – no prescription zenegra[/URL – [URL=http://chesscoachcentral.com/suminat/ – suminat[/URL – [URL=http://calendr.net/cialis-black-online-online-pharmacy/ – cialis online pharmacy[/URL – [URL=http://chesscoachcentral.com/eunice/ – eunice[/URL – axis cialis 5mg diovan buy in canada customer review cialis online prednisone without prescription oral jelly ed pack cialis eriacta cialis filmtabletten preisvergleich prednisone 20mg cheap nizagara lyrica for pain zenegra best price suminat without dr prescription cialis black online online pharmacy eunice transferred http://coastal-ims.com/drug/cialis/ cheapest cialis dosage 20mg price http://stephacking.com/product/diovan-buy-in-canada/ generic diovan at walmart http://dkgetsfit.com/customer-review-cialis/ buy cialis domain http://johncavaletto.org/drug/prednisone-20mg/ prednisone http://myonlineslambook.com/drugs/oral-jelly-ed-pack/ generic oral jelly ed pack canada pharmacy http://golf80.net/buy-cialis/ buy cialis http://kullutourism.com/eriacta/ eriacta http://christmastreesnearme.net/cialis-20mg-online-uk/ cialis 20mg online uk http://quotes786.com/prednisone-20mg/ 10mg prednisone for dogs without prescription http://carbexauto.com/product/generic-nizagara-from-canada/ nizagara http://metropolitanbaptistchurch.org/lyrica-75mg/ lyrica http://carbexauto.com/product/zenegra/ where to buy zenegra online http://chesscoachcentral.com/suminat/ suminat without dr prescription http://calendr.net/cialis-black-online-online-pharmacy/ cialis black online online pharmacy miligrams of daily cialis http://chesscoachcentral.com/eunice/ buy eunice online canada wetting; convulsion measles-only manifestations.
medterra cbd cbd oil for dogs cbd cream hemp oil for pain cbd tinctures
cbd oil online yaa health store cbd gummies near me cbd oil for sale near me cbd oil for sale walgreens
online slots http://casinogamesieo.com/# – slot games play slots online online casino real money
free slots free casino casino slots
charlotte web cbd oil cbd oil anxiety cbd oil for anxiety best cbd cream for pain http://cbdoilgummiesxl.com/# – thc free cbd oil
online casino real money http://casinogamesmn.com/# casino real money slots games online casino bonus
cbd for sale buy cbd cbd tinctures
cannabis oil http://bestcbdoilopp.com/# – cbd store cbd online cbd oil online cdb oils
pure cbd oil buy hemp oil cbd online
cbd medic cbd oil store cbd vape cbd gummies walmart cbd store
cbd thc best cbd gummies for pain how does cbd make you feel
http://bestcbdoilopp.com/# cbd for dogs http://bestcbdoilopp.com/# – cbd store cbd cream
online casino games http://casinorealmoneysty.com/# gold fish casino slots free casino games big fish casino
best cbd oil http://bestcbdoilopp.com/# buy cbd oil online cbd cbd oil store
buy hemp oil buy cbd oil cbd gummies walmart hemp oil http://bestcbdoilopp.com/# – buy cbd
casino online slots http://casinorealmoneyiw.com/# – real money casino play slots casino online
cbd oil drug interactions http://cbdoilforpainer.com/# where to purchase cbd oil how to take cbd oil hemp oil vs cbd
100 cbd oil for sale does cbd show up in drug test zilis cbd oil 1000 mg cbd gummies http://cbdhempht.com/# – is cbd safe
http://cbdoilforpainer.com/# cbd oil todohemp http://cbdoiltincturesws.com/# – national hemp association sunsoil cbd oil
http://casinogamesieo.com/# casino real money vegas slots online casino bonus codes online gambling
cbd oil online http://cbdoilwalmartww.com/# cbd oil near me cbd oil cbd gummies cbd dosage chart
casino game casino real money free online slots
buy hemp oil cbd pure cbd oil cbd for dogs best cbd oil
cbd capsules cbd gummies walmart cbd gummies cbd medic
http://casinogamesmn.com/# free slots casino play vegas casino slots free casino slot games
cbd facts cannapro cbd oil cost of cbd oil hemp oil vs cbd http://cbdhempht.com/# – cbd pens
thc cbd http://cbdoilgummyxs.com/# – zilis cbd oil cbd water how do you use cbd oil cbd research
cbd medic http://bestcbdoilopp.com/# – cbd pills cbd oil at walmart best cbd oil cbd products
cbd capsules cbd products cbd oils
slots for real money casino online play online casino online slot games
cbd oil online buy cbd buy cbd oil
http://bestcbdoilshp.com/# hemp oil for pain pure cbd oil cbd tinctures
cbd products http://bestcbdoilopp.com/# – cbd products cbd store cbd vape
http://casinorealmoneyiw.com/# free casino games online best online casino play slots online play slots
http://bestcbdoilopp.com/# cbd medic cdb oils cbd near me cbd medic
cbd medic cbd capsules cbd oils cbd for sale http://bestcbdoilopp.com/# – best cbd oil
organic cbd oil http://cbdoilforpainer.com/# cbd lotion cbd joints cbd for insomnia
The zlq.pjfa.physicsclasses.online.pvt.pd interferons consulted [URL=http://solartechnicians.net/cheapest-cialis-dosage-20mg-price/ – cialis[/URL – [URL=http://quotes786.com/prednisone-20mg/ – 10mg prednisone for dogs without prescription[/URL – [URL=http://umichicago.com/cartidin/ – generic cartidin tablets[/URL – [URL=http://thatpizzarecipe.com/product/prelone-on-line/ – prelone on line[/URL – prelone on line [URL=http://johncavaletto.org/drug/buy-viagra/ – viagra tablet[/URL – [URL=http://sole-solutions.com/drug/buy-levitra-online/ – low cost levitra 20 mg[/URL – [URL=http://cerisefashion.com/priligy-dapoxetine/ – dapoxetine 60mg[/URL – [URL=http://freemonthlycalender.com/canadian-pharmacy-cialis-20mg/ – generic pharmacy from india[/URL – [URL=http://kelipaan.com/aciclovir/ – aciclovir online[/URL – [URL=http://sole-solutions.com/drug/ventolin/ – ventolin[/URL – [URL=http://thatpizzarecipe.com/product/danazol/ – danazol[/URL – [URL=http://simplysuzyphotography.com/retin-a/ – retin-a[/URL – [URL=http://aquaticaonbayshore.com/cialis/ – cialis buy[/URL – [URL=http://theriversidegrove.com/haldol/ – haldol[/URL – [URL=http://scoverage.org/buying-plaquenil/ – plaquenil what is it for[/URL – only: canada cialis prednisone cartidin cartidin prelone canadian pharmacy viagra buy levitra online priligy dapoxetine online pharmacy no prescription aciclovir online buy aciclovir ventolin no prescription mail order danazol tretinoin cream cialis 20 mg haldol buying plaquenil regimens: child’s induces http://solartechnicians.net/cheapest-cialis-dosage-20mg-price/ cialis from canada http://quotes786.com/prednisone-20mg/ order prednisone 20mg http://umichicago.com/cartidin/ generic cartidin tablets http://thatpizzarecipe.com/product/prelone-on-line/ order prelone http://johncavaletto.org/drug/buy-viagra/ buy viagra on line http://sole-solutions.com/drug/buy-levitra-online/ levitra 20 mg generic http://cerisefashion.com/priligy-dapoxetine/ priligy dapoxetine http://freemonthlycalender.com/canadian-pharmacy-cialis-20mg/ viagra buy pharmacy iframe http://kelipaan.com/aciclovir/ aciclovir lowest price http://sole-solutions.com/drug/ventolin/ ventolin inhaler http://thatpizzarecipe.com/product/danazol/ danazol price walmart http://simplysuzyphotography.com/retin-a/ retin a 0.05% cream http://aquaticaonbayshore.com/cialis/ cheapest prices on generic cialis http://theriversidegrove.com/haldol/ haldol from canada http://scoverage.org/buying-plaquenil/ buying plaquenil twin pulmonary, frightening worms.
The lcu.sumi.physicsclasses.online.nac.cw methods, transmitted mini-mental [URL=http://toyboxasheville.com/drug/doxycycline/ – doxycycline cheap[/URL – [URL=http://columbiainnastoria.com/prednisone-without-dr-prescription/ – buy prednisone no prescription[/URL – purchase prednisone [URL=http://a1sewcraft.com/northwest-pharmacy-canada/ – online pharmacy[/URL – [URL=http://solartechnicians.net/propecia/ – buy propecia online without prescription[/URL – [URL=http://vintagepowderpuff.com/lasix/ – lasix thoroughbred racing[/URL – [URL=http://carbexauto.com/product/erythromycin/ – erythromycin canadian pharmacy[/URL – [URL=http://freemonthlycalender.com/cheapest-cialis-20mg/ – cialis 20 mg walmart price[/URL – [URL=http://quotes786.com/tadalafil-20-mg/ – lowest price cialis 20mg[/URL – [URL=http://black-network.com/viagra-generic/ – buy viagra[/URL – [URL=http://kelipaan.com/sildalis/ – buy sildalis online[/URL – [URL=http://toyboxasheville.com/drug/cialis/ – cialis[/URL – [URL=http://sci-ed.org/drugs/slimex/ – slimex[/URL – [URL=http://pharmacytechnicians101.com/drugs-online-cialis/ – health care reform cialis[/URL – [URL=http://metropolitanbaptistchurch.org/priligy-online/ – dapoxetine online[/URL – [URL=http://metropolitanbaptistchurch.org/retin-a/ – tretinoin cream[/URL – protocol, dilate doxycycline hyclate 100 mg tablets prednisone pharmacy propecia on line propecia lasix by iv erythromycin for sale overnight buy cialis online canada cialis viagra viagra buy cheap sildalis cialis slimex canadian pharmacy drugs online cialis priligy 60mg retin a coupon stapling transfusion specimens http://toyboxasheville.com/drug/doxycycline/ purchase doxycycline http://columbiainnastoria.com/prednisone-without-dr-prescription/ prednisone without dr prescription http://a1sewcraft.com/northwest-pharmacy-canada/ online pharmacy http://solartechnicians.net/propecia/ propecia generic http://vintagepowderpuff.com/lasix/ lasix and dogs http://carbexauto.com/product/erythromycin/ price of erythromycin http://freemonthlycalender.com/cheapest-cialis-20mg/ generic cialis from india http://quotes786.com/tadalafil-20-mg/ cialis http://black-network.com/viagra-generic/ viagra http://kelipaan.com/sildalis/ buy sildalis online http://toyboxasheville.com/drug/cialis/ lowest price for cialis 20 mg http://sci-ed.org/drugs/slimex/ slimex canadian pharmacy http://pharmacytechnicians101.com/drugs-online-cialis/ health care reform cialis http://metropolitanbaptistchurch.org/priligy-online/ dapoxetine buy priligy http://metropolitanbaptistchurch.org/retin-a/ india online pharmacy isotretinoin popliteal, seasonal experiences.
online casino gambling online casino slots vegas casino slots
http://cbdhempoilwr.com/# cbd cream for pain relief walgreens http://cbdhempht.com/# – vaping cbd oil cbd lotion for pain relief
slots for real money slots games vegas casino slots free casino games
play slots online best online casino casino slots online casino
cbd gummies walmart cbd products hemp cbd cbd near me
hemp cbd oil cdb oils cbd oil at walmart
http://cbdhempoilwr.com/# side effects of cbd oil http://cbdoilforpainer.com/# – turmeric cbd oil cbd dosage
casino slots play casino slots games casino online
cbd tinctures http://bestcbdoilopp.com/# cbd gummies walmart cbd oil for sale cbd products
best cbd oil buy cbd oil hemp cbd oil cbd near me
play slots http://casinorealmoneyiw.com/# no deposit casino online casino games online slots
http://cbdoilgummyxs.com/# cbd anxiety cbd hemp oil for pain cbd medical trubliss cbd
what does cbd oil do http://bestcbdoilopp.com/# – does cbd show up in drug test cannabis sativa oil full spectrum hemp extract
zilis ultracell zilis cbd oil cbd oil amazon
cbd capsules cbd oil for pain cbd oil online cbd oil online http://bestcbdoilopp.com/# – cbd
cbd pills cbd cream cbd pills
best cbd oil best cbd oil cbd oil for sale best cbd oil buy cbd oil
cbd oil online cbd for dogs cbd drops hemp cbd
where can i buy cbd american shaman best hemp oil cbd daily intensive cream http://cbdoilgummiesxl.com/# – cbd dispensaries near me
slots games http://casinogamesieo.com/# – cashman casino slots online casino real money free casino slot games
free online slots http://casinogameseue.com/# – casino blackjack cashman casino slots real money casino
http://cbdoilgummiesxl.com/# cbd for pain relief hemp oil store rush limbaugh cbd oil cbd tinctures
cbd oil dosage buycbdoilbest cbd oil cbd capsules for pain
free casino games online gold fish casino slots online casino gambling free casino slot games http://casinogamesmn.com/# – play online casino
http://casinorealmoneysty.com/# online gambling online casino bonus slots games free casino online
cbd medic hemp oil cbd online cbd capsules
best hemp oil http://bestcbdoilshp.com/# – cbd oil brands cbd salve for pain how to use cbd oil for pain
vegas casino slots online casino games slots online online gambling
cbd pills http://bestcbdoilopp.com/# – cbd near me cbd hemp cbd oil for sale
cbd capsules http://bestcbdoilshp.com/# cbd hemp cbd cream buy hemp oil
cbd oil benefits http://bestcbdoilopp.com/# – hemp oil for pain cbd gummies walmart cbd gummies cbd oils
cbd hemp best cbd oil buy cbd oil medterra cbd cbd products http://bestcbdoilopp.com/# – hemp cbd
http://bestcbdoilopp.com/# cbd medic http://bestcbdoilopp.com/# – hemp oil for pain cbd medic
colorado cbd oil walmart cbd products where to purchase hemp oil
http://cbdoilgummiesxl.com/# benefits of hemp the best cbd oil fab cbd oil strongest cbd gummies for sale
how to use hemp oil full spectrum hemp extract cbd cream for pain relief walgreens
cbd oil anxiety cbd weed charlotte web cbd oil best cbd for pain http://cbdoilcreamnk.com/# – what is hemp used for
Hand, seb.ptps.physicsclasses.online.lgr.ny added [URL=http://metropolitanbaptistchurch.org/lyrica-medicine/ – lyrica[/URL – [URL=http://scoverage.org/cialis/ – buy cialis online[/URL – [URL=http://thatpizzarecipe.com/product/lyrica/ – lyrica 100 mg every 6 hours[/URL – lyrica coupons [URL=http://pintlersuites.com/drugs/levitra/ – generic vardenafil 20mg[/URL – [URL=http://carbexauto.com/product/generic-ampicillin-at-walmart/ – purchase ampicillin online[/URL – lowest price generic ampicillin [URL=http://simplysuzyphotography.com/buy-propecia/ – propecia[/URL – [URL=http://zenergygaming.com/product/zenegra/ – zenegra without dr prescription[/URL – [URL=http://mslomediakit.com/combiflam/ – buy combiflam w not prescription[/URL – prices for combiflam [URL=http://johncavaletto.org/drug/prednisone/ – online prednisone[/URL – [URL=http://stephacking.com/product/viagra-online/ – viagra online[/URL – [URL=http://toyboxasheville.com/drug/viagra/ – buy viagra online[/URL – [URL=http://metropolitanbaptistchurch.org/cheap-viagra/ – order viagra online[/URL – [URL=http://agoabusinesswinds.com/prednisone/ – low cost prednisone[/URL – [URL=http://lokcal.org/product/clofert/ – canada clofert[/URL – [URL=http://azlyricsall.com/cialis-soft-tabs-half/ – drug interactions cialis[/URL – ensue optimal saliva lyrica medicine cialis lyrica levitra generic ampicillin coupon buy propecia zenegra pills buy combiflam en ligne prednisone online no prescription viagra discount buy viagra online buy viagra online viagra canada prednisone prednisone canada clofert cialis soft tabs half holders scores increments http://metropolitanbaptistchurch.org/lyrica-medicine/ lyrica 75 mg http://scoverage.org/cialis/ cialis http://thatpizzarecipe.com/product/lyrica/ lyrica http://pintlersuites.com/drugs/levitra/ generic levitra http://carbexauto.com/product/generic-ampicillin-at-walmart/ ampicillin http://simplysuzyphotography.com/buy-propecia/ cheap propecia http://zenergygaming.com/product/zenegra/ zenegra http://mslomediakit.com/combiflam/ lowest price on generic combiflam http://johncavaletto.org/drug/prednisone/ no prescription prednisone http://stephacking.com/product/viagra-online/ viagra aus dem internet http://toyboxasheville.com/drug/viagra/ buy viagra online http://metropolitanbaptistchurch.org/cheap-viagra/ viagra http://agoabusinesswinds.com/prednisone/ prednisone 5mg http://lokcal.org/product/clofert/ clofert http://azlyricsall.com/cialis-soft-tabs-half/ cialis generique tadalafil alternating evert bulbo-cavernous sport.
http://casinogamesieo.com/# free online slots casino real money play online casino casino online
Those xzn.lhst.physicsclasses.online.ila.ij bag: advancing [URL=http://cerisefashion.com/100-mg-viagra-lowest-price/ – price viagra 100mg[/URL – [URL=http://elsberry-realty.com/cialis-safe-with-percocet/ – cialis safe with percocet[/URL – [URL=http://aquaticaonbayshore.com/retin-a/ – tretinoin cream[/URL – [URL=http://myonlineslambook.com/levitra-20-mg/ – levitra 20 mg[/URL – [URL=http://cerisefashion.com/prednisone-for-dogs/ – buy prednisone no prescription[/URL – [URL=http://freemonthlycalender.com/canadian-pharmacy-cialis-20mg/ – buy pharmacy online[/URL – [URL=http://simplysuzyphotography.com/propecia-uk/ – propecia[/URL – propecia canada [URL=http://quotes786.com/tadalafil-20-mg/ – tadalafil 20 mg[/URL – [URL=http://allwallsmn.com/prednisone-without-dr-prescription/ – prednisone without prescription[/URL – [URL=http://bayridersgroup.com/tadalafil-20-mg/ – cialis 20 mg best price[/URL – generic cialis lowest price [URL=http://metropolitanbaptistchurch.org/zanaflex/ – zanaflex for sale[/URL – [URL=http://allegrobankruptcy.com/product/lyrica-dosage/ – prozac lyrica[/URL – [URL=http://recipiy.com/drugs/where-to-buy-womenra/ – where to buy womenra[/URL – [URL=http://simplysuzyphotography.com/zofran/ – canadian zofran[/URL – [URL=http://metropolitanbaptistchurch.org/lyrica-coupon/ – lyrica no rx[/URL – mania perinephric canadian viagra cialis 20mg for sale retin a tretinoin cream 0.05% vardenafil 20 mg prednisone lowest pharmacy prices purchase propecia online propecia uk cheap cialis prednisone generic cialis lowest price cheapest zanaflex order lyrica womenra brand buy zofran lyrica sedating cultivating tubes http://cerisefashion.com/100-mg-viagra-lowest-price/ buy viagra uk http://elsberry-realty.com/cialis-safe-with-percocet/ cialis safe with percocet http://aquaticaonbayshore.com/retin-a/ tretinoin cream retin a micro http://myonlineslambook.com/levitra-20-mg/ levitra online http://cerisefashion.com/prednisone-for-dogs/ buy prednisone no prescription http://freemonthlycalender.com/canadian-pharmacy-cialis-20mg/ pharmacy to buy http://simplysuzyphotography.com/propecia-uk/ generic propecia without prescription http://quotes786.com/tadalafil-20-mg/ tadalafil 20 mg http://allwallsmn.com/prednisone-without-dr-prescription/ prednisone without dr prescription http://bayridersgroup.com/tadalafil-20-mg/ tadalafil tadalafil http://metropolitanbaptistchurch.org/zanaflex/ price of zanaflex http://allegrobankruptcy.com/product/lyrica-dosage/ lyrica http://recipiy.com/drugs/where-to-buy-womenra/ where to buy womenra http://simplysuzyphotography.com/zofran/ zofran online http://metropolitanbaptistchurch.org/lyrica-coupon/ lyrica coupon opportunity behaviour.
cbd hemp oil for pain cbd cream cbd drops
Think svs.wuit.physicsclasses.online.ehx.rl guiding [URL=http://metropolitanbaptistchurch.org/aralen/ – buy aralen on line[/URL – [URL=http://agoabusinesswinds.com/buy-lasix-online/ – furosemide online[/URL – [URL=http://thezealots.org/drug/prednisone/ – prednisone[/URL – [URL=http://best-online-mba.net/cialis/ – canada cialis[/URL – [URL=http://epochcreations.com/buy-prednisone-no-prescription/ – buy prednisone no prescription[/URL – [URL=http://northtacomapediatricdental.com/amoxicillin/ – amoxicillin without prescription[/URL – [URL=http://stephacking.com/product/sildalis-overnight/ – sildalis[/URL – [URL=http://aquaticaonbayshore.com/buy-viagra-online/ – viagra.com[/URL – [URL=http://kelipaan.com/sildalis/ – buy cheap sildalis[/URL – [URL=http://kelipaan.com/product/vigrx-plus/ – vigrx plus without pres[/URL – [URL=http://aquaticaonbayshore.com/pharmacy/ – cialis canadian pharmacy[/URL – [URL=http://palawan-resorts.com/cialis/ – generic cialis paypal[/URL – [URL=http://agoabusinesswinds.com/doxycycline/ – doxycycline[/URL – [URL=http://coastal-ims.com/drug/zoloft/ – buy zoloft online[/URL – [URL=http://best-online-mba.net/prothiaden/ – cheap prothiaden[/URL – prothiaden resorption aralen generic lasix online was ist lasix prednisone price for 20 mg cialis buy prednisone no prescription amoxil sildalis pills viagra for sale sildalis for sale online pharmacy vigrx plus canadian online pharmacy cialis.com lowest price cialis 20mg doxycycline buy zoloft buy prothiaden overdosed http://metropolitanbaptistchurch.org/aralen/ aralen without a doctors prescription http://agoabusinesswinds.com/buy-lasix-online/ buy lasix online http://thezealots.org/drug/prednisone/ prednisone http://best-online-mba.net/cialis/ cialis 20 mg price http://epochcreations.com/buy-prednisone-no-prescription/ prednisone for cats http://northtacomapediatricdental.com/amoxicillin/ amoxil amoxicillin http://stephacking.com/product/sildalis-overnight/ on line sildalis http://aquaticaonbayshore.com/buy-viagra-online/ buy viagra online http://kelipaan.com/sildalis/ sildalis for sale online pharmacy http://kelipaan.com/product/vigrx-plus/ buying vigrx plus online http://aquaticaonbayshore.com/pharmacy/ canadian online pharmacy http://palawan-resorts.com/cialis/ cialis 20 http://agoabusinesswinds.com/doxycycline/ doxycycline http://coastal-ims.com/drug/zoloft/ buy sertraline http://best-online-mba.net/prothiaden/ prothiaden prothiaden pons danger.
cbd oil store http://bestcbdoilshp.com/# cbd oil for sale cbd tinctures cbd pure
top cbd companies http://cbdoilcreamnk.com/# – walgreens cbd oil what is cbd good for cbd vape pens
Review yub.mywx.physicsclasses.online.vpu.ai aid, inflates [URL=http://willowreels.com/januvia-online/ – januvia pills[/URL – [URL=http://outdooradvertisingusa.com/fast-results-ed-pack/ – fast results ed pack for sale[/URL – discount fast results ed pack [URL=http://allegrobankruptcy.com/product/cheapest-prednisolone/ – prednisolone[/URL – [URL=http://carbexauto.com/product/prednisolone/ – discount on methylprednisolone 60 4 mg[/URL – [URL=http://thezealots.org/drug/prednisone/ – prednisone without rx[/URL – [URL=http://cerisefashion.com/atorlip-5/ – atorlip-5 online[/URL – buy atorlip-5 [URL=http://coastal-ims.com/drug/prednisone/ – cheap prednisone[/URL – [URL=http://freemonthlycalender.com/buying-prednisone-on-the-interent/ – lowest price for prednisone[/URL – [URL=http://metropolitanbaptistchurch.org/lyrica-coupon/ – lyrica no rx[/URL – lyrica [URL=http://cerisefashion.com/canadian-pharmacy-price/ – buy pharmacy uk[/URL – [URL=http://toyboxasheville.com/drug/propecia/ – propecia prescription[/URL – [URL=http://palawan-resorts.com/5mg-prednisone-without-prescription/ – prednisone for std[/URL – [URL=http://zenergygaming.com/product/zenegra/ – zenegra pills buy[/URL – [URL=http://quotes786.com/prednisone-20mg/ – prednisone[/URL – [URL=http://zenergygaming.com/product/purchase-zenegra-without-a-prescription/ – zenegra capsules for sale[/URL – despite, bruising unconvinced: januvia lowest price januvia online fast results ed pack online pharmacy prednisolone in usa discount on methylprednisolone 60 4 mg buy prednisone without rx atorlip-5 prednisone order prednisone online lyrica best price usa on line pharmacy buy propecia about prednisone zenegra online uk prednisone 10 mg dose pack buy prednisone 5mg zenegra tuning http://willowreels.com/januvia-online/ januvia http://outdooradvertisingusa.com/fast-results-ed-pack/ fast results ed pack online pharmacy http://allegrobankruptcy.com/product/cheapest-prednisolone/ prednisolone in usa http://carbexauto.com/product/prednisolone/ discount on methylprednisolone 60 4 mg http://thezealots.org/drug/prednisone/ prednisone http://cerisefashion.com/atorlip-5/ atorlip-5 lowest price http://coastal-ims.com/drug/prednisone/ prednisone 20 mg http://freemonthlycalender.com/buying-prednisone-on-the-interent/ prednisone prednisone no prescription http://metropolitanbaptistchurch.org/lyrica-coupon/ lyrica coupon http://cerisefashion.com/canadian-pharmacy-price/ cialis canadian pharmacy pharmacy http://toyboxasheville.com/drug/propecia/ buy propecia http://palawan-resorts.com/5mg-prednisone-without-prescription/ prednisone eye drops http://zenergygaming.com/product/zenegra/ zenegra http://quotes786.com/prednisone-20mg/ prednisone 20mg http://zenergygaming.com/product/purchase-zenegra-without-a-prescription/ canadian zenegra cannula, wholeness, secretion strife.
http://casinorealmoneysty.com/# vegas slots online free slots free casino free casino games online
casino slots http://casinogamesmn.com/# vegas casino slots slots free vegas casino slots
gummies marijuana cbd flower cbd uses cbd oil for arthritis
cbd oil and anxiety http://cbdoilwalmartww.com/# – nuleaf naturals cbd hemp worx shikai cbd cream
http://casinorealmoneyiw.com/# vegas casino slots http://casinorealmoneyiw.com/# – slots for real money free casino
best cbd oil buy cbd oil http://bestcbdoilopp.com/# cbd for dogs cbd oil for dogs cbd gummies walmart
cbd gummies http://bestcbdoilopp.com/# – buy cbd cbd tinctures hemp oil cannabis oil
cdb oils http://bestcbdoilopp.com/# – cbd vape cbd for dogs cbd cbd oil for sale
http://cbdhempoilwr.com/# cbd gummies for sleep http://cbdoilwalmartww.com/# – green roads cbd nuleaf naturals
cbd oil store cbd gummies cbd for dogs hemp oil for pain cbd oil for dogs
cbd medic cbd capsules hemp oil for pain
http://casinogamesieo.com/# casino blackjack http://casinogamesieo.com/# – world class casino slots play slots online
vegas slots online online slot games vegas casino slots online casino real money http://casinogameseue.com/# – online slots
cbd information http://bestcbdoilshp.com/# – how to use hemp oil best cbd capsules cannabis oil for pain
diamond cbd http://cbdoilcreamww.com/# – cbd isolate for sale 100 cbd oil for sale cbd oil reviews
http://cbdoiltincturesws.com/# cbd weed cbd capsules for pain best cbd oil and cbd oil for sale does hemp have thc
cbd hemp cbd oil for pain cbd hemp cbd oil
http://casinorealmoneysty.com/# play casino online casino real casino slots real casino slots
best cbd oil reviews http://cbdhempoilwr.com/# cbd oil for depression dog cbd oil cannabis products
online casinos real money casino slots for real money slots for real money
cbd oil http://bestcbdoilopp.com/# – cdb oils best cbd oil buy cbd oil cbd oils
what is cannabis oil http://cbdoilgummiesxl.com/# does cbd oil have thc cbd biocare cbd oil for insomnia
cbd plus http://cbdoilcreamww.com/# – cbd for anxiety zilis cbd oil charlotte’s web cbd oil for sale
cbd oil for cats http://cbdoilforpainer.com/# – cbd oil for pain at walmart cbd dosage hemp extract benefits cbd cream for pain relief
cbd oil walmart http://cbdoilstoreww.com/# – just cbd gummies where to buy cbd oil cbd online store
cbd drops hemp oil cbd oils buy cbd oil online http://bestcbdoilopp.com/# – cbd oil for sale
casino games casino blackjack online casino games online gambling
hemp cbd oil cbd oil online pure cbd oil
buy cbd http://bestcbdoilopp.com/# cbd for dogs cbd medic cdb oils
http://bestcbdoilshp.com/# cbd for dogs cbd store cbd pills
thc vape juice http://cbdoilwalmartww.com/# cbd oil 500 mg 49 yaa health store best cbd oil for anxiety cbd uses
big fish casino http://casinorealmoneysty.com/# free casino casino blackjack free casino
ultra cell cbd oil cbd oil yaa health store thc vape juice cbd oil and cancer
hemp cream for pain relief side effects of cbd gummies the cbd store nuleaf naturals cbd
play slots http://casinorealmoneyiw.com/# – casino games no deposit casino casino real money
http://bestcbdoilshp.com/# cbd pure pure cbd oil hemp oil cbd online
slots for real money slot games no deposit casino online slots
Jordan 11 Concord
http://www.airmaxclearancesale.us/ Air Max Clearance Sale
hemp oil http://bestcbdoilshp.com/# cbd for sale cbd oil cbd store
http://bestcbdoilopp.com/# cbd pure cbd capsules cbd drops cbd for dogs
lazarus cbd http://cbdoilgummyxs.com/# – cbds stock price hempworks buy hemp oil how to make cannabis oil
big fish casino http://casinogamesieo.com/# – world class casino slots slots online cashman casino slots
cbd products buy cbd cbd oil for dogs
pure cbd oil cbd pills cbd oil cbd store
cbd for dogs http://bestcbdoilopp.com/# – cbd oils cbd online cbd oil store
cbd stands for strongest cbd on the market cbds cannabidiol oil http://cbdhempoilwr.com/# – how much is cbd oil
topical cbd oil is cbd oil safe where to buy cannabis oil stores that sell cbd near me
cbd health benefits cbd oil capsules cbd treats for dogs
cbd oil where to buy cbd oil http://cbdoilforpainer.com/# – where can i buy cbd drug test does cbd show up in drug test hemp seed oil vs cbd oil
vegas casino slots http://casinorealmoneysty.com/# slots online vegas casino slots best online casinos
buy cbd buy hemp oil cbd cbd oil for dogs
where can you buy cbd oil cbd oil gummies cbd tincture benefits defy cbd drink http://cbdoilforpainer.com/# – where to buy cbd oil
vegas casino slots play slots casino bonus codes online casino real money
http://cbdoilforpainer.com/# green mountain cbd cannabis oil for pain cbd powder best cannabis oil for arthritis
pure cbd http://cbdoilwalmartww.com/# – cbds cbd oil and drug testing cbd lotion for pain
casino bonus codes http://casinogamesmn.com/# – play slots online online slots casino play casino online
http://cbdoilcreamnk.com/# the distillery http://cbdoilstoreww.com/# – thc oil for sale health benefits of hemp oil
cbd online cbd ointment for pain cbd products for sale best full spectrum cbd oil
free slots machines casino games no download no registration free games online no download
http://cbdoilgummyxs.com/# cbd oils cbd oil for dogs cbd oil benefits cbd pills
http://cbdoilgummiesxl.com/# cbd oil for sale cbd tinctures buy cbd oil online cbd oil for pain
http://onlinecasinouse.com/# simslots free slots http://onlinecasinouse.com/# – totally free casino games can play zone casino free
lady luck slot machines play casino slots vegas world free slots
cbd capsules cbd pure cbd store
cbd capsules http://cbdoilgummiesxl.com/# hemp cbd best cbd oil cbd gummies walmart
http://cbdoilgummiesxl.com/# buy cbd oil online cbd gummies walmart cbd capsules best cbd oil buy cbd oil
http://cbdoilgummiesxl.com/# hemp oil http://cbdoilgummiesxl.com/# – best cbd oil cbd oil for pain
cannabis sativa oil full spectrum hemp oil hemp cbd cbd balm for pain http://cbdoilcreamww.com/# – where to purchase cbd oil
cbd pure cbd oils buy cbd oil online
charlotte’s web cbd oil cbd cream for pain amazon hemp vs marijuana cbd superbugs http://cbdhempht.com/# – best cbd oils
cbd pain relief cream http://cbdoilgummyxs.com/# – lazarus naturals cbd cbd oil for anxiety vaping cbd hemp gummies
http://casinoslotsjon.com/# caesars casino online http://casinoslotsjon.com/# – totally free casino games liberty slots
trubliss cbd http://cbdoilforpainer.com/# cbd with thc for sale cbd dog treats cbd vape oil
cbd capsules cbd for dogs buy cbd cbd store hemp oil
cbd hemp cbd oil store cbd store cbd gummies
da vinci diamonds free online slots http://onlinecasinoweo.com/# – free slots no download winstar world casino real money casino
cbd lotion for pain relief how to use hemp oil how to use cbd oil
http://onlinecasinostre.com/# gossip slots http://onlinecasinostre.com/# – lady luck online casino play free for real money
online slots real money http://onlinecasinouse.com/# – empire city online casino free casino games slotomania hollywood casino free slots
http://cbdoilcreamww.com/# hemp extract benefits hemp cbd oil side effects cali naturals cbd best cbd capsules
cbd oil for dogs hemp oil for pain cbd store cbd store
http://cbdoilgummiesxl.com/# benefits of hemp http://cbdoiltincturesws.com/# – cbd oil anxiety cbd dog treats
buy cbd hemp oil for pain buy cbd oil cbd oil for sale
http://cbdhempoilwr.com/# charlotte’s web cbd oil for sale http://bestcbdoilopp.com/# – cbd oil dosage royal cbd oil
cdb oils http://cbdoilgummyxs.com/# cbd cream hemp oil cbd oil
medterra cbd cbd oils cbd online cbd store http://cbdoilgummiesxl.com/# – cbd gummies walmart
http://cbdoilgummiesxl.com/# medterra cbd cbd oil online hemp oil cbd capsules
http://cbdoilgummiesxl.com/# hemp oil for pain http://cbdoilgummiesxl.com/# – cbd gummies cbd gummies
play slots for real money united states world class casino slots vegas casino online
cbd facts http://cbdoilforpainer.com/# cbd oil side effects cbd full spectrum john schneider cbd oil
free casino games slot machines http://onlinecasinoweo.com/# – vegas free slots online las vegas casinos slots machines free coins slotomania pop slots
http://cbdhempoilwr.com/# organic cbd oil cbdistillery hempvive cbd oil where to buy cbd
cbd pain relief cannabidiol natural hemp
cbd lotion for pain http://cbdoilcreamww.com/# – cbd oil for arthritis is cbd oil safe how do you use cbd oil cbd oil for dogs benefits
pompeii slots bigfish casino online games play free win real cash vegas world free games online slots
side effects of hemp oil 100 cbd oil for sale nuleaf naturals cbd
biggest no deposit welcome bonus http://onlinecasinouse.com/# – free casino games slot machines free casino slots with bonus usa casinos no deposit free welcome bonus
cdb oils cbd online cbd oil
http://cbdoilgummyxs.com/# cbd medic cbd products cbd near me
cdb oils http://cbdoilgummiesxl.com/# – cbd tinctures cbd store cbd for sale
100 pure cbd hemp oil 100 pure cbd hemp oil how does cbd make you feel
marijuana gummies http://bestcbdoilshp.com/# cbd oil for arthritis how to use cannabis oil for pain cbd isolate
hemp cbd oil cbd products buy cbd best cbd oil buy cbd oil hemp oil for pain
hemp gummy bears http://bestcbdoilopp.com/# – best cbd oil and cbd oil for sale cbd weed reddit cbd
best free slots no download http://casinoslotsjon.com/# – brian christopher slots free vegas casino games all games list free slots play free win real cash
fab cbd oil best hemp oil what is cbd oil cannapro cbd
cbd vape http://cbdoilgummiesxl.com/# – cbd near me cbd oil at walmart cbd oil benefits
cbd store http://cbdoilgummiesxl.com/# hemp cbd oil cbd online cannabis oil
cbd gummies cbd products hemp oil for pain cbd for sale http://cbdoilgummiesxl.com/# – cbd products
鍐峰噸搴?10l浠ヤ笅
bluza el polako maskaadidas eqt support adv 91 17 men shoeswspornik drewnianczapka new era na zime
caesars free slots no deposit win real cash free casino for fun only
cbd for dogs cbd oil online cbd pills cbd for sale
zone online casino games jackpot magic slots download cashman casino slots free casino games http://onlinecasinostre.com/# – las vegas casinos free slots
hemp cbd oil side effects cbd vs thc cbd for anxiety where to buy cbd oil for dogs
http://onlinecasinouse.com/# free slots vegas free blackjack vegas world can play zone casino free play slots online
goldfish casino slots free http://casinoslotsdns.com/# caesars free slots free casino games for fun slots games
organic cbd http://cbdoiltincturesws.com/# just cbd gummies cbd vs hemp walgreens cbd products
pure cannabis oil for sale http://cbdoilwalmartww.com/# – thc vape juice where can i buy cbd oil near me level select cbd
http://cbdoilgummiesxl.com/# cdb oils http://cbdoilgummiesxl.com/# – cbd oils medterra cbd
cbd retailers near me http://cbdoilgummyxs.com/# charlotte web cbd oil cannabidiol cbd honey
cbd gummies buy cbd hemp oil cbd oil store
http://cbdoilgummyxs.com/# cbd oil online cbd cream cbd for dogs cbd online
firekeepers casino casino slot machine games casino bonus all games list free slots
http://cbdhempoilwr.com/# turmeric cbd oil level select cbd 100 pure cbd oil walmart cbd products
cbd oil dogs http://cbdoilforpainer.com/# cbd hemp oil for pain cbd oil for diabetes cbd oil brands
casino games slots free http://onlinecasinoweo.com/# – play slots for real money united states free casino games for fun new online casinos cleopatra slots
http://cbdoilgummyxs.com/# medterra cbd cbd gummies walmart cbd capsules
http://cbdoilgummyxs.com/# cbd cream hemp cbd cbd oil online
http://cbdoilgummiesxl.com/# cannabis oil http://cbdoilgummiesxl.com/# – cbd oil cbd gummies walmart
penny slots for free online free 777 slots no download royal river casino best time to play slot machines
cbd pure cbd vape cbd store
who sells the best cbd oil cbd distributors cbd shop cbd online store
http://onlinecasinouse.com/# slot games free http://onlinecasinouse.com/# – vegas slots casino casino play
lady luck casino caruthersville freeslots.com slots turning stone online slots
best cbd oil cbd vape cbd online
hemp gummies http://bestcbdoilshp.com/# – where to buy cbd oil for dogs what is cbd oil good for level select cbd oil cbd for insomnia
hemp oil for pain buy cbd oil cbd oil cbd vape http://cbdoilgummiesxl.com/# – cbd oil for dogs
http://cbdoilgummiesxl.com/# best cbd cream for pain http://cbdhempht.com/# – nuleaf naturals cbd hemp oil for pain
what is cbd good for http://cbdhempht.com/# – what is cbd oil cbd and anxiety cbd cartridges spruce cbd oil
new no deposit casinos accepting us players http://casinoslotsjon.com/# slots online free free casino blackjack games casino slots
cannabinol cbdoilaide best cbd oil cbd distributors cbd companies http://cbdhempht.com/# – thc oil for sale
cbd wholesale http://bestcbdoilopp.com/# hemp lotion just cbd gummies best rated cbd cream for pain relief
http://cbdoilgummyxs.com/# cbd vape cbd medic buy cbd
http://cbdoilgummyxs.com/# cbd cream hemp oil for pain cdb oils cbd gummies walmart
free slots just for fun no money http://onlinecasinoweo.com/# – slots free online casino 100 free casino no deposit online casino games
slots for real money http://onlinecasinostre.com/# real casino slot machine games casinos online free penny slots no download
http://onlinecasinouse.com/# 888 casino online free slot games hollywood slots hollywood casino online
cbd oil for inflammation cbd lotion for pain what is hemp extract cbd gel caps
online casinos real money free slots no download no registration free video slots online casino
http://bestcbdoilopp.com/# cbd oil in texas http://cbdhempht.com/# – what is hemp extract cbd oil cannabidiol for pain
cbd tinctures cbd for dogs buy cbd cbd capsules http://cbdoilgummiesxl.com/# – buy cbd oil
http://cbdoilgummiesxl.com/# cbd oil http://cbdoilgummiesxl.com/# – cbd oil store cbd for sale
http://cbdoilgummiesxl.com/# buy cbd http://cbdoilgummiesxl.com/# – cbd store best cbd oil buy cbd oil
http://cbdoilgummiesxl.com/# cbd oil online cbd oil hemp cbd oil cbd oil
cdb oils cbd vape cbd oils buy cbd
http://cbdoilgummyxs.com/# buy cbd hemp cbd oil cbd oil for sale
what is cannabis oil hemp gummy bears hempworx cbd daily intensive cream http://cbdoilcreamww.com/# – defy cbd drink
walmart cbd oil for pain cbd oil for cats cbd cannabis cbd superbugs http://cbdoilgummyxs.com/# – cbd drug test
cbd oil store http://cbdoilgummiesxl.com/# cbd online cbd pills cbd oil online
free slots hollywood slots games free vegas casino free online games online casino slots no download
buy hemp oil cbd vape cbd hemp cannabis oil cbd hemp
cbdoilaide best cbd oil http://cbdoilforpainer.com/# – cbd oil stocks best cbd oil for pain cbd oil for dogs benefits
does cbd show up in drug test http://cbdoilcreamnk.com/# – cbd gummies amazon walmart cbd oil where to buy cannabis oil does cbd oil work
http://onlinecasinoweo.com/# casino game free slots vegas free slot play free slots no registration
penny slots for free online doubledown casino online casino no deposit free welcome bonus free slots online no download no registration
free 777 slots no download new no deposit casinos accepting us players online casino bonus
free slots games slotomania free slots free casino slots with bonus no deposit win real cash http://casinoslotsdns.com/# – vegas slots online
cbd oil gummies http://bestcbdoilopp.com/# – hemp extract benefits what is hemp your cbd store hemp products
cbd cbd pills hemp cbd buy cbd oil online
cbd oil at walmart hemp cbd cbd hemp best cbd oil buy cbd oil
cbd oil online cbd gummies walmart buy hemp oil cbd oil benefits http://cbdoilgummiesxl.com/# – cbd oil store
http://cbdoilgummiesxl.com/# cbd oils cbd oil at walmart buy hemp oil cbd vape
cbd gummies walmart http://cbdoilgummiesxl.com/# – cbd oil store hemp oil best cbd oil
http://cbdoiltincturesws.com/# where to buy cbd cbd oil tincture where to purchase hemp oil hemp products
best cbd gummies cbd oil best cbd oil select cbd hempworks
cbd balm for pain http://cbdoiltincturesws.com/# – cbd tinctures vaping cbd oil cbd stores near me
rock n cash casino slots http://casinoslotsjon.com/# real casino 888 casino nj slots games vegas world
buy cbd oil online cdb oils hemp cbd oil best cbd oil
cannabis oil http://cbdoilgummyxs.com/# cbd pills cbd oils cbd gummies
cbd side effects http://cbdoilgummiesxl.com/# most reputable cbd oil supplier cbd anxiety cbd gummies amazon
free games online no download hearts of vegas free slots foxwoods casino online
http://cbdoilgummiesxl.com/# charlottes web cbd oil http://bestcbdoilshp.com/# – caligarden cbd oil reviews where to find cbd oil in walmart
200 free slot casino games http://onlinecasinostre.com/# tropicana online casino online gambling casino foxwoods casino online
old vegas slots http://onlinecasinouse.com/# – real vegas casino games free best free slots vegas world casino games online
http://cbdoilgummyxs.com/# hemp cbd cbd oil online hemp oil for pain
cbd dog treats cbd medical hemp gummy bears
dakota sioux casino http://casinoslotsdns.com/# hot shot casino slots free video slots online casino slots no download
http://cbdoilcreamnk.com/# where to buy cbd http://bestcbdoilopp.com/# – cbd capsules walmart cbd gummies dosage
cbd gummy http://cbdoilstoreww.com/# what is cbd oil used for cbd oil drug interactions how to make cbd oil
http://cbdhempht.com/# top cbd companies http://cbdhempht.com/# – dog cbd oil best cbd capsules
cannapro cbd http://cbdoilgummyxs.com/# – cbd treats for dogs defy cbd drink cbd distributors
cbd store http://cbdoilgummiesxl.com/# cbd capsules cbd oil online buy cbd
cbd gummies buy cbd oil cbd hemp cdb oils http://cbdoilgummiesxl.com/# – cbd capsules
hemp oil for pain cbd tinctures best cbd oil cbd store http://cbdoilgummiesxl.com/# – cbd tinctures
free slot games http://casinoslotsjon.com/# vegas casino online lady luck casino vicksburg free casino games
cbd gummies cbd cream buy hemp oil hemp oil for pain http://cbdoilgummiesxl.com/# – cbd pure
cbd oil benefits cbd oil at walmart best cbd oil cbd for sale
buy cbd pure cbd oil cbd store cbd oil store
hemp oil cbd tinctures cbd drops cbd oils
best cbd capsules for pain cbd oil 500 mg 49 yaa health store cbd cream for pain relief walgreens cbd oil stocks
strongest cbd gummies for sale http://bestcbdoilopp.com/# – cbd lotion for pain relief does cbd show up in drug test cbd information cbd vape juice
http://onlinecasinoweo.com/# totally free slots no download free slot machine games vegas casino online play casino
cbd gummy bears http://bestcbdoilopp.com/# – cbd oil for anxiety best cbd capsules cbd stores near me
Air Jordan 1 Mid
http://www.airmaxclearancesale.us/ Air Max Clearance Sale
online gambling casino slots free online casinos near my location jackpot magic slots http://onlinecasinostre.com/# – free games online no download
free slot games http://onlinecasinouse.com/# – free casino games best online casinos slot games
trubliss cbd what is hemp oil used for cbd lotion for pain relief cbd oil reviews
http://casinoslotsdns.com/# da vinci diamonds free online slots http://casinoslotsdns.com/# – list of las vegas casinos connect to vegas world
cbd tinctures cbd oil online cbd pure buy cbd oil online
what is cannabis oil cbd distillate cbd md
cbd oil cbd capsules cbd drops cbd gummies cbd pure
lazarus naturals defy cbd cbd oil drops national hemp association http://bestcbdoilopp.com/# – trubliss cbd gummies
cbd oil benefits cbd online cbd cbd cream
cbd side effects http://cbdhempoilwr.com/# – hempvive cbd oil benefits of hemp buycbdoilbest cbd oil
http://casinoslotsjon.com/# real money casinos http://casinoslotsjon.com/# – 100 best usa casinos with best codes vegas casino games slots free
cbd pure http://cbdoilgummiesxl.com/# – cbd tinctures hemp oil buy cbd oil
buy cbd cbd for dogs cbd oil for sale cbd
cbd tinctures cbd products cbd near me
http://onlinecasinoweo.com/# play free lucky 777 slots http://onlinecasinoweo.com/# – free online casino games vegas free slot games for fun
hemp cbd http://cbdoilgummiesxl.com/# cbd oils cbd online cbd oil for sale
cbd definition cbd pure hemp oil cbd lotion for pain relief how to use hemp oil
cbd cartridges http://cbdoilcreamww.com/# – uses for cbd oil lazarus cbd oil thc oil for sale
medterra cbd cbd medic cbd pills
slots farm http://onlinecasinostre.com/# best online casino konami free slots hollywood casino
hemp oil for pain cbd drops cbd
cbd capsules cbd cream cbd gummies walmart hemp cbd
slots of vegas casino vegas world casino games online casino games
cbdpure cbd marijuana the cbd store
cbd oil cbd gummies http://bestcbdoilopp.com/# – cbd meaning best cbd cream for pain relief hemp seed oil
slots lounge penny slots for free online empire city online casino
cannabis sativa oil cbd medical abbreviation cbd store near me cbd dosage http://cbdhempoilwr.com/# – cannabis oil for pain
cbd topicals for pain my daily choice hempworx zilis cbd oil
free slot games no download no registration http://casinoslotsjon.com/# atari vegas world free slots zone online casino vegas world
http://cbdoilcreamnk.com/# cbd thc http://cbdhempht.com/# – cbd cartridges hemp bombs cbd
buy cbd oil online pure cbd oil buy cbd oil online
http://cbdoilgummyxs.com/# buy hemp oil hemp cbd buy hemp oil
where to find cbd oil in walmart cbd side effects 100 pure cbd hemp oil how to use cbd oil http://cbdhempoilwr.com/# – pure cannabis oil for sale
http://cbdoilstoreww.com/# does cbd work http://cbdoilwalmartww.com/# – cbd plus cbd heroin addiction
play free slots casino games free free slots no download online casino gambling
cbd products http://cbdoilgummiesxl.com/# best cbd oil buy cbd oil hemp oil cbd capsules
best cbd oil buy cbd oil http://cbdoilgummiesxl.com/# – best cbd oil pure cbd oil cbd for dogs cbd tinctures
http://cbdoilstoreww.com/# cbd cream for pain relief charlotte web cbdistillery does cbd get you high
http://cbdoilgummyxs.com/# medterra cbd cbd medic cbd gummies walmart cannabis oil
http://cbdoilgummiesxl.com/# cannabis oil cbd oils cbd vape hemp cbd oil
cbd for sale http://cbdoilgummiesxl.com/# – medterra cbd buy hemp oil cbd drops pure cbd oil
http://onlinecasinostre.com/# play free slot 200 no deposit bonus usa slots for free free slots online no download no registration
cbd medic cbd pure cbd oil store cbd vape
http://onlinecasinouse.com/# vegas slots http://onlinecasinouse.com/# – bovada casino play online casino
100 best usa casinos with best codes free casino blackjack games free casino games slotomania
http://cbdoilgummyxs.com/# best cbd oil for pain cbd oil full spectrum yaa health store best cbd gummies on amazon how to take cbd oil
how much is cbd oil http://cbdoilcreamww.com/# – does cbd oil have thc zilis ultracell green mountain cbd
http://cbdoilgummyxs.com/# hemp cbd oil cbd capsules cbd tinctures cbd hemp
buy cbd oil buy cbd oil buy cbd
http://casinoslotsjon.com/# slots free http://casinoslotsjon.com/# – jack online casino borgata online casino
http://cbdhempoilwr.com/# hempworx cbd http://cbdoilcreamnk.com/# – hemp worx strongest cbd gummies for sale
online casino bonus free slots hollywood hollywood casino casino games free slots
cbd oil 500 mg 49 yaa health store http://cbdoilcreamnk.com/# – cbd gummies for sale nutrax cbd oil how do you use cbd oil natural hemp
cbd tinctures http://cbdoilgummiesxl.com/# hemp oil hemp cbd oil cbd oil store
benefits of hemp seed oil health benefits of cbd oil thc cbd does walgreens sell cbd oil
http://cbdoilgummiesxl.com/# best cbd oil buy cbd oil http://cbdoilgummiesxl.com/# – cbd drops cbd near me
free casino games online http://onlinecasinouse.com/# – free casino slots with bonuses hollywood casino online sugarhouse casino online
casino slot machine games http://casinoslotsdns.com/# – vegas slots online scatter slots big fish casino slots zone online casino games
http://cbdoilgummiesxl.com/# cbd tinctures http://cbdoilgummiesxl.com/# – cbd oil online cbd oil
http://cbdoilforpainer.com/# top cbd companies cbd oil stores near me cbd topical cream does cbd oil work
cbd drops hemp cbd oil cbd oil at walmart cbd capsules cbd pills
totally free casino games http://casinoslotsjon.com/# – casino bonus slot machine free free slots
thc cbd http://cbdoilgummyxs.com/# best rated cbd cream for pain relief cbd stores near me where to buy cbd oil
walmart cbd products http://cbdoilgummiesxl.com/# – cbd daily intensive cream cbd softgels cbd distillery
http://cbdoilforpainer.com/# hempworx 750 http://cbdoilforpainer.com/# – dog cbd cbd oil walmart
best cbd gummies for pain http://cbdoiltincturesws.com/# cbd and sleep cbd full spectrum full spectrum cbd oil
http://onlinecasinoweo.com/# caesars free slots parx online casino free online casino games free slots casino games
Affordable. Practical.
cbd for dogs cbd for dogs cbd oil online
cbd stands for http://cbdoilgummiesxl.com/# – best cannabis oil for arthritis cbs oil cbd drug test
cbd oil for sale buy cbd oil cbd for dogs cbd oil store
100 best usa casinos with best codes http://onlinecasinostre.com/# – free online slots no download maryland live casino online 100 free casino no deposit
http://onlinecasinouse.com/# free slots online 888 casino download free games online slots plainridge casino
caesar casino online slot games http://casinoslotsdns.com/# – da vinci diamonds free online slots free slot games no download no registration hearts of vegas free slots
cbd cbd oil store cbd drops pure cbd oil
http://cbdoilgummiesxl.com/# cbd oil for dogs cbd tinctures cbd tinctures cbd capsules
project cbd justcbd cbd for pain relief how much is cbd oil http://cbdoilgummyxs.com/# – cbd oil where to buy cbd oil
cbd drops http://cbdoilgummiesxl.com/# cbd capsules cbd capsules cbd oil for pain
uses for cbd oil http://cbdoilforpainer.com/# does hemp have thc just cbd cbd information
tru bliss cbd gummies cannabidiol cbd strongest cbd gummies for sale cbd powder
hemp oil for pain http://cbdoilgummiesxl.com/# cdb oils pure cbd oil cbd store
http://cbdoilgummyxs.com/# buy hemp oil cbd oil for sale cannabis oil
medterra cbd cannabis oil cbd medic cbd oil store
free vegas casino games http://casinoslotsjon.com/# – free slots no download no registration zeus caesars casino online slotomania free slots us online casinos for real money
hemp cigarettes cbd oil near me is cbd oil safe thc and cbd http://cbdoilgummyxs.com/# – cbd definition
http://cbdoilgummyxs.com/# cbd cream buy hemp oil cbd gummies
casino near me chumba casino slots casino games
charlotte’s web cbd oil cbd heroin addiction cbd oil and cancer legitimate cbd oil companies
caligarden cbd oil reviews what is cbd full spectrum cbd cbdistillery http://bestcbdoilshp.com/# – cbd health benefits
cbd benefits http://cbdoiltincturesws.com/# – walmart cbd products cbd cream for pain where to buy cbd cream for pain where to purchase hemp oil
cbd tincture benefits http://cbdoilwalmartww.com/# – best cbd oil for anxiety highest rated cbd oil for sale select cbd oil
cbd cbd oil benefits cbd oil store cbd http://cbdoilgummiesxl.com/# – cbd cream
free coins slotomania http://onlinecasinostre.com/# free games for casino slots hollywood big slots games for free pch slots tournament
penny slots best time to play slot machines play free vegas casino games
cbd medic cbd gummies cbd oil for sale cbd drops cbd oils
cbd tinctures cbd tinctures cbd drops cbd medic buy hemp oil
cbd oil http://cbdoilgummiesxl.com/# – cbd tinctures cbd products hemp cbd oil
what is hemp oil used for cbd oil vape cannabis oil benefits cbd cream for arthritis
best cbd oil buy cbd oil http://cbdoilgummiesxl.com/# – cbd oil for pain cbd oil for dogs cbd pills
cbd gummies walmart buy cbd oil online cdb oils
Hi would you mind letting me know which webhost you’re utilizing?
I’ve loaded your blog in 3 completely different web browsers and I must say
this blog loads a lot quicker then most. Can you suggest a good internet hosting
provider at a fair price? Many thanks, I appreciate it!
cbd for sale cbd oil at walmart cbd oil online
best online gambling sites for real money http://casinoslotsjon.com/# free slots online no download free buffalo slots real casino slot machine games
http://cbdoilgummyxs.com/# hempworx cbd oil http://cbdoilforpainer.com/# – how does cbd work the best cbd oil on the market
cbd oil dosage recommendations global cannabinoids koi cbd oil cbd pain relief http://cbdhempoilwr.com/# – cbd vs hemp oil
http://cbdoiltincturesws.com/# hemp oil for pain relief water soluble cbd cbd oil prices cbd salve
vegas world free games world class casino slots can play zone casino free
cbd gummies cbd online hemp cbd oil hemp oil for pain
cbd hemp cbd oil at walmart cbd store
cbd oil online http://cbdoilgummiesxl.com/# cbd cbd online cannabis oil
http://cbdoilgummiesxl.com/# cannabis cream http://cbdhempht.com/# – cbd stocks does cbd oil show up on drug test
download free casino games doubledown casino free slots vegas world casino games free
free casino for fun only online casino games free empire city casino online free zone online casino games
best online gambling sites for real money real casino slot machine games big fish casino free online
http://cbdoilgummyxs.com/# buy cbd oil cbd oils cbd cbd tinctures
Air Max Clearance Sale
http://www.adidasyeezyofficialwebsite.com/ Adidas Yeezy Official Website
cbd capsules walmart best cbd cream for pain relief cannativa cbd oil reviews cbd shop http://bestcbdoilopp.com/# – defy cbd drink
dog cbd oil cbd cream for pain relief pure cbd cbdoil
trubliss cbd cbd salve for pain best hemp oil cali naturals cbd http://cbdoilgummyxs.com/# – cbd and anxiety
cbd pure pure cbd oil hemp oil cbd pure http://cbdoilgummiesxl.com/# – cbd drops
where to buy cbd oil near me http://cbdoiltincturesws.com/# – cbd oil and drug testing just cbd medterra cbd oil health benefits of cbd oil
http://cbdoilgummyxs.com/# cbd vape cbd oil store best cbd oil
online slot machines http://onlinecasinoweo.com/# goldfish casino slots free grand falls casino wizard of oz slots
http://bestcbdoilopp.com/# cbd vape cartridges http://cbdhempoilwr.com/# – cbd distributors cbd oil 1000 mg 79 yaa health store
cbds http://cbdoilwalmartww.com/# – cbdoilaide best cbd oil cbd store near me legitimate cbd oil companies cbd gel caps
http://onlinecasinostre.com/# free online casino games da vinci diamonds free online slots slot games free play slots for real money
hemp cbd oil http://cbdoilgummiesxl.com/# – hemp oil hemp cbd cbd for sale
hemp lotion http://cbdoilcreamnk.com/# – pure hemp benefits of hemp seed oil cbd md vaping cbd
hollywood casino online slots http://onlinecasinouse.com/# – free online casino games vegas slots online free caesars slots
http://casinoslotsdns.com/# borgata online casino online casinos for us players liberty slots casino absolutely free slots
cbd oil online cbd gummies cbd oil at walmart cbd store cbd oil benefits
cbd store cbd store cbd drops hemp cbd oil cbd oil store
http://cbdoilgummiesxl.com/# pure cbd oil cbd store pure cbd oil cbd
http://casinoslotsjon.com/# vegas world casino games free http://casinoslotsjon.com/# – caesar casino free slots games hollywood casino online slots free
cbd tinctures http://cbdoilgummyxs.com/# cbd gummies walmart cannabis oil cbd oil for dogs
http://cbdoilwalmartww.com/# cbd clinic products cbd information cbd cream for pain relief cbd pen
cbd dosage for dogs cbd oil drug interactions cbd dosage cbd for pets
buy hemp oil cbd for sale cbd gummies
cannativa cbd oil reviews http://bestcbdoilshp.com/# cbd stands for nuleaf naturals cbd organic cbd
cbd cream http://cbdoilgummiesxl.com/# – hemp cbd best cbd oil buy cbd oil cbd online hemp oil for pain
http://cbdoilgummiesxl.com/# cbd products http://cbdoilgummiesxl.com/# – buy cbd oil cbd store
http://onlinecasinoweo.com/# casino vegas world online slots free no deposit bonus codes for usa players free slots casino games
http://cbdoilstoreww.com/# hemp extract benefits cbd definition my daily choice hempworx cbd edibles
keen sandalen sale
outdoor cat house with heat lampnike mercurial superfly 111adidas advertising campaign historyrg59 coaxial cable diameter
cbd oil drug interactions cbd oil where to buy cbd oil best cannabis oil for arthritis best price for cbd oil http://cbdoilforpainer.com/# – cbd hemp oil benefits
cbd oil at walmart cbd oil cbd oil for sale pure cbd oil
cbd gummies walmart buy hemp oil hemp oil cbd for sale
slot machine free free casino blackjack games 200 no deposit bonus usa free slots slotomania
cbd products cbd oil store cbd gummies walmart
play real casino slots free turning stone online slots free slots slot machines http://onlinecasinouse.com/# – las vegas free penny slots
hollywood casino online slots free http://casinoslotsdns.com/# – 200 free slot casino games list of online casinos for us players online casinos for us players gambling sites
cbd pills for pain cbd gummies amazon cbd oil anxiety cbd distributors
what is cbd oil good for http://bestcbdoilopp.com/# – cbd gummies reviews cbd oil effects cbd retailers near me
http://casinoslotsjon.com/# online slot machines list of online casinos for us players play free slots for fun caesars free slots online
best cbd oil buy cbd oil http://cbdoilgummiesxl.com/# – cbd store cbd near me cbd capsules
cbd oil benefits http://cbdoilgummyxs.com/# cbd pills buy cbd cbd near me
http://cbdoilgummyxs.com/# cbd products cbd oil benefits buy cbd cbd gummies
online gambling casino gossip slots free casino blackjack games
hemp oil for pain cbd hemp cbd oil for dogs cbd vape
cbd oil for insomnia http://cbdoilcreamnk.com/# hemp seed oil benefits cbd dispensaries near me zilis cbd oil
buy cbd oil http://cbdoilgummiesxl.com/# – cbd cbd vape cbd store
best cbd oil on amazon http://cbdoilstoreww.com/# – cbd balm for pain cannapro cbd hempoil cbd superbugs
cbd meaning http://cbdhempht.com/# – cbd oil full spectrum yaa health store hemp vs cbd cbd oil full spectrum yaa health store cbd marijuana
pure cbd oil cbd oil cbd oils cbd oil at walmart
http://cbdoilforpainer.com/# cbd clinic products http://cbdoilcreamww.com/# – where can i buy cbd oil where to find cbd oil in walmart
hemp oil http://cbdoilgummiesxl.com/# cbd gummies walmart cbd online cbd oil for pain
real money casino bonus casino slots online free free las vegas slot machines
pala casino online big fish casino slots hollywood casino free slot play chumba casino
free casino slots no download http://casinoslotsdns.com/# – posh casino online slots games vegas world play free lucky 777 slots no deposit casino
http://cbdoilwalmartww.com/# cbd powder http://cbdoilgummyxs.com/# – best cbd gummies for pain cbd pure hemp oil
best cbd oil cbd cream http://cbdoilgummyxs.com/# – cbd lotion for pain relief cbdoil does walgreens sell cbd oil cbd oil drug interactions
cbd store cannabis oil cannabis oil buy cbd oil
parx casino online casino slots las vegas free penny slots casino games free online http://casinoslotsjon.com/# – casinos online
Howdy! canadian online pharmacy viagra online pharmacies with out prescriptions online pharmacies canada legitimate
cbd stock http://cbdoiltincturesws.com/# – where to purchase cannabis oil cbd tincture benefits cbd hemp oil cbd for cats
cbd capsules for pain http://bestcbdoilshp.com/# – cbd research cbd distillate best cbd cream for pain
cbd oil store cbd online cannabis oil best cbd oil http://cbdoilgummiesxl.com/# – cbd tinctures
cbd distillery cbd isolate powder organic cbd oil cbd oil for weight loss http://cbdoilcreamww.com/# – what are the benefits of cbd oil
sugarhouse casino online http://onlinecasinoweo.com/# – free slots no download no registration needed free online slots no download free online slots no download
cbd tinctures cbd oil cbd vape cbd for dogs http://cbdoilgummiesxl.com/# – cbd gummies
hemp oil for pain http://cbdoilgummiesxl.com/# buy hemp oil cbd gummies cbd oil benefits
cannabinol hemp products cbd gel caps how do you use cbd oil http://bestcbdoilopp.com/# – cbd roll on
http://cbdoilgummyxs.com/# cbd tinctures best cbd oil cbd oil for sale cbd
http://cbdoilgummyxs.com/# hemp cbd cbd online hemp cbd oil
free slots games http://onlinecasinostre.com/# play free slots 300 free slots no download free casino games slot machines
cbd oil for pain cbd online cbd gummies walmart
marijuana gummies http://cbdoiltincturesws.com/# – royal cbd oil how to make cbd oil where to buy cbd gummies
http://onlinecasinouse.com/# grand falls casino hollywood casino online slots msn games zone online casino hot shot casino slots
play online casino games http://casinoslotsdns.com/# free slots no download vegas casino slots vegas world casino games
oregon cbd http://cbdoilgummiesxl.com/# – cbd distillate cbd oil price benefits of cbd
http://cbdoilgummyxs.com/# cbd oil store best cbd oil buy cbd oil cbd oil for sale
http://casinoslotsjon.com/# las vegas casinos free slots with no download or registration free slots with no download or registration high five casino slots
pure cbd oil cbd capsules pure cbd oil cbd tinctures http://cbdoilgummiesxl.com/# – cbd store
cbd oil prices hempworx cbd oil pure kana
online slot games http://onlinecasinoweo.com/# – slot machines for home entertainment online casino reviews best online casino best online gambling sites for real money
pijama suit how i met your mother
macbook pro 鍏呴浕 鍣?绱旀銈儍銉椼儷 銉炪偢銉冦偗 銉炪偊銈?2椋熷櫒娲椼亜 姗?瀹夈亜銉嬨儱銉笺儛銉┿兂銈?m635
hempworx cbd http://bestcbdoilopp.com/# best cbd capsules hemp oil vs cbd oil cbd wax
http://cbdoilgummyxs.com/# cbd near me hemp cbd oil buy cbd oil online
http://cbdoilgummiesxl.com/# cbd for dogs http://cbdoilgummiesxl.com/# – best cbd oil cbd
cbd oil for dogs http://cbdoilgummiesxl.com/# – cbd drops buy cbd buy cbd oil
cbd oil online cbd oil store cbd gummies walmart cbd oil for sale
http://cbdoiltincturesws.com/# trubliss cbd cbd gel caps where to purchase cbd oil cbds
http://onlinecasinostre.com/# lady luck casino free games free penny slots online vegas casino games slots free download free casino games
las vegas free slots http://onlinecasinouse.com/# free games online no download big slots games for free play slots for real money
cbd oil benefits cbd pills cbd oil at walmart pure cbd oil http://cbdoilgummiesxl.com/# – cbd oil online
free games online no download no registration free slots no registration goldfish casino slots free
http://cbdoiltincturesws.com/# cbd oil where to buy cbd oil http://cbdoilcreamww.com/# – dog cbd oil cbdpure
http://cbdoilgummyxs.com/# cbd for sale cbd tinctures medterra cbd
http://cbdhempht.com/# royal cbd oil http://bestcbdoilshp.com/# – cbd oil interactions with medications caligarden cbd oil reviews
thc oil http://cbdoilgummiesxl.com/# – cbd stocks cbd oil for diabetes cbd cream for pain relief
cbd cbd oil online buy cbd cbd pills
las vegas free slots wizard of oz slots play free slot
cbd oils http://cbdoilgummiesxl.com/# – hemp cbd oil hemp cbd cbd oil online
http://onlinecasinoweo.com/# slotomania free slots free casino slots no download free slots games online casino games no download no registration
windows mac 鐒$窔 銉嗐兂銈兗
air max 1 kaki femmeadaptateur casque pc ps4tapis citroen c3 aircrosslunette de soleil homme alain afflelou
http://cbdoilgummyxs.com/# cbd gummies walmart pure cbd oil buy hemp oil cbd oil for pain
http://cbdoilcreamnk.com/# how to use cbd oil for pain http://cbdoilforpainer.com/# – cbd dispensaries near me cbd tincture benefits
cdb oils http://cbdoilgummiesxl.com/# buy cbd oil online cbd vape cdb oils
cannabis oil cbd drops cbd online
cbd pure cbd products cbd cannabis oil http://cbdoilgummiesxl.com/# – cbd online
slots free games http://onlinecasinostre.com/# hollywood casino online slots free online casino reviews all free casino slots
http://onlinecasinouse.com/# slots online usa online casino free slots no registration pch slots tournament
http://casinoslotsdns.com/# free slots no download http://casinoslotsdns.com/# – empire city casino online free all free casino slot games
http://cbdoilgummyxs.com/# cbd medic cbd pills cbd oils
cannabis oil buy cbd oil online cbd store
free online games that pay real money http://casinoslotsjon.com/# – free casino games online empire city casino online free pop slots casino real casino games slots free
Nike Outlet
http://www.airjordan-1.us/ Air Jordan 1
cbd gummies http://cbdoilgummiesxl.com/# buy cbd oil best cbd oil buy cbd oil hemp cbd
cbd near me cbd pure hemp oil
http://cbdoilgummyxs.com/# cbd pure hemp cbd cbd capsules
http://onlinecasinostre.com/# free slots no download http://onlinecasinostre.com/# – buffalo slots no download no registration slots
gambling sites vegas casino games free slots no download no registration caesars free slots online http://onlinecasinouse.com/# – free 777 slots no download
http://cbdoilgummiesxl.com/# cbd near me cbd pure best cbd oil cbd pills
http://casinoslotsdns.com/# zone online casino free casino games no registration no download casino vegas world free casino games slot machines
cbd for dogs http://cbdoilgummiesxl.com/# cbd hemp best cbd oil buy cbd oil cbd tinctures
http://cbdoilgummyxs.com/# hemp cbd hemp cbd buy hemp oil
cbd oil http://cbdoilgummiesxl.com/# – cbd store hemp cbd cbd store cbd near me
http://cbdoilgummiesxl.com/# cbd http://cbdoilgummiesxl.com/# – cbd oil at walmart cbd gummies
free casino blackjack games vegas casino games slots free 200 no deposit bonus usa online gambling casino
free games online no download no registration http://onlinecasinoweo.com/# – maryland live casino online vegas world free slots games free casino games slot vegas free slots online
http://cbdoilgummyxs.com/# cbd vape cbd oils cbd gummies hemp cbd
hemp cbd oil cbd cream hemp cbd oil
free vegas world slots play free mr cashman slots lady luck casino vicksburg firekeepers casino http://casinoslotsdns.com/# – online casino games free
http://cbdoilgummiesxl.com/# cbd near me cbd pure hemp cbd cbd for dogs
buy cbd oil online cbd pills hemp cbd oil
cbd oil benefits http://cbdoilgummiesxl.com/# cbd gummies cbd pills cannabis oil
http://casinoslotsjon.com/# vegas world casino games caesar casino free slots games list of online casinos for us players hypercasinos
cbd oil store http://cbdoilgummiesxl.com/# cbd oil for sale cbd cbd oils
new online casinos accepting usa http://onlinecasinoweo.com/# – da vinci diamonds free online slots hollywood casino free slots online best free slots no download best place to gamble in vegas
cbd http://cbdoilgummiesxl.com/# – cbd oil for sale cbd oil benefits cannabis oil
cbd gummies walmart cbd oil for sale buy cbd cbd for dogs cbd oils
http://cbdoilgummyxs.com/# cbd cbd cream cbd oil benefits cbd pure
gold fish casino slots http://onlinecasinostre.com/# – free casino slot games online casino bonus free slots games casino games
slot games http://casinoslotsdns.com/# vegas slots online free casino games free slots
free casino games http://casinoslotsjon.com/# – free casino games casino real money casino blackjack
cbd oil for sale http://cbdoiltincturesws.com/# – cbd oil for sale cbd oil for pain cbd medic cbd for sale
cbd products http://cbdoiltincturesws.com/# cbd tinctures cbd medic cbd oil for sale
free casino http://onlinecasinoweo.com/# – play slots slots games free real money casino free casino games
buy cbd http://cbdoiltincturesws.com/# – cbd for sale cbd cbd near me buy cbd oil online
best cbd oil buy cbd cbd capsules cbd cbd oil online
cbd for dogs http://cbdoiltincturesws.com/# cbd tinctures cbd oil store cbd cream
http://cbdoiltincturesws.com/# cbd hemp http://cbdoiltincturesws.com/# – cdb oils pure cbd oil
http://onlinecasinostre.com/# real casino slots http://onlinecasinostre.com/# – casino game gold fish casino slots
free casino slot games http://onlinecasinouse.com/# casino slots vegas slots online online casino gambling
wiggle nike pegasus 30
銉戙偨銈炽兂 銉儖銈裤兗 瑙掑害銈炽儫銉?銈ゃ兂銉娿兗銈︺偋銈?hit air 銉忋兗銉嶃偣銉┿兂銉?銈枫偋銉笺儔 寮垫浛銇?/a>銈兗銈?銉戙兂銉?浼哥府 鎬?/a>
casino slots slots games casino bonus codes online casino real money http://casinoslotsdns.com/# – casino game
cbd medic cbd oil online cbd near me
http://cbdoilcreamww.com/# cbd oil for sale cbd for sale medterra cbd
casino games http://casinoslotsjon.com/# – slots online slots online slots games online casinos
free online slots big fish casino slots for real money
cbd hemp http://cbdoiltincturesws.com/# – hemp cbd pure cbd oil cbd pure
http://cbdoiltincturesws.com/# cannabis oil cbd pills cbd store best cbd oil
cbd hemp http://cbdoiltincturesws.com/# – hemp oil for pain cbd hemp buy cbd oil online
cbd medic cbd oil for sale best cbd oil buy cbd oil cbd cream
hemp cbd oil http://cbdoilcreamww.com/# buy hemp oil cbd oil benefits cbd cream
hemp cbd http://cbdoilcreamww.com/# buy cbd oil cbd oil for pain cbd capsules
cashman casino slots free slots games play slots
sudadera hombre sportswear tech fleece nike
銈广優銉?鏈涢仩閺?鍘熺悊銉曘儵銈ゃ儜銉?瑁?姹氥倢鍐峰噸搴?鏍煎畨銈点兂 銉戙儵銈姐儷 銈搞儯銈ゃ偄銉炽儓 銈般儶銉笺兂 銈兗銉嗐兂
best cbd oil buy cbd oil cbd oil for pain cbd medic cbd oil benefits
real money casino online casino slots for real money
Hi there! I could have sworn I’ve visited this blog before but after browsing through some of the articles
I realized it’s new to me. Anyhow, I’m definitely pleased I found it and I’ll be book-marking it
and checking back often!
cbd oil for dogs cbd tinctures cbd oil for pain best cbd oil buy cbd oil buy hemp oil
best cbd oil buy cbd oil cbd pills cbd online buy cbd cbd gummies
http://cbdoiltincturesws.com/# cbd oil at walmart http://cbdoiltincturesws.com/# – cbd oil for dogs buy cbd
cbd hemp cbd online buy cbd oil buy cbd oil http://cbdoiltincturesws.com/# – buy hemp oil
http://cbdoiltincturesws.com/# cbd oil online http://cbdoiltincturesws.com/# – cbd tinctures hemp oil
http://cbdoiltincturesws.com/# cbd gummies cbd oil online cbd hemp cbd cream
free casino games online http://onlinecasinostre.com/# – play slots free casino games online casino play gold fish casino slots
hemp oil for pain best cbd oil buy cbd oil cbd medic cbd vape cbd tinctures
http://casinoslotsdns.com/# online casino slots http://casinoslotsdns.com/# – online casino bonus free casino games online
Yeezy
http://www.kobebryantjersey24.us/ Kobe Bryant Jersey 24
hemp oil for pain cbd for sale cbd cdb oils
cbd online http://cbdoilcreamww.com/# cbd oil at walmart cbd products cbd oil store
casino blackjack http://onlinecasinouse.com/# – slots games free slots online online casino gambling play casino
http://cbdoiltincturesws.com/# cbd store http://cbdoiltincturesws.com/# – buy cbd oil online cbd tinctures
cbd oils hemp cbd oil cbd drops cbd products http://cbdoiltincturesws.com/# – cbd pure
best cbd oil buy cbd oil http://cbdoiltincturesws.com/# – cbd oil for dogs cbd online cbd tinctures buy cbd oil
http://cbdoiltincturesws.com/# cbd oil for pain http://cbdoiltincturesws.com/# – medterra cbd cbd oil store
world class casino slots http://onlinecasinoweo.com/# – free slots online casinos gold fish casino slots
buy cbd oil hemp cbd cbd oil for pain
cbd capsules cbd oil store cbd pills cbd vape
buy cbd cbd vape hemp cbd oil cbd oil http://cbdoiltincturesws.com/# – cbd hemp
no deposit casino casino play casino real money play online casino
online casino slots free casino games online free casino free slots
cannabis oil cbd oil store cbd tinctures cdb oils cbd products
best cbd oil http://cbdoiltincturesws.com/# – cbd oils cbd products cbd oil
slots for real money real money casino online casino slots casino online slots
http://cbdoiltincturesws.com/# cbd pills cbd for sale cbd capsules cbd oil for sale
cbd for sale http://cbdoiltincturesws.com/# cbd vape cbd for sale cbd oil for pain
cbd oils http://cbdoilcreamww.com/# cbd online cbd for dogs cbd for dogs
http://onlinecasinouse.com/# play online casino http://onlinecasinouse.com/# – casino play free casino slot games
free casino http://onlinecasinouse.com/# – online casino real money slots games casino games
casino real money no deposit casino online casino slots
Do you mind if I quote a few of your posts as long as I provide credit and sources back to your site? My blog is in the exact same area of interest as yours and my users would really benefit from some of the information you provide here. Please let me know if this alright with you. Cheers!
Air Max 97
http://www.retrojordan.us.com/ Jordan Retro
medterra cbd http://cbdoiltincturesws.com/# hemp oil cbd cream cbd pills
http://cbdoilcreamww.com/# cbd oil for sale buy cbd oil online cbd gummies
http://cbdoilcreamww.com/# cbd gummies walmart cbd products cbd near me hemp oil
vielzahn xzn satz amazon
air max 90 premium femme dore roseveste en tweed homme avec coudierejordan 1 white black on feetmaillot de bain culture sud
cbd products http://cbdoilcreamww.com/# cbd store cbd pills cbd oil online
polar skate co golf club pullover jacket 2.0 black
lila adidas schuhenew balance cycling shoes menscowboystiefel wei脽schal silber glitzer
cbd pure cbd oils cbd hemp cbd tinctures cbd vape
cannabis oil cbd oil online cbd store cbd oil hemp oil
cbd capsules http://cbdoiltincturesws.com/# – cbd oil for dogs hemp cbd best cbd oil
cbd for sale http://cbdoilcreamww.com/# cbd pure cbd for dogs cbd oil store
buy cbd cbd oil for sale cbd oil at walmart cbd oil for dogs http://cbdoilcreamnk.com/# – cbd gummies
Instagram product and service.
This design is steller! You most certainly know how to keep a reader entertained. Between your wit and your videos, I was almost moved to start my own blog (well, almost…HaHa!) Great job. I really enjoyed what you had to say, and more than that, how you presented it. Too cool!
cbd pills http://cbdhempoilwr.com/# cbd for dogs cdb oils buy cbd oil
http://onlinecasinouse.com/# hypercasinos http://onlinecasinouse.com/# – online casino reviews win free money no deposit
cbd oil benefits hemp cbd oil cbd oil for dogs cbd oil online http://cbdoilcreamnk.com/# – medterra cbd
cbd gummies cbd capsules cbd near me
nike revolution 3 ladies trainers
primeroutlet jeansnike air force volt off white retronike why not white
http://cbdhempoilwr.com/# cbd oil for dogs cbd oil benefits cbd vape buy cbd
Jordan Retro
http://www.jordanaj1.us/ Jordan AJ 1
cbd capsules http://cbdoilcreamnk.com/# – cannabis oil cbd products cbd gummies best cbd oil
kate spade 銉愩儍銈?amazon
dior 鏈€ 瀹夊€?/a>adidas originals cp 80sdiesel 璨″竷 x05660 p1752 h6818銉愩儸銉炽偡銈偓 銈儯銉冦儣 鍋界墿 瑕嬪垎銇?/a>
cbd cbd gummies cbd online
boitier auto amazon
montre homme moderne chicadidas superstar multicolor pride packcombinaison pantalon asymetriquesac aspirateur e201badidas campus black and white mensnike vapormax flyknit 4sortie de bain eponge hommechausson antiderapant enfantboots bout carr茅 hommecei…
cbd oils http://cbdoilcreamnk.com/# – cbd for dogs cbd tinctures cbd drops
buy cbd oil online buy cbd cbd products best cbd oil
cbd online pure cbd oil cbd oil for sale cbd store
best cbd oil http://cbdoilcreamnk.com/# – cbd medic cbd near me cbd pure
http://cbdoilcreamnk.com/# cbd products http://cbdoilcreamnk.com/# – cbd cream cbd pure
cbd oil online http://cbdoilcreamnk.com/# – cbd capsules pure cbd oil cbd pure cbd vape
http://cbdoilcreamnk.com/# hemp cbd cbd near me buy cbd cbd oil
medterra cbd http://cbdhempoilwr.com/# cdb oils medterra cbd cbd near me
cbd oil at walmart cbd products best cbd oil buy cbd oil buy cbd http://cbdoilcreamnk.com/# – cbd for dogs
chapeau de paille png
swissgear 6677 scansmart tsa laptop backpackasics gel nimbus juniorgeox respira kindernike tn running shoes
Jordan Retro 4
http://www.jerseys-nba.us/ Cheap NBA Jerseys
http://cbdhempht.com/# cbd cream http://cbdhempht.com/# – cbd oil for sale cbd oils
cbd buy hemp oil cbd vape cannabis oil cbd near me
samsonite notebook backpack
zapatillas adidas mana bouncejunior ropa infantilvestido mono lunaresadidas zx 8000
hemp cbd cdb oils pure cbd oil cbd oil for dogs hemp cbd oil
Howdy! buy valtrex no rx buy valtrex cheap valtrex online
cbd oil for dogs best cbd oil cbd gummies cbd drops cbd pills
nike cortez rose gold noir
doschool marc o polobms gummistiefel kinderchevrolet caprice fuel economyamy vermont design
NBA Store
http://www.nbaoutletstore.us/ NBA Store
Many posts that are old or outdated can be brought back to life with fresh, up-to-date information.
cbd cbd drops cbd hemp cbd hemp
cbd pure best cbd oil buy cbd oil cbd oil for sale cbd hemp cbd gummies
casino play for free dakota sioux casino can play zone casino free play free blackjack against computer http://onlinecasinouse.com/# – igt free slots
free slots online http://onlinecasinostre.com/# – liberty slots casino slotomania free slots play free slots
monopoly slots free casino games with bonus online slots play free casino games online
100 best usa casinos with best codes http://casinoslotsjon.com/# – casino slot free stn play online casino empire city casino online
Cheap product! Excellent seller!
play free slots buffalo slots slot machine free borgata online casino
http://cbdoilforpainer.com/# cbd oil online cbd oil for pain cbd tinctures cbd gummies
no deposit games online for real cash free slot games no download no registration casino vegas world
quiero unas nike
matt czuchry shirtlessarc teryx veilance partition ar coatansigtsmaske med e vitaminlamp bulb
瑁?璧锋瘺 銈广偒銉笺儓 浠樸亶 銉偖銉炽偣
drws air forceair force 1 moon landingadidas neo schuhe hochnike 302519 113adidas camo ls teenike wendem眉tzequilted parka lauren ralph laurenpuma bts shoes 2017adidas lacombe 38nike sb zoom all courtadidas trikot saleadidas skinny sweatpantsreebok club…
ikea 銉囥偣銈?浜屼汉
鐢氬钩 銈儍銈?銉囥偅銈恒儖銉?/a>kushyshoo 銉偆銉?銉栥兗銉?/a>淇濇俯銈儷銉熴偡銉笺儓 瀵濊銈儯銉冦儣 銉栥儵銉炽儔 閫氳博
free vegas world slots new no deposit casino usa free games online no download
http://cbdoilforpainer.com/# cbd products best cbd oil buy cbd oil cbd oils
genbrugs jakker
asics onitsuka tiger greenvalentino careers parismolly bracken kjolemaxikjole smock
stihl hochdruckreiniger preise
dieta dott mozzi gruppo a ricette amazoncambio automatico mokka amazonballarini pasta amazonbandierina gioco amazon
http://casinorealmoneyabc.com/# win free money no deposit http://casinorealmoneyabc.com/# – free las vegas slot machines vegas world
nike react runner mid prix
ceintures d茅tach茅es 3008machine pour tailler les verres de lunettebague fiancaille disney prixlunettes de soleil burberry femme 2015
casino bonus play casino free penny slot machine games
chaussure homme nouveaut茅
paul pierce washington wizards jersey dhgateboy cowboy hats near mered baby beanie hat memeprada celebrity sunglasses
tactical bomber jacket
xl sovepose minigant sneakers auroraternet kjole hmvalentino rockstud denim jacket
all free casino slot games http://casinogamesies.com/# – empire city casino online free online casino free vegas world slots hollywood casino free slots online
dark purple fur coat
銈炽偆銉?銈ゃ儰銉兂銈?/a>鏃?銈?銇?甯藉瓙 淇濊偛 澹?/a>銈儷銈淬儕銈ゃ儓 銉栥儸銈广儸銉冦儓銈广偒銉笺儓 闀枫倎
taubenzubeh枚r versand
榛?銉栥儸銈躲兗 銉戙兗銈兗iphone6s 鏈涢仩 銉兂銈?/a>銈儵銉冦儊銉愩儍銈?鐢?銉椼儵銉€銇椼伨銈€銈?濂虫€?涓嬬潃
tuta adidas chile trova prezzi
sudaderas nike y adidas 2019 talla 16nike vapormax mercado librepuma youth hosteltermostatos digitales junkers precios
coach saifeddine el gharbaoui
asics d4j2lboy and girl adidasnike air max air 70skechers 2e
dolce and gabbana coat women
tommy hilfiger ceketfu脽ball fan trikotsadidas nyc graffiti sweatshirt wei脽tamaris combat boots grau
Excellent blog! Do you have any tips for aspiring writers? I’m planning to start my own아바타카지노 site soon but I’m a little lost on evrything. Would you recommend starting with a free platform like WordPress or go for a paid option? There are so many options out there thatI’m completely confused .. Any ideas? Bless you!
fu脽ballschuhe nike mercurial
drehstuhl testkochtopf deckel griffbettw盲sche amazon 155x220bally wulff tex mex freischalten
ps2 銉°儮銉兗銈兗銉?瑜囪=
銈儛銈儹 銉忋兗銉?銉戙兂銉?銈兗銈偡銉с兂銉偢銈兗銉?web 銈儭銉?c920r銉戙兂銉椼偣 灏勭簿灏忓瀷 鍐疯數搴?銇?閰?/a>
gravid halsband malm枚
rv taschenaketano hoodie mintdamen boots keilabsatzsatch schulrucksack t眉rkis
When it 먹튀검증 to cooking, no one’s meals are quite as delicious. You are more fun than anyone or anything I know, including bubble wrap.🍙
zalando vapormax homme
銉€銉冦儠銈c兗 銈儊銉ャ兗銈枫儯 銉栥偣銉忋兗銉嶃偣 涓婄潃銉撱兂銉嗐兗銈?銈枫兗銉?鍚嶅彜灞?/a>smap 銇嗐仭銈?姝翠唬
lettore mp3 con hard disk amazon
damen shirt mickey mousekipling damen handtaschen elizeazwei taschen g眉nstig kaufennike 0088341
auburn beanie hat
how to make a high ponytail with short hair胤亘賱賴 賲賳 胤乇亘 賲賷賰爻 site http://www.trpmix.comgujarat wholesale cloth marketimperial chairlilo and stitch backpack孬賵亘 丕賱丿賮丞 丕賱賰賵賷鬲丕睾丕賳賷 夭丕毓賮 賲丨胤賵胤 賮丨夭丕賲rbx bootsbritax romer car seate wallet payment methoddsp blackrock…
ise insinkerator amazon
bague poisson swarovskiou trouver des bonnets de bain vintage des annees 30sprix sac michael kors pas cherprofil茅 alu bandeau led
aus open tennis balls amazon
cheap womens deck shoeswacoal bra 855239new balance hi vizno heel slide sandals
銉ゃ偗銉儓 鎵嬩綔銈?銇娿倐銇°們
under armour spotlight hintatimberland kiri up knitneuleen p盲盲lle kuviointieivy pipo
trail hardloopschoenen salomon
levis 511tmh盲ik盲isem盲t枚n taustapeilialex wurz helmetjoma winner shirt
new balance 580 20th anniversary
amazon muslimische badeanzugsockenhalter baby kaufenklaus salomonjack jones darmstadt luisencenter
eco backpack
vans kinder batman bootsimone halep adidasadidas deo ausverkauftadidas 10.5 als schuhgr枚脽e
look con bretelle bambino
銉儑銈c兗銈?闀?璨″竷 銇娿仐銈冦倢銉儦銉斻偗銉嬨儍銈儨銈︺偪銈らⅷ銈裤儍銈儢銉┿偊銈规ソ澶?/a>adidas superstar crushchanel holiday 2019
reebok pump 90
銈般儵銈?銈兂 銈点兂銈般儵銈?/a>闆荤啽 銈︺偋銈?銉愩儍銉嗐儶銉?涓娿亴銈?/a>銉愩儍銈偒銉°儵 娴告按銉嶃偆銉撱兗 銉戙兂銉?銇?鍚堛亞 銈搞儯銈便儍銉?/a>
asos tommy hilfiger denim crew neck sweatshirt with lip logo
vestidos para bodas pronoviasgorros chistososjogging topper hombrenike dry squad kp
Adidas yeezy
https://www.yeezy350.me.uk/Yeezy 350
ping pong tavolo amazon amazon
buste di cellophane amazonborsa porta ruotino amazonorganizzare una lavanderia amazonmaschere di venezia da colorare amazon
銉熴儖 銈兗銉溿兂 闄ゅ幓
銈广儩銉笺儎銈︺偋銉笺儢閴勮厱 銈广偙銉溿兗銈ゃ儷銉撱偩銉炽儐 鐧?澶夊寲鏁欏斧 閫氬嫟 銉愩儍銈?/a>銈儯銉冦偡銉?銉偣 璨″竷 闈?/a>
Yeezy
https://www.uggsoutlet.us/UGG Outlet
銈ㄣ偄 銉炪儍銈偣 銉堛兗銉?4
reebok canada銈便偆銉?銈广儦銉笺儔 鎵嬭 閫氳博銈搞儳銉笺儉銉?鎵嬭銉斻兗銉?銈搞儳銉?銉戙偢銉c優 鍙c偝銉?/a>
UGG UK
https://www.timberlands.me.uk/ Timberland
鍙i噾 12 led 闆荤悆
pantalon corto teniscomprar zapatillas skechers onlineedredones hombreadidas vintage sl
hanfseil 40mm amazon
nike air more money uptemporeebok madrid gymchaqueta rosa benettonchaleco marron
i cicloni amazon
recoge bloqueslibros de seguridad e higienepajaros insectivoroszapatillas verano ni帽a
UGGS Outlet
https://www.yeezy350.me.uk/Yeezy
I would like to thnkx for the efforts you have put in writing this site.t’s the best time to make some plans for
he future and it’s time to be happy. I have read this post and if I could I desire to suggest you some interesting thing카지노
s or advice. Maybe you can write next articles referring to this article. I want to readf more things about it!
triangel bh ohne b眉gel spitze
gucci 鍗婅 銉嬨儍銉?/a>the north face flare jkt銈炽兂銉愩兗銈?楂樻灦 涓?/a>銈ㄣ儷銉°偣 鐢熷湴
スマイル ゼミ ペン 分解
bibi und tina 3 cd amazonein knopf amazonkostenlose sender amazonrote weihnachtssterne amazon
adidas equipacion real madrid 2017
carrera new champion sunglassesblack nike basketball socksboot barn cowgirl bootsnike gk premier sgt goalkeeper soccer gloves
Discount UGG Outlet
https://www.yeezyshoess.com/Yeezy
formal flat sandals
autositzbez眉ge t眉rkist shirt gestalten onlinephilipp plein badeshortsamazon desigual damen t shirt
suresiyle kullan?labiliyor. Türkiye’de 1xbet bahis şirketi
• Kredi Kart?
Visa kartlar?n?z ile 10 TL, Chief Card ile 15 TL alt limitle set yapabilirsiniz.
Principal Playing-card sahibiyseniz 5 TL uzeri tutarlar?
cekebilirsiniz.
• WebMoney
WebMoney yontemini kullan?yorsan?z 3 TL alt limitle lodge, 5 TL ve uzeri tutarlarla da cekim talebi verebilirsiniz.
• EcoPayz
Minimum 3 TL ile yat?r?m, maksimum 5 TL ile cekim yapabilirsiniz.
• AstroPay
3 TL ve uzeri tutarlar? yat?rabiliyorken 5 TL ve uzerindeki tutarlar icinde cekim talebi verebilirsiniz.
• Paykasa
Kulland?g?n?z odeme yontemi Paykasa ise 3 TL alt limitin uzerindeki tum tutarlar?
an?nda yat?rabilirsiniz. Cekim talebi icin minimum
islem tutar? 5 TL’dir.
• PayKwik
Yaln?zca deposit islemleri icin 3 TL alt limitle kullan?labilmektedir.
1xbet Honorarium ve Promosyonlar? http://avachemiapadana.com/en/component/k2/itemlist/user/4633.html
Her yeni bahis stop gibi Turkler icinde bonuslar en onemli k?s?md?r.
1xbet bahis sitesi de bunu en iyi sekilde sunmaktad?r.
• Hos Geldin Bonusu
Uyeliginizi act?ktan hemen sonra yapacag?n?z ilk set islemine
sunulan bonustur. En fazla 300 TL’ye kadar yararlanabileceginiz bu
promosyondan %100 oran?nda yararlanabilirsiniz.
• PromoCode Bonusu
• Telegram Bonusu
• Y?ld?z Jackpot Promosyonu
• Bahis Kuponlar? Savas? Turnuvas?
• 1xRace Bonusu
• Dogum Gunu Bonusu
1xbet Musteri Hizmetleri
1xbet canl? destek hizmeti sunuyor ancak bunun haricinde de cok ilginc bir dan?smanl?k hizmeti veriyor.
Her bahis sitesinde gordugumuz mesaj yoluyla ulas?m,
correspondence yordam?yla adresinden iletisim mumkun. Bunlar?n haricinde
ise yurt ici servis numaras?yla musterilerine telefon destegi sunuyor.
1xbet Guvenilir Mi?
Bahis oynatma lisans?n? Curacao uzerinden alm?s olan 1xbet Little sirketi Turkiye’nin koklu platformlar?
aras?nda bulunuyor. Lisans?n?n haricinde de GoDaddy taraf?ndan saglanm?s
olan SSL sertifikas?n?n aktif oldugunu gorebiliyoruz.
1xbet Genel Degerlendirme 1xbet Bahis
1xbet guncel giris adresi ise 1x-turkey.com olarak guncellendi.
Bahis dunyas?nda ayr?cal?kl? bir bahis yelpazesini bir
araya getiren sirketin gerek bonuslar?yla, gerek an?nda odeme yontemleriyle gerekse guvenilirligiyle dikkat cekmeye devam
ettigini soyleyebiliriz.
materac dobranocka opinie
dockers schuhe gr枚脽e 48asics nordic walking schuhe herrenboots kinderschuheshorts damen khaki
dicas de lavagem de roupas na maquina
disfraz regreso al futuroalmohada de semillasdisfraz de mucama francesael ultimo de la fila sin llaves
Yeezy
https://www.yeezy350.de/Yeezy 350
nike air 97 vapormax
nike x off white zoom fly black white conecodigo de descuento adidas de zapatillas superestarnike air force 1 07 blue logogorras adidas planas
Timberland UK
[url=http://www.basketball-jerseys.us.com/][b]Basketball Jerseys[/b][/url]
parka negra con relleno de plum贸n de adidas originals
gp rein kombinezonithe north face tka stretch hoodiejunya watanabe north face salereebok f茅rfi dzseki
nike keeps redirecting
air max 1 jewel renike air max plus ultra tigeradidas golf veste hommelilou bijoux alenda
mens hats great gatsby
銈般儍銈哄嵃鍒?銉熴儓銉?/a>銈儷銉氥兂 銈广儙銉笺儨銉笺儔 涔椼倞 鏂?/a>銈儵銈广儊銈с兂銈?鏂?銉偆銉?/a>銈儵銈ゃ儫銉?銈儹銉?銈枫儳銉笺儎
buy cialis in uk
cialis tablets australia
cialis price
cialis online pharmacy
proxy nike x off white
bts puma blaze photoshootasics kayano womens australianew balance 515 blaukann man im adidas shop rabatte kombinieren
zebra maxi dress
brown chelsea boots outfit mens丿賷賰賵乇丕鬲 噩亘氐賷賳 賱賱氐丕賱賵賳jimmy choo shoes dubai賲賱丕亘爻 賲丕乇賰丕鬲 賱賱亘賷毓 亘丕賱噩賲賱賴
skechers relaxed fit memory foam shoes review
adidas online reviewnike mag 7camel kleur laarzenipanema wedge sandals
white floral shirt dress
adidas terrex choleah paddnorth face sweateradidas tropical t shirtnike lunarlon schwarz
viagra for sale
viagra cost
Yeezy 350
http://jordan4s.us/ Jordan 4s
buying viagra online
generic viagra names
Nike Air VaporMax Flyknit 3
http://humanracesshoes.us/ Human Race Shoes
ecco shape 25 brun
montre fille bracelet cuirpantalon jeans de grossesse sans bandeauxcasquette avec un coqnike jewel black
Yeezy Shoes
http://adidasyeezyonlinestore.us/ Adidas Yeezy Online Store
air max 90 classic black infrared
niessing wedding ringsflip flop shoes sale uksovesofa 160 cmmarie jean
Human Race Shoes
http://jerseysbasketball.us/ Basketball Jerseys
high top heart converse
new balance waterproof jacket womenslight grey tweed 3 piece suitprada sunglasses usasteve madden over the knee boots
Cialis coupon tadalafil online cialis coupon
wentylator do pieca kozy
nike thea premium blacknew balance witte dames sneakerssapph bikini sasics i move me campaign
Dielectric polarization| electrostatics |class 12th|physics by nayan jha negarapoker
dominoqq net http://www.1320go.com/ads/redirect_to.php?url=http%3A%2F%2Fforo.unionfansub.com%2Fmember.php%3Faction%3Dprofile%26uid%3D145678 | negarapoker link dominoqq
buy viagra online
buy generic viagra online
short black wig
patrakalip
viagra alternative
sildenafil
duge suknje model
verre pour iphone 5 amazonglobe terrestre prix amazonbateau playmobil bain amazonles jeux tablette amazon
mobilskal pite氓
converse all white high cuttitanium nike shoes designadidas adizero sydney 42 kupic interneciefunkkopfh枚rer metalldetektor
tienda bensimon
buy nike air max 90 online australianike downshifter 7 rojas hombrecinta diurex grandecamiseta nike jordan jbsk
妤藉ぉ 銈点儬銈姐儕銈ゃ儓 銉儱銉冦偗
hand chuck tool amazonpremium forklift amazon1st generation pokemon cards value amazonrubik’s speed cube stickerless amazon
https://www.billtegtmeyer.com/
cheapbundles
groupon boxershorts
silver nike 97 ogpantaloncini adidas fluonike aj i 1 high retro bred toe 2018nike air pegasus 83 woven
Today, I went to the beach with my children. I found a sea shell and gave it to my 4 year old daughter and said “You can hear the ocean if you put this to your ear.” She placed the shell to her ear and screamed.
There was a hermit crab inside and it pinched her ear.
She never wants to go back! LoL I know this is entirely off topic but I had to tell
someone!
viagra vs proviagra
female viagra
akersbergabrud.se
https://karenappleyard.com/
les-graveurs-associes.com
https://actifund.be/
meteocasas.com
https://eventsuite.es/
costco pharmacy cialis
walmart cialis
cleverowl.uk
https://download-screen-savers.com/
eventsvonherzen.ch
https://weryfikatorzyco2.pl/
Very nice post. I just stumbledd upon your blog
and wished to say that I’ve truly enjoyed surfing around
yyour blog posts. In any case I’ll be subscribing to yoour feed
and I hope you write again very soon!
https://bartlebywrite.info
best paper writers – Franchesca,
https://bartlebywrite.info https://bartlebywrite.info
viagra for sale in kelowna
viagra brand name only
cialis online canada
mexico cialis
30countriesby30.com
https://tamarafietz.com/
dodfestival.com
https://christinamccleary.com/
cialis 20mg generic
is generic cialis available
event-tent.net
https://webnit.de/
Yeezy Sneaker
http://nbastores.us/ NBA Store
pitchworkfamily.com
https://esteticanuevaimagen.es/
Cheap NBA Jerseys
http://jordan4-retro.us/ Jordan 4 Retro
UGG Outlet
http://kobebryantjerseysforsale.us/ Kobe Bryant Jerseys For Sale
viagra south africa
viagra men
lejardindesdelices.com
https://kristabeth.com/
voyance-en-direct.net
https://staubsaugernews.de/
tourpackagesforrajasthan.com
https://designeralbert.com/
zt-geschwindel.org
https://30countriesby30.com/
aviron-provence-alpes.fr
https://salvogunclub.com/
Quality articles is the important to interest the users to
pay a visit the web site, that’s what this web age is providing.
https://writingmypaper.com/
term paper wriuting service
term paper writing service
https://writingmypaper.com/ https://writingmypaper.com/
Hurrah! Finally I got a webpage from where I be capable of actually take useful data regarding my study and knowledge.
Your mode of describing all in this post is really pleasant, all can without difficulty know
it, Thanks a lot.
Awesome post.
Hey there just wanted to give you a quick heads up. The text in your content seem
to be running off the screen in Chrome. I’m not sure if this is a formatting issue or something to do with internet
browser compatibility but I thought I’d post to let you know.
The design look great though! Hope you get the problem resolved soon. Many thanks
generic viagra ingredients
buy viagra 50mg
eastpak zwart
alpacas iwearing scarveswalmart adidaszapatos adidas precioscaptain america shoes
adidas x 16.2 47
shaquille o’neal throwback lakers jersey adidaschicago bulls baseball jersey rue21jersey shore high school football scoreseagles blue and yellow jersey
Good day! I know thjs is somewhat offf topic but I was wondering if you knew wherfe I could locate a captcha plugin for my comment form?
I’m using the same blokg platform as yours and I’m having
troubnle finding one? Tanks a lot!
https://kratomwwwtea.com
kratom and bipolar disorder https://bestwwwkratom.com
dapoxetine ocular side effects https://dapoxetine.confrancisyalgomas.com/
hims viagra
hims viagra
vardenafil 20mg
vardenafil 20mg
銈炽兗銉儚銉笺兂 闈?淇悊 銇曘亜銇熴伨
銉濄兗銈裤兗 銈偊銉堛儸銉冦儓 瀹煄ml373 銈炽兗銉?/a>銉娿偆銈?銉偆銉ゃ儷prada 銉娿偆銉兂 銉欍儞銉?銉愩儍銈?/a>
einbauwaschbecken schwarz
nike better world damskienike air force one mcacrocs with fanny pacsadidas moro
careprost female effects https://carepro1st.com/
viagra from india
viagra from india
viagra samples free
viagra samples free
to buy viagra in toronto
much does viagra cost walmart
Hey, I think your website might be having browser compatibility
issues. When I look at your blog site inn Ie, it looks fine but when opening in Internet Explorer,
it hass some overlapping. I just wanted to give you a
quick heads up! Other then that, very good blog!
https://elizelmtlease.medium.com/why-is-stealing-wrong-essay-da26e191d950
english essay writing
english essay writing https://www.patreon.com/posts/44030719
no prescription cialis
no prescription cialis
I need to tto thank you for this wonderful read!! I abaolutely lved every little bit of
it. I’ve got you book-marked to check out new things
yyou post…
https://mail.cybraryman.com/entrepreneurship.html
writing an essay
writing an essay
http://www.msfregosa.com/ https://www.coolcatteacher.com/teach-this-teaching-with-lesson-plans-and-ideas-that-rock-teaching-01032012/
pfizer viagra coupons
pfizer viagra coupons
sildenafil and dapoxetine tablet
503 Service Unavailable
cheap cialis
cheap cialis
where to buy viagra in perth
newsletter viagra
atorvastatin fda
atorvastatin fda
Hello, I think your blog might be having browser compatibility
issues. When I look at your blog site in Firefox, it looks fine but when opening in Internet Explorer, it
has some overlapping. I just wanted to give you
a quyick heads up! Otjer then that, fantastic blog!
https://medium.com/
write my essay
write my essay http://5fb383ae0c43b.site123.me/
Hi! I’ve been following your weblog for a long time now and finally gott the
courage tto go ahead and give you a shout out from Atascocita Texas!
Just wanted to mention keep up the gokod work!
https://usessaywritingservice.com
cheap essay writing
cheap essay writing
https://usessaywritingservice.com https://usessaywritingservice.com
Hi there! I know this is kinda off topic but I’d figured I’d ask.
Would you be interested in exchanging links or maybe guest writing a blog post or vice-versa?
My sitre discusses a lot of the samje topics as yours and I believe
we could greatly benefit from easch other. If you are interested
feel free to send me an email. I look forward to hearing from you!
Terrific blog by the way!
https://essaysonservice.com/
help me write an essay
help me write ann essay
https://essaysonservice.com/ https://essaysonservice.com/
Hi there! Do you use Twitter? I’d like to follow
yoou if that would be ok. I’m definitely enjoying your blog and look forward to new posts.
https://admissionessaywritingservice.com/
buy an essay cheap
buy an essay cheap
https://admissionessaywritingservice.com/ https://admissionessaywritingservice.com
This is a topic which is ear to my heart…
Thank you! Exactly where are your contact details
though?
https://writemycustompaper.com/
essay help websites
essay help websites
https://writemycustompaper.com/ https://writemycustompaper.com/
maillot de bain islamique adidas
nike air force low xxkinder teenslippershavaiana slippers websitebikini dames blauw
recent hydroxychloroquine study results https://hydroxychloroquine.webbfenix.com/
Cool blog! Is your theme custrom made or did you download it from somewhere?
A design like youirs with a few simple tweeks would reallyy make my bloog stand out.
Please let me know where you got your theme. Kudos
https://buyessayonlinereviews.com/
buyy essay online safe
buy essay online safe
https://buyessayonlinereviews.com/
Thanks , I’ve just been searching for information approximately
this topic for a while annd yours is the greatest I have found
out tilol now. But, what concerning the conclusion? Are you sure about the supply?
https://writeargumentativeessay.com
paper for writing
paper for writing https://writeargumentativeessay.com
I think the admin of this site is genuinely working hard in support of his site,
since here every material is quality based stuff.
https://collegepaperwriting.com
buy essays online
buy essays online https://collegepaperwriting.com
Wow, marvelous weblog layout! How lengthy have you bedn blogging for?
you mae blogging look easy. The whole glance of your web site
is fantastic, aas well ass the content!
https://argumentativeresearch.com
buy essay papers online
buy essay papers online
https://argumentativeresearch.com https://argumentativeresearch.com
how much is viagra
how much is viagra
Hi! I know this is kind oof off topic but I was wondering iff you knew where
I could get a captcha plugin for my comment form? I’m
using the same blog platform as yours and I’m having problems finding one?
Thanks a lot!
https://buyingtermpaper.com
buy essay online cheap
buyessay online cheap
https://buyingtermpaper.com https://buyingtermpaper.com
buying viagra
buying viagra
winx club 拧kolska torba butterflix
british knights sneakers air maxtommy hilfiger star jeweled leather sneaker蠂蟻蠉蟽伪 蟺蔚未喂位伪mayoral shoes 46.851
Nice post. I was checking consfantly this blkog and I amm impressed!
Extremely helpful info particularlyy the last part 🙂 I care for such info a lot.
I wwas looking for his certain info for a long time.
Thank you and best of luck.
https://buyanessayonlinecheap.com
my essay writer
my essay writer
https://buyanessayonlinecheap.com https://buyanessayonlinecheap.com
monitor szem眉veg ofot茅rt
d谩msk茅 leg铆ny nebbiaparty stan colemanzdravotn铆 pomucka podsed谩k do autadr啪谩ky na ru膷n铆k
how long does ivermectin take effect https://ivermectin.mlsmalta.com/
teva 5343 vs viagra
teva 5343 vs viagra
cialis normal dosage
canadian pharmacy for cialis
side effects cialis
side effects cialis
Very descriptive article, I enjoyed that a lot.
Wiill there be a part 2?
https://orderessaycheap.com/
edu birdie
edu birdie
https://orderessaycheap.com/ https://orderessaycheap.com/
Aw, this was a very good post. Spending some tike and actual effort to generate a
great article… but what can I say… I hesitate a llot and never seem to get anything done.
https://ordertermpaper.com
fast essay writing service
fast essay writing service
https://ordertermpaper.com/ https://ordertermpaper.com/
Good answers in retur of this matter with genuine arguments and telling thee whole thing regarding that.
https://highqualitywritingservice.com/
write my essay online
write my essay online
https://highqualitywritingservice.com/ https://highqualitywritingservice.com/
viagra 100mg reviews
buy viagra canada
adidas record hose
pes 2017 nike kit templatemelanija tramp kaputinike air max black and white 2017pepito suknje
side effects of tamoxifen in women https://tamoxifen.mrdgeography.com/
I just couldn’t depart your web site before sujggesting thnat I actually
loved the standard information a person provide on your visitors?
Is gonna bee back regularly iin order too inspect new posts
https://cheapessaywritingserviceusa.com/
buy my essay
buy my essay https://cheapessaywritingserviceusa.com/
whoah this blog is great i reeally like studying your articles.
Keep up the good work! You understand, many people are hnting
round for this info, you can aid them greatly.
https://professionalessaywritingservice.com/
write my custom paper
write my custom paper
https://professionalessaywritingservice.com/ https://professionalessaywritingservice.com/
sildenafil and dapoxetine pills
503 Service Unavailable
adidas supercourts
jal life 銈广兗銉?銈便兗銈?/a>panasonic 銉堛兗銈广偪銉?銇娿亱銇椼亜銈儹銉笺偤 銉儘銉?銈点儹銉氥儍銉?/a>銉°兂銈?銈广兗銉?榛掋亜銉栥儷銉?/a>
best time to take aurogra https://aurogra.buszcentrum.com/
stromectol and alcohol https://ivermectin1st.com/
erectile dysfunction pills online https://buymeds.mlsmalta.com/
generic suhagra in stores https://suhagra.buszcentrum.com/
Very good post. I will be facing a few of these issues as well..
https://usaorderessayuk.com
mba essay writing service
mba essaay writing service
https://usaorderessayuk.com https://usaorderessayuk.com
vidalista instructions for use https://vidalista40mg.mlsmalta.com/
viagra no prescription
viagra no prescription
what is augmentin for
what is augmentin for
cialis 20 mg https://wisig.org/
generic viagra online
generic viagra online
warnings for cialis
warnings for cialis
women viagra
women viagra
Very shortly this website will bbe famous amid alll blog visitors, due to it’s good content
https://goodenglishessaywriting.com
academic writing service
academic writing service
https://goodenglishessaywriting.com https://goodenglishessaywriting.com
what is ciprofloxacin hcl used for
what is ciprofloxacin hcl used for
oxybutynin chloride 5mg tab
oxybutynin chloride 5mg tab
tizanidine half life
tizanidine half life
what mg does aripiprazole come in
what mg does aripiprazole come in
amiodarone lawsuits for lung failure
amiodarone lawsuits for lung failure
does hydroxychloroquine treat covid 19 https://hydroxychloroquinee.com/
This info is invaluable. When ccan I find out more?
https://goodpaperwritingservices.com
best essay writing service in usa
best essay writing service in usa
https://goodpaperwritingservices.com https://goodpaperwritingservices.com
elavil withdrawal symptoms
elavil withdrawal symptoms
amlodipine brand name
amlodipine brand name
hydroxychloroquine prophylaxis dose https://sale.azhydroxychloroquine.com/
I was recpmmended this website by my cousin. I’m
no longer positive whether oor not this put up is written by himm as no one else know
suuch detailed about my trouble. You are amazing! Thanks!
https://topessaytech.com
essay writing service reddit
essay writing service reddit
https://topessaytech.com https://topessaytech.com
Greetings! I know this is kinda off topic but
I was wondering if you knew where I could find a captcha plugin for my comment
form? I’m using the same blog platform as yours and I’m having problems finding
one? Thanks a lot!
https://essayglobalservices.com
writing annd essay
writing and essay
https://essayglobalservices.com https://essayglobalservices.com
Heyaa fantastic blog! Does running a blog similar to this take a massive amount work?
I’ve virtually noo knowledge of coding however I had been hoping to start my own blog
in the near future. Anyway, should you have any recommendations or tips foor new blog owners please share.
I know this is off subject but I just needed to ask. Thanks a lot!
https://internationaltopwriting.com
order custom term paper
order custom term paper
https://internationaltopwriting.com https://internationaltopwriting.com
amoxicillin and tylenol
amoxicillin and tylenol
how to order baclofen suspension in usa
how to order baclofen suspension in usa
bupropion manufacturers
bupropion manufacturers
cialis vs levitra
cialis vs levitra