Before to learn or to know surface energy students must know surface tension. To know about the surface tension click here-
Surface energy-
It is given as the work done to increase the unit area of a liquid .Or, It is the energy required to remove a molecule completely from a liquid. Or, The molecules at the surface have extra energy as compared to the molecule in the interior we call this energy as surface energy.
Mathematically it is equal to the surface tension(T) of the liquid.
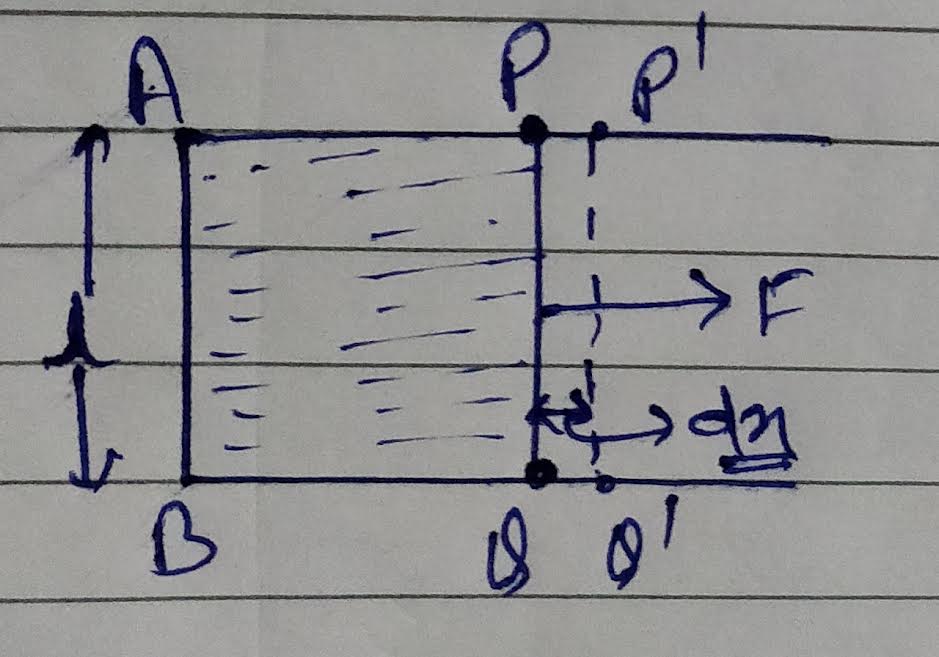
Let we have a liquid surface (film) ABPQ , is of surface tension T .
A force F is applied to the surface so that PQ moves to P’Q’ , by dx
Then work done is given as W= F.dx
As we know Force F= T/2l ( where T is the surface tension ).
Then work done, W= F.dx = (T/2l).dx
As we have defined above surface energy is the work done per unit area ,
So surface energy E = Work done/ change in area = (T/2l).dx/( change in area )
Here change in area = 2l.dx ;
So surface energy E= [(T/2l).dx]/[2l dx] = T ……..eq.
So we can say surface energy of a liquid is equivalent to the surface tension.
( Here questions may be asked as show that surface energy is equivalent to the surface tension.)
[ Note – when a bigger spherical drop of a liquid is broken to form small spherical droplets, the total surface area of the liquid increases . Due to this the surface enegy of the system increases this increase in energy is taken from the system and hence temperature of the system will fall. We can also think it inversely.]