Surface tension– It is the property of a free surface of liquid to get minimum surface area .
The property of a liquid at rest by virtue of which its free surface behaves like a stretched elastic membrane and tries to occupy as small an area as possible called surface tension.
As we know in a liquid , the molecules are not as close to each other as they are in solid . The result in a liquid not having a strong enough cohesive force to have a rigid structure as a solid . But these intermolecular forces are strong enough to give the liquid a definite shape. These molecular force is effective upto certain distance called molecular range . The uppermost layer of the liquid which thickness is equal to the molecular range is called surface film.
To watch the most important derivation of surface tension click on the video link given below-
We can define the surface tension in another way mathematically the surface tension of a liquid is the force of tension acting across a unit length of an imaginary line drawn on the free surface of a liquid at rest . The direction of this force is perpendicular to the line and tangential to the surface.
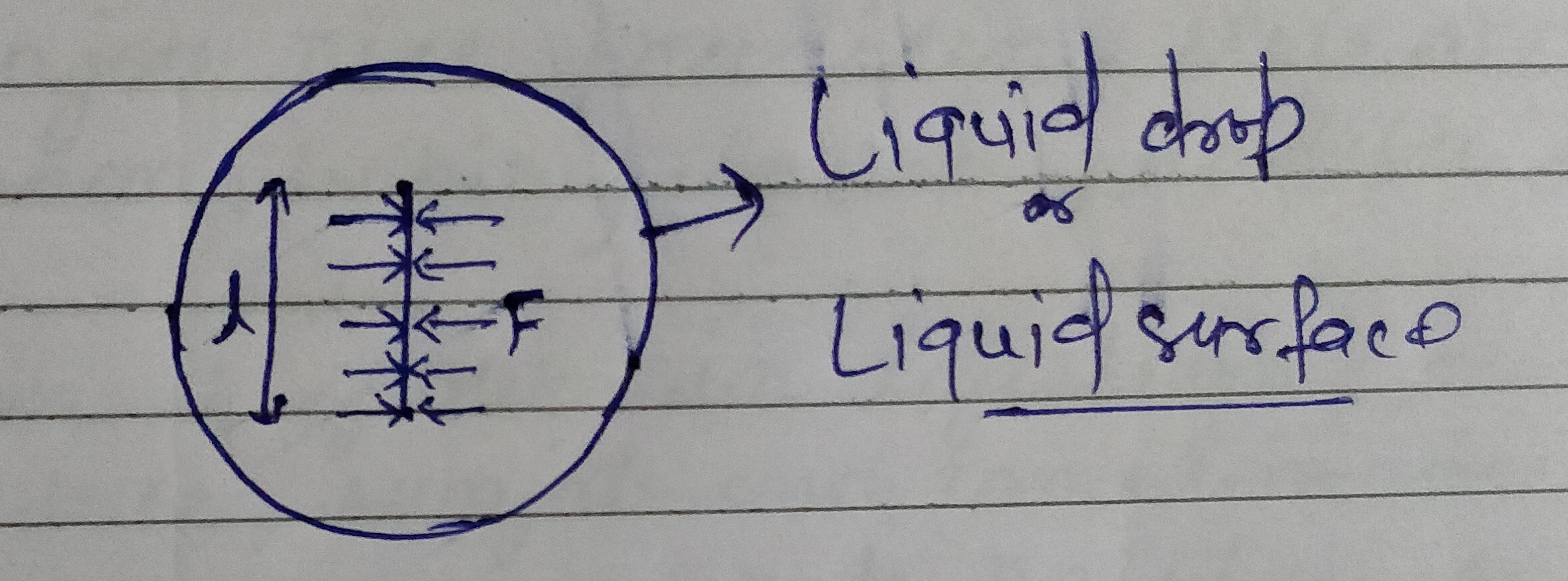
If F is the force and l is the length of an imaginary line on the surface of liquid then surface tension T is given as
T = F/ l .
But, for a surface film it is given as the
Surface tension ; T = F/2l ;
Unit of surface tension is , Newton/meter or , kgs-2
Dimension of surface tension is ; [ MT-2] or [M L0 T-2]
Note- 1. Temperature of the liquid increases surface tension decreases .
2. The surface tension arises due to the intermolecular cohesive forces .
3. The surface tension doesn’t depend on the area of the surface.
4. Soap or hot water helps in better cleaning of cloths because it reduces the surface tension of water.
After completing the surface tension students must know surface energy.
to get the notes on Surface energy click here